Open Access Article
Open Access ArticleStructural analysis of potassium borate solutions†
Fayan
Zhu
 ab,
Daniel T.
Bowron
ab,
Daniel T.
Bowron
 b,
Sabrina
Gärtner‡
b,
Sabrina
Gärtner‡
 b,
Chunhui
Fang
a,
Yongquan
Zhou
b,
Chunhui
Fang
a,
Yongquan
Zhou
 a,
Hongyan
Liu
a and
Alex C.
Hannon
a,
Hongyan
Liu
a and
Alex C.
Hannon
 *b
*b
aKey Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources, Qinghai provincial Key Laboratory of Resources and Chemistry of Salt Lakes, Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining Qinghai 81008, China
bISIS Facility, STFC, Rutherford Appleton Laboratory, Chilton, Didcot, Oxon OX11 0QX, UK. E-mail: alex.hannon@stfc.ac.uk
First published on 3rd April 2023
Abstract
In this work, H/D isotopic substitution neutron diffraction was combined with empirical potential structure refinement (EPSR) and DFT-based quantum calculations to study the interactions between B(OH)3 boric acid molecules, B(OH)4− metaborate ions, water molecules, and potassium cations in borate solutions. The results show that the solute ions and molecules have a marked effect on the second coordination shell of the water molecules, causing a greater deviation from a tetrahedral structure than is observed for pure water. Potassium ions and trans-B(OH)3 tend to form a monodentate contact ion pair (MCIP) with a K–B distance ∼3.8 Å, which remains constant upon changing the solution concentration. Potassium ions and cis-B(OH)3 form both a MCIP at K–B ∼3.8 Å and a bidentate contact ion pair (BCIP) at K–B ∼3.4 Å. As the solution concentration increases, there is a BCIP to MCIP transformation. Boric acid molecules can undergo hydration in one of three ways: direct hydration, interstitial hydration, and axial hydration. The energetic hydration preference is direct hydration → interstitial hydration → axial hydration. Nine water molecules are required when all water molecules directly interact with the –OH groups of B(OH)4−, and a tenth water molecule is located at an interstitial position. The hydrogen bonding between boric acid molecule/metaborate ion and water molecules is stronger than that between water molecules in the hydration layer.
Introduction
Boron-containing compounds have important applications in medical treatment, agriculture, and chemical products,1–4 and they also have significant academic research value. The electron-deficient B atom has two structural units, BO3 and BO4 in borate anions, which can transform between themselves upon changing the atomic and molecular stoichiometry of the solution, or the species of cation within the mixture. Wright et al.5,6 defined five basic structural units of BO3 and BO4 according to the bonding between the oxygen atoms and the boron atom. They also defined superstructural units for boron-containing cyclic species. This diversity of borate structures leads to a variety of interesting properties of borate materials.A unique property of borate solutions is that a variety of boron-containing species can coexist in water. A significant amount of research has been performed using various techniques, including Raman spectroscopy,7,8 nuclear magnetic resonance (NMR),9,10 X-ray diffraction,11,12 and near-edge X-ray absorption fine structure (NEXAFS).13 There are at least six boron-containing species in borate solutions: B(OH)3, B(OH)4−, B3O3(OH)4−, B3O3(OH)52−, B4O5(OH)42−, and B5O6(OH)4−.14,15 New borate species have also been discovered in recent studies. For example, diborate [B2O(OH)5]− was shown to exist in solution using Raman spectroscopy and quantitative calculations,16,17 and the pentaborate ion [B5O6(OH)4]− was also shown to exist. The hexaborate [B6O7(OH)6]2− ion has also been found in magnesium borate solutions.18 Researchers have suggested that the cation greatly influences the structure of ion clusters in highly-concentrated solutions.19,20 For example, a complex ion cluster is composed of two potassium ions and one metaborate ion, B(OH)4−, in potassium metaborate solutions, while complex ion clusters dissociate into a sodium ion and two metaborate ions in sodium metaborate solutions. X-ray diffraction has recently been used to study the structure of borate solutions, the hydration number, and the distance between borate ions and water.11,12,21 Although X-ray diffraction can provide direct structural information, it has certain limitations when used to study the hydration structure of borate solutions because of the weaker scattering power of lightweight atoms such as H and B.
Recently, Pye et al.22 studied the structure and vibrational frequencies of boron-containing species and proposed the most stable structures of their isomers, and a variety of boric acid isomers were proposed. It was pointed out that the most stable boric acid molecules had C3h and Cs symmetries (trans-B(OH)3 and cis-B(OH)3), with similar energies. The species distribution map also showed that polyborate ions hydrolyzed to B(OH)4− and B(OH)3 in dilute solutions.23 This hints that the two B(OH)3 isomers may coexist in borate solutions, but few experiments have reported these two structures. For this reason, we used quantitative calculation methods to study the hydrolysis mechanism of polyborate ions24,25 and found that B5O6(OH)4− has two hydrolysis pathways: water-poor and water-rich. During water-poor hydrolysis, the products are B(OH)3 and [B3O3(OH)4]−. [B3O3(OH)4]− further hydrolyzes to B(OH)4− and B(OH)3 in a water-rich process, and all boric acid molecules are cis-B(OH)3. [B4O5(OH)42−] finally hydrolyzes to cis-B(OH)3 and trans-B(OH)3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. This was the first time that these two isomers were found in borate solutions.
1 molar ratio. This was the first time that these two isomers were found in borate solutions.
Researchers26–29 have studied the structures of boric acid molecules and B(OH)4− using quantitative calculations. The bond length, bond angle, Raman spectra, and water number of boric acids that formed Lewis bases were given, but few studies have shown whether trans-B(OH)3 and cis-B(OH)3 molecules coexist in solution, and their structure details remain unclear. The hydration structure of boric acid molecules and metaborate ions was studied using NEXAFS13 and it was found that the hydrated structure of borates had little effect on their spectra. The K-edge NEXAFS spectrum of B was not very sensitive to hydrogen bonds, solution environment, or interactions between solute and water molecules; therefore, the interaction between solute and water molecules should be further studied using other techniques.
Here, neutron diffraction measurements with hydrogen/deuterium (H/D) isotopic substitution30,31 (NDIS) were analysed using empirical potential structure refinement (EPSR) simulations to obtain atomic-scale information on aqueous borate solutions. The effects of solution concentration, type, and structure of boron species on the hydration structure are discussed. H/D substitution is used to change the scattering ability of hydrogen atoms and hence to obtain more structural information related to the hydrogen atoms in the borate solutions. Replacing natural boron with 11B eliminates the strong neutron absorption of 10B, which otherwise would be a great experimental handicap. The 11B neutron scattering length is 6.06 fm, which is comparable to the O scattering length of 5.80 fm.31 This study provides a method to accurately analyze the hydration structure of borate species. DFT-based quantum calculations are also used to reveal the structural details of hydration of borate species.
Neutron diffraction experiment and data analysis
Sample preparation
The first stage of the sample preparation was to make hydrous K11BO2·1.33H2O and K211B4O7·4H2O crystals by evaporation from solution, using information from the B2O3–K2O–H2O ternary phase diagram,32 and full details of the sample preparation are given in the ESI.† The crystals were then dehydrated, yielding anhydrous powders of K11BO2 and K211B4O7 respectively. The solutions were made from these crystals with the aim of ensuring that the K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio and the concentrations of potassium and boron in the solutions were as intended.
B ratio and the concentrations of potassium and boron in the solutions were as intended.
Hydrogen–deuterium isotopic substitution was used to obtain the structural information about hydrogen bonding.33 Potassium borate solutions were prepared by dissolving the above anhydrous crystals in H2O (double-distilled water), D2O (Sigma-Aldrich 99.9 atom %D), and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O and D2O. Solution densities were measured using a digital display instrument (Anton Paar) (Table 1) calibrated using pure water. The concentrations of the samples are specified by their water-salt molar ratio (WSR). The relationship between WSR and concentration (mol L−1) is shown in Fig. S4 (ESI†). The detailed composition information is shown in Table 1.
1 mixture of H2O and D2O. Solution densities were measured using a digital display instrument (Anton Paar) (Table 1) calibrated using pure water. The concentrations of the samples are specified by their water-salt molar ratio (WSR). The relationship between WSR and concentration (mol L−1) is shown in Fig. S4 (ESI†). The detailed composition information is shown in Table 1.
| System | WSR | K11BO2 (g) | K211B4O7 (g) | D2O (g) | H2O (g) | Density/g cm−3 |
|---|---|---|---|---|---|---|
| K[B(OH)4]_20 | 20 | 0.8494 | 0 | 4.1554 | 0 | 1.2993 |
| 20 | 0.8488 | 0 | 2.1876 | 1.9758 | 1.2396 | |
| 20 | 0.9282 | 0 | 0 | 4.0791 | 1.1957 | |
| K[B(OH)4]_60 | 60 | 0.3192 | 0 | 4.6980 | 0 | 1.1751 |
| 60 | 0.3206 | 0 | 2.4660 | 2.2179 | 1.1186 | |
| 60 | 0.3532 | 0 | 0 | 4.6550 | 1.0695 | |
| K2B4O7_60 | 60 | 0 | 0.8156 | 4.2124 | 0 | 1.2624 |
| 60 | 0 | 0.8164 | 2.2091 | 1.9901 | 1.2028 | |
| 60 | 0 | 0.8918 | 0 | 4.1139 | 1.1582 | |
| K2B4O7_100 | 100 | 0 | 0.5239 | 4.4770 | 0 | 1.2038 |
| 100 | 0 | 0.5231 | 2.3570 | 2.1280 | 1.1466 | |
| 100 | 0 | 0.5756 | 0 | 4.4317 | 1.0988 | |
| WSR: the molar ratio of water to salt. | ||||||
Neutron diffraction experiments
For neutron diffraction measurements, samples of volume ∼1.4 cm3 were transferred to a Ti0.677Zr0.323 null alloy container with internal dimensions of 1 mm thickness × 35 mm width × 40 mm height. The composition of the alloy was chosen so that ideally coherent scattering from the container is eliminated, because the average coherent scattering length of the alloy is zero (![[b with combining macron]](https://www.rsc.org/images/entities/i_char_0062_0304.gif) Ti = −3.438 fm and
Ti = −3.438 fm and ![[b with combining macron]](https://www.rsc.org/images/entities/i_char_0062_0304.gif) Zr = 7.16 fm31). Filled cells were loaded onto an automatic sample changer on the small-angle neutron diffractometer for amorphous and liquid samples (SANDALS) at the ISIS pulsed neutron source of the Rutherford Appleton Laboratory, UK.34 The temperature of each sample was controlled to 25 ± 0.1 °C. The experimental data were corrected for background scattering, absorption, and multiple scattering using the Gudrun routines.35 Finally, the diffraction data were normalized with reference to the measured scattering from a V-Nb null alloy plate. The corrected interference differential scattering cross-sections, F(Q), and pair distribution functions, G(r), are shown in Fig. 1.
Zr = 7.16 fm31). Filled cells were loaded onto an automatic sample changer on the small-angle neutron diffractometer for amorphous and liquid samples (SANDALS) at the ISIS pulsed neutron source of the Rutherford Appleton Laboratory, UK.34 The temperature of each sample was controlled to 25 ± 0.1 °C. The experimental data were corrected for background scattering, absorption, and multiple scattering using the Gudrun routines.35 Finally, the diffraction data were normalized with reference to the measured scattering from a V-Nb null alloy plate. The corrected interference differential scattering cross-sections, F(Q), and pair distribution functions, G(r), are shown in Fig. 1.
Data analysis
The total differential scattering cross-section of a sample contains structural information about pair correlations between the constituent atoms of the sample, and is expressed as the total structure factor, F(Q), defined by eqn (1), in which Q is the magnitude of the momentum transfer vector of the scattering process: | (1) |
![[b with combining macron]](https://www.rsc.org/images/entities/i_char_0062_0304.gif) α are the atomic concentration and the Q-independent coherent neutron scattering length of each atom type.
α are the atomic concentration and the Q-independent coherent neutron scattering length of each atom type.
The partial structure factors are related to the partial pair distribution functions, gαβ(r), via a Fourier transform weighted by the atomic density of the system, ρ, defined by eqn (2):
 | (2) |
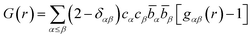 | (3) |
The EPSR program is a tool for extracting structural information from the measured F(Q).36,37 EPSR first performs a standard Monte Carlo simulation of a system using the intramolecular structure, the bulk atomic density, and a set of Lennard-Jones atomic reference potentials. It is predicted from the equilibrium constants that the metaborate ion B(OH)4− is the dominant ion for metaborate solutions,38 and these predictions are supported by experimental results from Raman spectroscopy and X-ray diffraction.39 Therefore the only borate anions in the EPSR models of metaborate solutions were B(OH)4−. Equal numbers of B(OH)4− and B(OH)3 anions were used in the EPSR modelling of K2B4O7 solutions because DFT calculations24 show that the tetraborate anion [B4O5(OH)42−] finally hydrolyses to equal numbers of B(OH)4− and B(OH)3. The relative numbers of trans-B(OH)3 and cis-B(OH)3 are not known, and thus they were assumed to be equal in the EPSR modelling of these solutions. The details of the structural models and simulation box are shown in Table S3 (ESI†) and Table 2. EPSR modelling was performed using the three experimental structure factor measurements for each composition up to a maximum momentum transfer of 30 Å−1, and the minimum momentum transfer of the structure factor measurements was 0.125 Å−1. Fig. 1 shows the experimentally-determined and EPSR-simulated F(Q) and G(r) functions for potassium borate solutions. There is good agreement between the experimental and simulated data, which indicates that the simulated structures are close to the real structures.
| WSR | Number of K+ | Number of [B(OH)4−] | Number trans-[B(OH)3] | Number of cis-[B(OH)3] | Number of water molecules | Length of box (Å) | Number density (atoms A−3) |
|---|---|---|---|---|---|---|---|
| Owater and Hwater refer to oxygen and hydrogen sites in water molecules, and the subscripts 4, trans and cis refer respectively to atom sites in B(OH)4−, trans-[B(OH)3], and cis-[B(OH)3]; the charge parameters are the Mulliken charge from DFT calculation at m062x/6-311++g(2df,2pd); WSR: molar ratio of water to salt.a Aqueous K2B4O7 solution. | |||||||
| 20 | 150 | 150 | 0 | 0 | 3000 | 47.2 | 0.099853 |
| 60 | 50 | 50 | 0 | 0 | 3000 | 45.6 | 0.10019 |
| 60a | 112 | 112 | 56 | 56 | 2968 | 47.6 | 0.10021 |
| 100a | 64 | 64 | 32 | 32 | 2976 | 46.4 | 0.10026 |
| Atom | ε (kJ mol−1) | σ (Å) | q (e) | Mass [amu] |
|---|---|---|---|---|
| K40 | 0.5000 | 3.000 | 1.0000 | 39.0981 |
| B441 | 0.3970 | 3.5180 | 0.71101 | 10.810 |
| O441 | 0.7200 | 3.1200 | −0.6594 | 15.9990 |
| H441 | 0.000 | 0.000 | 2.000 | 0.2316 |
| Btrans | 0.7113 | 3.5000 | 0.8505 | 10.810 |
| Otrans | 0.8803 | 3.1000 | −0.5706 | 15.9990 |
| Htrans | 0 | 0 | 0.2871 | 2.0 |
| Bcis | 0.8000 | 3.2000 | 0.7500 | 10.810 |
| Ocis | 0.6500 | 3.100 | −0.5000 | 15.9990 |
| Hcis | 0 | 0 | 0.2500 | 2.0 |
| Owater42 | 0.6500 | 3.160 | −0.8476 | 16.0 |
| Hwater42 | 0 | 0 | 0.4238 | 2.0 |
Computational methods for hydrated clusters
Density functional theory (DFT) is an effective method for studying boron-containing systems.11,12,19,20,24,25 The M06-2X method43–45 and 6-311++G(2df,2pd) Pople-style basis set46 were used to optimize hydrated structures in the gas phase, with as many initial structures as possible. Then, the optimized equilibrium structures were further optimized in the liquid phase to obtain the final equilibrium structure. Single-point energy calculations and frequency calculations were conducted on these structures using the same basis set to determine the zero-point energy (ZPE) values and to confirm that the optimized structures had reached a local minimum (zero imaginary frequency). Furthermore, self-consistent field (SCF) energy, zero-point vibrational energy (ZPE) and energy correction terms associated with the basis set superposition error (BSSE) were also involved in the definition of the calculated total energies of the hydrated clusters. After this, the optimized isomers were ranked in order of energy, and the low energy structures were chosen for discussion. The hydration shape of the chosen structures is similar to the spatial density functions and their hydration distance B–O(W) is close to the NDIS result. The optimized structures and their energy data are shown in Fig. S5 and Table S4 (ESI†). The Raman spectrum of the boric acid molecule was also calculated under the same conditions. The calculated Raman spectrum was compared with the Raman spectrum of boric acid solution, as shown in Fig. S6 (ESI†), showing that the calculated frequency of the stretching vibration of the boric acid molecule is close to the experimental value. Therefore, although the acidity medium was not considered during the DFT calculation, the results can support our experimental conclusion. All calculations were carried out using the Gaussian16 package.47 The electrostatic potentials and average reduced density gradient analysis were performed using Multiwfn 3.6 (dev).48Results and discussion
Structure around water molecules
Fig. 2 shows the intermolecular pair distribution functions for Owater⋯Owater (gOwater…Owater) (a), Hwater⋯Owater (gHwater⋯Owater) (b), and Hwater⋯Hwater (gHwater⋯Hwater) (c) between pairs of atoms in pure water and different water molecules in the solutions. Fig. 2 and Table 3 show that the Owater⋯Owater distances (rOwater⋯Owater) are in the range 2.2–3.3 Å. The peak position of ∼2.71 Å remains constant, and the coordination number (4.1–3.6) has an inverse relationship with the concentration. The peak rOwater⋯Owater distance 2.71 Å is slightly shorter than the distance 2.73 Å obtained by Soper for pure water.49 Using X-ray diffraction, Zhou et al.11,12 reported that rOwater⋯Owater is in the range 2.76–2.82 Å in sodium/potassium metaborate solution, which is larger than the hydration distance observed in this study. This difference probably arises from the superior ability of neutron diffraction with H/D substitution to locate the atoms in an aqueous solution. Skinner et al.50 reported that the coordination number of the first hydration layer is 4.3 ± 0.1 in pure water when the cutoff value of the Owater⋯Owater pair distribution function is 3.3 Å, which is close to the value of 4.1 ± 1.1 found in this work. Zhou et al.11,12 reported that the Owater⋯Owater coordination numbers were 4.9 ± 1.1 and 4.7 ± 1.4 in pure water and dilute sodium/potassium metaborate solutions, respectively, which are higher than the values in pure water and in this work. Again this difference may be due to the advantageous use of NDIS. The second hydration distance (Owater⋯Owater) in the solutions is about 3.8 Å (Fig. 2), shorter than the distance of 4.5 Å for pure water,51 and there is greater shortening of the distance as the potassium borate concentration increases.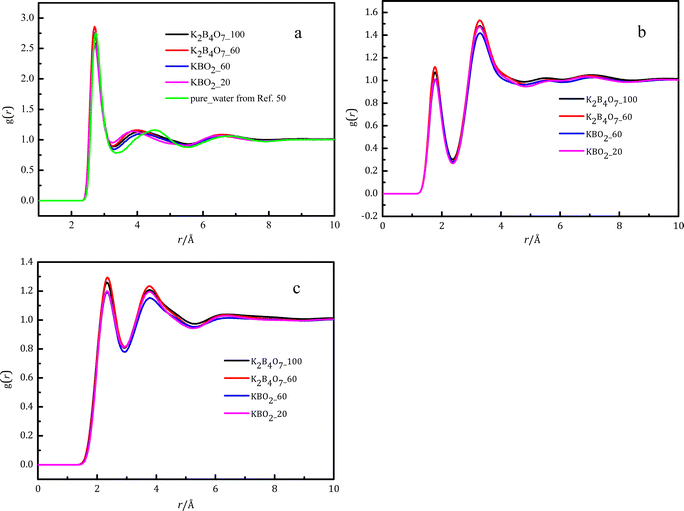 | ||
| Fig. 2 Intermolecular pair distribution functions at various concentrations, obtained by EPSR modeling: Owater–Owater (a), Hwater⋯Owater (b), and Hwater⋯Hwater (c). | ||
| K2B4O7_100 | K2B4O7_60 | K[B(OH)4]_60 | K[B(OH)4]_20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r peak | r min–rmax | CN | r peak | r min–rmax | CN | r peak | r min–rmax | CN | r peak | r min–rmax | CN | |
| Owater–Owater | 2.71 | 2.2–3.3 | 4.1 ± 1.2 | 2.71 | 2.2–3.25 | 3.6 ± 1.2 | 2.71 | 2.2–3.3 | 4.1 ± 1.1 | 2.71 | 2.2–3.3 | 3.6 ± 1.2 |
| Hwater–Owater | 1.77 | 1–2.3 | 1.1 ± 0.7 | 1.77 | 1–2.3 | 1.0 ± 0.7 | 1.77 | 1–2.4 | 1.1 ± 0.7 | 1.77 | 1–2.4 | 1.0 ± 0.7 |
| Otrans–Owater | 3.12 | 2.3–4.2 | 6.1 ± 2.5 | 3.12 | 2.3–4.1 | 4.6 ± 2.2 | ||||||
| Ocis–Owater | 3.12 | 2.3–4.1 | 6.1 ± 2.3 | 3.12 | 2.3–4.1 | 5.0 ± 2.1 | ||||||
| OB–Owater | 2.79 | 2.3–3.6 | 3.7 ± 1.2 | 2.79 | 2.3–3.6 | 3.5 ± 1.4 | 2.79 | 2.3–3.75 | 4.5 ± 1.3 | 2.79 | 2.2–3.75 | 4. 3 ± 1.4 |
| Btrans–Owater | 3.98 | 2.6–5.2 | 13.6 ± 4.4 | 4.02 | 2.6–5.2 | 12.2 ± 3.7 | ||||||
| Bcis–Owater | 3.85 | 2.6–5.2 | 12.9 ± 3.5 | 3.85 | 2.6–5.2 | 15.1 ± 4.6 | ||||||
| B–Owater | 3.56 | 2.6–4.8 | 11.6 ± 2.7 | 3.56 | 2.6–4.8 | 10.9 ± 2.3 | 3.56 | 2.7–4.55 | 15.1 ± 2.5 | 3.56 | 2.7–4.75 | 12.1 ± 1.9 |
| K–Btrans | 3.93 | 2.6–4.6 | 0.1 ± 0.2 | 3.95 | 2.6–4.6 | 0.1 ± 0.4 | ||||||
| K–Bcis | 3.42 | 2.6–4.6 | 0.2 ± 0.4 | 3.93 | 2.6–4.6 | 0.2 ± 0.4 | ||||||
| K–B | 3.32 | 2.6–4.6 | 0.8 ± 0.9 | 3.31 | 2.6–4.6 | 0.9 ± 0.8 | 3.30 | 2.6–4.6 | 0.4 ± 0.6 | 3.31 | 2.6–4.6 | 1.2 ± 0.8 |
| K–Otrans | 2.82 | 2–3.5 | 0.23 ± 0.6 | 2.79 | 2–3.5 | 0.17 ± 0.4 | ||||||
| K–Ocis | 2.80 | 2–3.4 | 0.05 ± 0.2 | 2.80 | 2–3.4 | 0.1 ± 0.4 | ||||||
| K–O | 2.68 | 2–3.4 | 1.1 ± 1.5 | 2.70 | 2–3.4 | 1.3 ± 1.4 | 2.70 | 2–3.5 | 0.5 ± 0.9 | 2.68 | 2.2–3.5 | 1.8 ± 1.6 |
| K–Owater | 2.63 | 2–3.45 | 5.6 ± 1.8 | 2.63 | 2–3.4 | 5.2 ± 1.5 | 2.63 | 2–3.5 | 6.2 ± 1.2 | 2.63 | 2–3.45 | 5.1 ± 1. 4 |
The EPSR simulations show that the ratio of the second to first oxygen-oxygen distances in the borate solutions are 1.52 and 1.48 (in K2B4O7 solution with WSR = 100 and 60), and 1.52 and 1.43 (in KBO2 solution with WSR = 60 and 20), compared to a value of 1.66 for pure water.51 The higher the concentration of the solutions, the more deviation from the ratio of pure water. This indicates that the dissolved ions distort the tetrahedral structure of water. The second coordination shell of water molecules is disrupted by the metal ion as the solution concentration increases, which causes water molecules in this outer shell to move to the first (inner) coordination shell. Soper and Ricci reported that external pressure caused the second hydration shell to move to smaller r in pure water.51 This implies the second shell is collapsing into some of the free space in a non-bonded fashion. Recently Woutersen et al.52 investigated three-dimensional structural density plots of the H-bond acceptor atoms (water O, TFA O, and hydrazinium N) in the first and second coordination shells of a water molecule in neat water at 1 bar, in neat water at 6 kbar, and in N2H5 TFA solution at 1 bar. The three-dimensional structural density surface of pure water at normal pressure is different from that under 600 MPa pressure and the N2H5 TFA solution under normal pressure. This is similar to that in pure water and in salt solutions reported by Soper et al.42,53 These phenomena again demonstrate that environmental pressure and added ions have the same effect on the second hydration shell of a water molecule. gHwater⋯Owater and gHwater⋯Hwater are shown in Fig. 2(b and c). Their first peak positions are at 1.77 Å and 2.33 Å, and their coordination numbers are 1.0 and 1.1.
Fig. 3 shows the spatial density function (SDF) of water molecules in aqueous K2B4O7 solutions for WSR = 100. The SDFs for other samples are shown in Fig. S7 (ESI†). The figure shows that the inner hydration layer has a tetrahedral structure and is less affected by concentration, which is consistent with rOwater⋯Owater remaining constant at various concentrations. There is a slight difference in the SDF surfaces of the outer sphere (Fig. 3) because the dissolved ions in the solutions damage the second hydration layer. The B(OH)3 and B(OH)4− species have –OH groups and prefer to form a tetrahedral structure. Therefore, this may reduce the disruption of the second hydration layer to some extent. Moreover, the Hwater⋯Owater and Hwater⋯Hwater distances of the second hydration layer remained nearly unchanged. These phenomena also confirm that the disruption of the second hydration layer is small, and this shell is resilient to change.
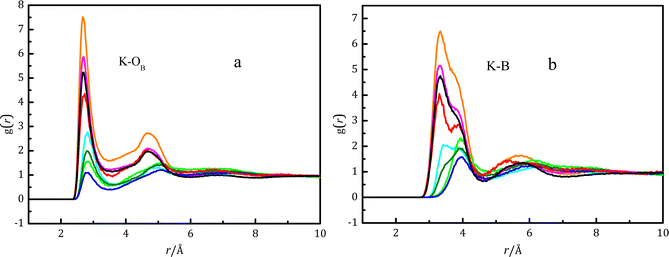
Fig. 4(a) shows the pair distribution function gK–OB between potassium ions and oxygen atoms in various boron-containing species, whilst Fig. 4(b) shows the gK–B between potassium ions and boron atoms in various boron-containing species. The intensity of gK–OB for B(OH)4− is greater than B(OH)3 due to strong electrostatic interactions between ions.  is shorter than rK–B[B(OH)3], which is also caused by the strong electrostatic attraction between anions and cations. In Fig. 4(b), there is a strong peak at 3.3 Å with a shoulder at 3.85 Å in gK–B for B(OH)4−. The main peak at 3.3 Å in gK–B of cis-B(OH)3 gradually weakened, and the shoulder at 3.85 Å increased with the potassium borate concentration. For trans-B(OH)3, only a peak at 3.85 Å was observed. We have previously studied the structure of potassium metaborate solutions and found that the peak at 3.85 Å is caused by the K–B distance of a monodentate ion pair (MCIP), while the peak at 3.3 Å is caused by a bidentate ion pair (BCIP).12Fig. 4(b) shows that the K–B distance (rK–B) is ∼3.8 Å in K–B(trans-B(OH)3), and the distance does not change with potassium borate concentration. When a contact ion pair is formed between the potassium ion and cis-B(OH)3, the peak at ∼3.4 Å is the dominant peak in dilute solutions, and the peak at ∼3.8 Å becomes the dominant one in concentrated solutions.
is shorter than rK–B[B(OH)3], which is also caused by the strong electrostatic attraction between anions and cations. In Fig. 4(b), there is a strong peak at 3.3 Å with a shoulder at 3.85 Å in gK–B for B(OH)4−. The main peak at 3.3 Å in gK–B of cis-B(OH)3 gradually weakened, and the shoulder at 3.85 Å increased with the potassium borate concentration. For trans-B(OH)3, only a peak at 3.85 Å was observed. We have previously studied the structure of potassium metaborate solutions and found that the peak at 3.85 Å is caused by the K–B distance of a monodentate ion pair (MCIP), while the peak at 3.3 Å is caused by a bidentate ion pair (BCIP).12Fig. 4(b) shows that the K–B distance (rK–B) is ∼3.8 Å in K–B(trans-B(OH)3), and the distance does not change with potassium borate concentration. When a contact ion pair is formed between the potassium ion and cis-B(OH)3, the peak at ∼3.4 Å is the dominant peak in dilute solutions, and the peak at ∼3.8 Å becomes the dominant one in concentrated solutions.
The DFT-based ab initio calculations were used to verify the above ion–pair structure. The structure and energy of the contact ion pair between a potassium ion and a boric acid molecule is shown in Fig. S9 and Table S5 (ESI†). Many initial models containing MCIPs and BCIPs were built to optimize their structures, but the BCIPs for trans-B(OH)3 transformed to MCIPs during the optimization process. Thus, a BCIP between K+ and trans-B(OH)3 cannot be found in Fig. S9 (ESI†). The lack of BCIPs for trans-B(OH)3 provides an explanation of the results from EPSR modelling of the NDIS data shown in Fig. 4b; in this figure the K–B functions for trans-B(OH)3 have a single peak at ∼4.0 Å, which is explained as arising solely from MCIPs (in the DFT calculations, the K–B distance for trans-B(OH)3 MCIPs is predicted to be ∼3.8 Å, see Table S5, ESI†). For cis-B(OH)3, BCIPs also transformed to a MCIP during the optimization process, except for n = 1. As shown in Table S5 (ESI†), for cis-B(OH)3 and n = 1 the enthalpy for a BCIP (−21.0 kJ mol−1) is lower than that of a MCIP (−17.1 kJ mol−1), which shows that the BCIP is more stable than the MCIP. The Gibbs energy for a BCIP (7.1 kJ mol−1) is lower than that of a MCIP (22.6 kJ mol−1), which also shows that the BCIP is relatively more stable. Nevertheless, a BCIP may transform to a MCIP with the addition of water molecules. For cis-B(OH)3, the K–B functions from EPSR modelling of the NDIS diffraction data shown in Fig. 4b have two peaks at ∼3.5 Å and ∼4.0 Å, and meanwhile the DFT calculations (Table S5, ESI†) predict that cis-B(OH)3 has K–B distances of ∼3.3 Å and ∼3.85 Å for a BCIP and a MCIP respectively. This shows that K+ and cis-B(OH)3 tend to form both monodentate and bidentate pairs in the solution. The DFT calculated K–O(B) distance rK–O is 2.8 Å, which is consistent with the EPSR result, but longer than rK–OW. The weaker interactions between K+ and boric acid molecules and stronger interactions between K+ and water cause this phenomenon. The small coordination numbers (see the CN values for K–Otrans and K–Ocis in Table 3) for K–O(B) and K–B also confirm the weaker interactions between K+ and boric acid. Duffin et al.13 also reported that increasing the concentration of cations (Na+, K+) did not significantly affect the NEXAFS spectra of boric acid aqueous solutions.
Hydration of borate anions
Fig. 5(b and c) shows the pair distribution functions OB⋯Owater (gOB⋯Owater) and OB⋯Hwater (gOwater⋯Hwater) between borate units and water. For boric acid molecules, gOB⋯Hwater is weaker than that between metaborate ions and water, and the O⋯Hwater distance of 2.1 Å is 0.2 Å longer than that of metaborate ions (Fig. 5(c)). Duffin et al.13 also reported that the interaction between boric acid and water molecules is weak. The peak of gOB⋯Owater for boric acid molecules is at 3.1 Å (Fig. 5(b)), which is weaker than that of metaborate ions, and the OB⋯Hwater peak (Fig. 5(c)) is especially weak, further demonstrating the weak interactions between boric acid and water molecules.
Fig. 6(a–d) shows the SDF of the first hydration layer of boric acid isomers in solution, and different views of the SDFs are shown in Fig. S10 (ESI†). The blue area represents the SDF surfaces where the hydration distance between boric acid molecules and water molecules ranges from 2.6–4.5 Å. The hydration within this distance range is referred to as direct hydration. The green part represents interstitial hydration, and the hydration distance between boric acid molecules and water molecules ranges from 4.6–5.2 Å. The difference between the SDF surfaces of these two hydrated boric acid isomers is mainly in the direct hydration layer due to their different symmetries. There is a clear separation between the axial and equatorial hydrated SDF surfaces in dilute solutions, and the two SDF surfaces merge together as the solution concentration increases. This situation is similar to the SDF surfaces between water molecules when an external pressure is applied to a pure water system.56 Both boric acid molecules have axial hydration, and the axially-hydrated SDF area decreases as the solution concentration increases. Axial hydration is mainly caused by water molecules in the empty 2pz orbital, which is perpendicular to the plane of the boric acid molecule. This is the first time that axial and interstitial hydration water molecules have been observed in hydrated boric acid molecules. Researchers have also observed axial hydration of the Pd(II) aqua ion in solution.40,57
Fig. 6(e–h) shows the SDF of hydration water molecules with respect to a central boric acid molecule and [B(OH)4−], and the SDFs with a different view are shown in Fig. S10 (ESI†). The blue block represents the SDF map with a B–Owater distance of cis/trans-B(OH)3 in the range 2.6–4.5 Å, and the green block represents the SDF map with a hydration distance range 4.6–5.2 Å. The blue block of the B(OH)4− SDF map (Fig. 6(e–h)) is in the range of 2.6–3.9 Å, and the green block is in the range of 3.7–4.8 Å. Therefore, there are two types of hydration in the hydration sphere of B(OH)4−: direct and interstitial hydration layers. The details of these hydration clusters were further studied using DFT calculation.
DFT study of hydration structure
| Species | n | B–O | B–Owater-dir | B–Owater-indir | B–Owater-axial | OB–Owater | Owater–Owater |
|---|---|---|---|---|---|---|---|
| trans-B(OH)3(H2O)n | 6 | 1.367 | 3.653 | 2.721 | 2.715 | ||
| 9 | 1.367 | 3.625 | 4.446 | 2.753 | 2.786 | ||
| 10A | 1.367 | 3.619 | 4.167 | 2.729 | 2.755 | 2.782 | |
| 10B | 1.367 | 3.628 | 4.576 | 2.757 | 2.824 | ||
| cis-B(OH)3(H2O)n | 5 | 1.367 | 3.630 | 2.798 | 2.791 | ||
| 8 | 1.369 | 3.489 | 3.884 | 2.755 | 2.782 | ||
| 9A | 1.368 | 3.521 | 4.191 | 4.401 | 2.795 | 2.802 | |
| 9B | 1.368 | 3.478 | 4.21 | ||||
| B(OH)4−(H2O)n | 9 | 1.473 | 3.488 | 2.810 | 2.841 | ||
| 10 | 1.473 | 3.482 | 4.595 | 2.812 | 2.839 | ||
As shown in Fig. 8 and Fig. S6 (ESI†), the hydrated structure of cis-B(OH)3 is similar to that of trans-B(OH)3. Five water molecules directly interact with cis-B(OH)3. The hydration number of cis-B(OH)3 is one less than trans-B(OH)3 because the two hydrogen atoms in cis-B(OH)3 simultaneously provide protons to water molecules. The similarity of the two isomers is that they have all three kinds of hydration modes in their first layers (direct hydration, interstitial hydration, and axial hydration). When the hydration number is in the range 6 ≤ n ≤ 8, the interstitial hydration layer appears. A ninth water molecule is found to hydrate the axial position of the trans-B(OH)3 molecule according to the energy difference of n9A and n9B (Table S5, ESI†). The B(OH)3 molecule is a planar structure, so it has an axial position both below and above the plane. The interstitial hydration occludes the axial position from one side, breaking the molecular symmetry. Therefore, only one of these sites is occupied. Table 4 shows that the distances rB–Owater-dir and rB–Owater-indir are roughly constant at ∼3.5 Å and ∼4.2 Å, respectively. rB–Owater-dir is within the distance range of the direct hydration layer of the SDF diagram (2.6–4.5 Å), whilst rB–Owater-indir is close to the range of the interstitial hydration layer of the SDF diagram (4.6–4.2 Å).
The rB–Owater distance for various borate ions in solution is ∼3.6 Å,11,12,21,57 as measured using X-ray diffraction. This shows that only direct interaction information can be obtained using X-ray diffraction, whereas more hydration information can be extracted using NDIS. The NDIS and DFT results show that the energetic hydration preference of water molecules around boric acid is: direct hydration > interstitial hydration > axial hydration. As shown in Table 4, the DFT calculations show that the OB⋯Owater distance between boric acid and water molecules is shorter than the Owater⋯Owater distance between water molecules of the hydration layer. This indicates that the hydrogen bonding between boric acid and water is stronger than that between water molecules in the hydration layer. This is why rB–Owater-dir is less affected by solution concentration. Neutron diffraction experiments (Table 3) show that the peak for Owater–Owater distances between free water molecules is at 2.71 Å, which is shorter than OB⋯Owater. This shows that the hydrogen bonding between free water molecules is the strongest, then the hydrogen bonding between boric acid molecules and water is second strongest, whilst the hydrogen bonding between water molecules in the hydration layer is relatively the weakest.
The reduced density gradient (RDG)59 diagram in Fig. 7 shows the distribution and size of various weak interactions in hydrated clusters. The definition of RDG is shown in the ESI.† Since there is no axial hydration in n6 and n9, the effect of steric hindrance is small, while the situation in n10A is the opposite. The interaction between the boron atom and the axial water molecules are van der Waals interactions, which are weaker than hydrogen bonds. The hydrogen bond between boric acid and water molecules is the strongest in n10A and n9A, both appearing (dark blue circles) in the RDG diagrams, while the hydrogen bonds between interstitial hydrated water molecules are weaker (light blue circles).
Conclusions
In this work, neutron diffraction experiments were combined with quantum calculations to study interactions between boric acid molecules, metaborate ions, water molecules, and cations in borate solutions. The boric acid molecule has three hydration modes: direct hydration, interstitial hydration, and axial hydration. The distance rB–Owater was in the range 2.6–3.9 Å for direct hydration, and the main hydration peak was at ∼3.6 Å, while it is in the range 4.0–4.8 Å for interstitial hydration with the main peak at ∼4.4 Å. The distance between boron and the axial hydration water molecules is 2.73 Å for trans-B(OH)3 and 4.4 Å for cis-B(OH)3. Hydrogen bonding between axial water and interstitial hydration waters is a reason for such a short axial hydration distance for trans-B(OH)3. The energetic hydration preference of water molecules around boric acid is: direct hydration > interstitial hydration > axial hydration. DFT-based calculations indicated that the hydrogen bonds between boric acid and water molecules were stronger than those between interstitial hydration water molecules. Axial hydration water and B atoms mainly underwent van der Waals interactions. The hydrated B(OH)4− has spherical symmetry, and 9 water molecules directly interacted with –OH of B(OH)4−, in which each –OH group form a four-coordinate hydration structure that is similar to water molecules. A tenth water molecule is located at the interstitial position. The hydrogen bond between B(OH)4− and water is stronger than the hydrogen bond between water molecules in the hydration layer.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank the Natural Science Foundation of Qinghai (No. 2020-HZ-811), the Qinghai Provincial Program for High-Level Innovative Talents (No. E240HX1001), and the National Natural Science Foundation of China (No. U1607106) for financial support. We also thank the Science and Technology Facilities Research Council (STFC) for beam-time on SANDALS (DOI: http://10.5286/ISIS.E.RB1910244).References
- M. T. Sabatini, L. T. Boulton and T. D. Sheppard, Sci. Adv., 2017, 3, e1701028 CrossRef PubMed.
- M. Mutailipu, M. Zhang, Z. H. Yang and S. L. Pan, Acc. Chem. Res., 2019, 52, 791–801 CrossRef CAS PubMed.
- X. L. Li, S. C. Wu, G. Q. Dong, S. Q. Chen, Z. L. Ma, D. Liu and C. Q. Sheng, ACS Med. Chem. Lett., 2020, 11, 439–444 CrossRef CAS PubMed.
- Q. Qin, R. H. Guo, E. H. Ren, X. X. Lai, C. Cui, H. Y. Xiao, M. Zhou, G. Yao, S. X. Jiang and J. W. Lan, ACS Sustainable Chem. Eng., 2020, 8, 10335–10344 CrossRef CAS.
- A. C. Wright, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2010, 51, 1–39 CAS.
- A. C. Wright and N. M. Vedishcheva, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2013, 54, 147–156 CAS.
- L. Maya, Inorg. Chem., 1976, 15, 2179–2184 CrossRef CAS.
- R. Janda and G. Heller, Spectrochim. Acta, Part A, 1980, 36, 997–1001 CrossRef.
- A. K. Covington, J. Inorg. Nucl. Chem., 1973, 35, 3257–3262 CrossRef CAS.
- C. G. Salentine, Inorg. Chem., 1983, 22, 3920–3924 CrossRef CAS.
- Y. Q. Zhou, S. Higa, C. H. Fang, W. Q. Zhang and T. Yamaguchi, Phys. Chem. Chem. Phys., 2017, 19, 27878–27887 RSC.
- F. Y. Zhu, T. Yamaguchi, K. Yoshida, W. Q. Zhang, Ho. Y. Liu, Y. Q. Zhou and C. H. Fang, Analyst, 2020, 145, 2245–2255 RSC.
- A. M. Duffin, C. P. Schwartz, A. H. England, J. S. Uejio, D. Prendergast and R. J. Saykally, J. Chem. Phys., 2011, 134, 154503 CrossRef PubMed.
- M. Maeda, J. Inorg. Nucl. Chem., 1979, 41, 1217–1220 CrossRef CAS.
- P. Wang, J. J. Kosinski, M. M. Lencka, A. Anderko and R. D. Springer, Pure Appl. Chem., 2013, 85, 2117–2144 CrossRef CAS.
- L. M. S. G. A. Applegarth, C. C. Pye, J. S. Cox and P. R. Tremaine, Ind. Eng. Chem. Res., 2017, 56, 13983–13996 CrossRef CAS.
- S. Sasidharanpillai, H. Arcis, L. Trevani and P. R. Tremaine, J. Phys. Chem. B, 2019, 123, 5147–5159 CrossRef CAS PubMed.
- J. Y. Peng, B. Zhang, J. Chen, Y. P. Dong and W. Li, Chin. J. Inorg. Chem., 2019, 35, 1821–1833 CAS.
- F. Y. Zhu, W. Q. Zhang, H. Y. Liu, X. F. Wang, Y. Q. Zhou, C. H. Fang and Y. H. Zhang, Spectrochim. Acta, Part A, 2020, 224, 117308 CrossRef CAS PubMed.
- F. Y. Zhu, W. Q. Zhang, H. Y. Liu, Y. Q. Zhou, X. F. Wang and Y. H. Zhang, Spectrochim. Acta, Part A, 2020, 230, 118039 CrossRef CAS PubMed.
- F. Y. Zhu, C. H. Fang, Y. Fang, Y. Q. Zhou, H. W. Ge and H. Y. Liu, J. Mol. Struct., 2015, 1083, 471–479 CrossRef CAS.
- C. C. Pye, Prog. Theor. Chem. Phys., 2018, 31, 143–177 CAS.
- H. W. Ge, Y. Fang, C. H. Fang, Y. Q. Zhou, F. Y. Zhu, H. Y. Liu, Z. X. Yang and Y. L. Tang, J. Chem. Eng. Data, 2014, 59, 4039–4048 CrossRef CAS.
- H. X. Zhou, F. Y. Zhu, H. Y. Liu, W. Q. Zhang, Y. Q. Zhou and C. H. Fang, Int. J. Quantum Chem., 2020, 120, e26118 CAS.
- H. X. Zhou, F. Y. Zhu, H. Y. Liu, W. Q. Zhang, Y. Q. Zhou, C. H. Fang and H. B. Li, Chem. Phys. Lett., 2020, 739, 136930 CrossRef CAS.
- M. Tachikawa, J. Mol. Struct.: THEOCHEM, 2004, 710, 139–150 CrossRef CAS.
- D. Stefani, I. Pashalidis and A. V. Nicolaides, J. Mol. Struct.: THEOCHEM, 2008, 853, 33–38 CrossRef CAS.
- M. A. Beckett, R. A. Davies and C. D. Thomas, Comput. Theor. Chem., 2014, 1044, 74–79 CrossRef CAS.
- Y. Q. Zhou, Y. Fang, C. H. Fang, F. Y. Zhu, H. W. Ge and H. Y. Liu, Chem. Phys. Lett., 2015, 636, 97–102 CrossRef CAS.
- A. C. Wright, A. C. Hannon, R. N. Sinclair, W. L. Johnson and M. Atzmon, J. Phys. F: Met. Phys., 1984, 14, L201–L205 CrossRef CAS.
- V. F. Sears, Neutron News, 1992, 3, 26–37 CrossRef.
- P. Toledano and A. Benhassaine, Rev. Chim. Miner., 1970, 7, 287–291 CAS.
- M. D. Gioacchino, M. A. Ricci, S. Imberti, N. Holzmann and F. Bruni, J. Mol. Liq., 2020, 301, 112407 CrossRef.
- (2019) for information: https://www.isis.stfc.ac.uk/Pages/sandals.aspx.
- A. K. Soper and >Rutherford Appleton Laboratory Technical Report, RAL-TR-2011-013, 2011 Search PubMed.
- A. K. Soper, Chem. Phys., 1996, 202, 295–306 CrossRef CAS.
- A. K. Soper, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 104204 CrossRef.
- F. Y. Zhu, C. H. Fang, Y. Fang, Y. Q. Zhou, H. W. Ge and H. Y. Liu, J. Mol. Struct., 2014, 1070, 80–85 CrossRef CAS.
- C. H. Fang, F. Y. Zhu, Y. Fang, Y. Q. Zhou, S. Tao and S. Xu, Phys. Chem. Liq., 2013, 51, 218–232 CrossRef CAS.
- D. T. Bowron, E. C. Beret, E. Martin–Zamora, A. K. Soper and E. S. Marcos, J. Am. Chem. Soc., 2012, 134, 962–967 CrossRef CAS PubMed.
- F. Bruni, S. Imberti, R. Mancinelli and M. A. Ricci, J. Chem. Phys., 2012, 136, 064520 CrossRef CAS PubMed.
- R. Mancinelli, A. Botti, F. Bruni, M. A. Ricci and A. K. Soper, Phys. Chem. Chem. Phys., 2007, 9, 2959–2967 RSC.
- Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2006, 110, 5121–5129 CrossRef CAS PubMed.
- H. J. Zhang, W. Wang, H. Li, R. Gao and Y. S. Xu, RSC Adv., 2022, 12, 5501–5508 RSC.
- T. Shinkai, P. J. Hsu, A. Fujii and J. L. Kuo, Phys. Chem. Chem. Phys., 2022, 24, 12631–12644 RSC.
- M. J. Frisch, J. A. Pople and J. S. Binkley, J. Chem. Phys., 1984, 80, 3265–3269 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian, Gaussian 16, Revision A. 03, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- T. Lu and F. W. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- A. K. Soper, Chem. Phys., 2000, 258, 121–137 CrossRef CAS.
- L. B. Skinner, C. J. Benmore, J. C. Neuefeind and J. B. Parise, J. Chem. Phys., 2014, 141, 214507 CrossRef CAS PubMed.
- A. K. Soper and M. A. Ricci, Phys. Rev. Lett., 2000, 84, 2881–2884 CrossRef CAS PubMed.
- S. Woutersen, B. Ensing, M. Hilbers, Z. F. Zhao and C. A. Angell, Science, 2018, 359, 1127–1131 CrossRef CAS PubMed.
- A. K. Soper, Chem. Phys., 2000, 258, 121–137 CrossRef CAS.
- P. E. Mason, L. Tavagnacco, M. L. Saboungi, T. Hansen, H. E. Fischer, G. W. Neilson, T. Ichiye and J. W. Brady, J. Phys. Chem. B, 2019, 123, 10807–10813 CrossRef CAS PubMed.
- H. Ohtaki and N. Fukushima, J. Solution Chem., 1992, 21, 23–38 CrossRef CAS.
- S. Imberti, A. Botti, F. Bruni, G. Cappa, M. A. Ricci and A. K. Soper, J. Chem. Phys., 2005, 122, 194509 CrossRef CAS PubMed.
- J. M. Martinez, F. Torrico, R. R. Pappalardo and E. S. Marcos, J. Phys. Chem. B, 2004, 108, 15851–15855 CrossRef CAS.
- W. Q. Zhang, C. H. Fang, W. Li, Y. Q. Zhou, F. Y. Zhu and H. Y. Liu, J. Mol. Struct., 2019, 1194, 262–270 CrossRef CAS.
- E. R. Johnson, S. Keinan, P. Mori-Sanchez, J. Contreras-Garcia, A. J. Cohen and W. T. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cp05331d |
| ‡ Current address: Miltenyi Biotec B.V. & Co. KG, (R&D Engineering, Biophysics), Friedrich-Ebert-Straße 68, 51429 Bergisch Gladbach, Germany. |
| This journal is © the Owner Societies 2023 |