Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An automated reaction route mapping for the reaction of NO and active species on Ag4 clusters in zeolites†
Shunsaku
Yasumura
 a,
Taisetsu
Kato
a,
Takashi
Toyao
a,
Taisetsu
Kato
a,
Takashi
Toyao
 a,
Zen
Maeno
a,
Zen
Maeno
 b and
Ken-ichi
Shimizu
b and
Ken-ichi
Shimizu
 *a
*a
aInstitute for Catalysis, Hokkaido University, N-21, W-10, Sapporo, 001-0021, Japan. E-mail: kshimizu@cat.hokudai.ac.jp
bSchool of Advanced Engineering, Kogakuin University, Tokyo, 192-0015, Japan
First published on 8th March 2023
Abstract
A computational investigation of the catalytic reaction on multinuclear sites is very challenging. Here, using an automated reaction route mapping method, the single-component artificial force induced reaction (SC-AFIR) algorithm, the catalytic reaction of NO and OH/OOH species over the Ag42+ cluster in a zeolite is investigated. The results of the reaction route mapping for H2 + O2 reveal that OH and OOH species are formed over the Ag42+ cluster via an activation barrier lower than that of OH formation from H2O dissociation. Then, reaction route mapping is performed to examine the reactivity of the OH and OOH species with NO molecules over the Ag42+ cluster, resulting in the facile reaction path of HONO formation. With the aid of the automated reaction route mapping, the promotion effect of H2 addition on the SCR reaction was computationally proposed (boosting the formation of OH and OOH species). In addition, the present study emphasizes that automated reaction route mapping is a powerful tool to elucidate the complicated reaction pathway on multi-nuclear clusters.
1. Introduction
Automated reaction route mapping is considered a powerful method for elucidating the mechanism of chemical reactions.1–10 Maeda et al. developed an artificial force-induced reaction (AFIR) method implemented in the global reaction route mapping (GRRM) program as an efficient reaction path-searching method; additionally, they demonstrated the rational design of chemical reactions in homogeneous systems.11–16 Investigating the reaction mechanism over multinuclear clusters in heterogeneous systems is challenging17–21 because various active sites are present in one cluster (e.g., on-top, bridge, and hollow sites) and their shapes change during the reaction.22–25 Thus, the exploration of the reaction mechanism over the clusters, based on transition state calculations, focuses typically only on some critical steps expected from previous results and experiences, which hinders the identification of more plausible reaction pathways.26–33 Gao et al. employed the AFIR method to investigate the structural transformation of neutral Aux clusters (x = 3–12) induced by O2 adsorption and reported that the transformation of Au clusters was promoted by O2 adsorption.34 Similarly, Iwasa et al. investigated the relationship between the structural transformation of a Cu13 cluster and NO dissociation using the AFIR method.35 Although the aforementioned studies reveal the nature of metal clusters and adsorbed molecules by the AFIR method, the detailed reaction mechanism in the catalytic processes over metal clusters has not been reported.Ag-loaded Al2O3 and Ag-exchanged zeolites have been studied for the selective catalytic reduction of NO to N2 (SCR).36–39 Interestingly, adding a small amount of H2 improved the SCR performance of these catalysts; many researchers have intensively studied the reaction mechanism of H2-assisted SCR on Ag-loaded Al2O3 and unraveled that the oxidized Ag species was promoted to be reduced into the metallic state by H2.40–48 In the case of Ag-exchanged zeolite, in the presence of H2, the formed Agnδ+ clusters improved the SCR performance.49–53 Shibata et al. performed UV-vis and XAS measurements, which revealed that the average structure of the formed cluster in the zeolite was Ag42+.54 Using in situ IR measurements, Shimizu et al. reported that the formed Agnδ+ cluster activates O2 and H2 to yield OOH species that are responsible for the drastic improvement in ammonia- (NH3−) and hydrocarbon-(HC-) SCR performance.52,55 For decades, numerous experimental50–52,55,56 and theoretical53 investigations have been reported on the characterization of active Ag clusters and plausible SCR mechanisms. However, these studies still lack a molecular-level understanding of the reaction pathways based on computational techniques.
In this study, using automated reaction route mapping, the reaction of H2 and O2 was investigated over Ag42+ clusters confined in CHA zeolite (a relatively small number of atoms are required to describe its periodic model). The formation of OOH and OH species from H2 + O2 requires a lower activation barrier than OH formation from H2O dissociation over the Ag42+ cluster. Subsequently, the reactivity of NO with the active species formed on Ag42+ was assessed. The results indicated that minimal activation barrier was required to produce the HONO intermediate, which then migrated into the Brønsted acid sites to give N2 + H2O via reaction with NH3 (discussed in our previous reports57). Similarly, the NO + H2 reaction required a relatively high activation barrier to produce NH3, thus indicating that NO preferentially reacts with the active species, OH, and OOH species on the Ag42+ cluster. The present study demonstrates the effective use of an automated reaction path-searching method to explore catalytic reactions in multinuclear clusters.
2. Computational details
Spin-polarized density functional theory (DFT) calculations were performed using the Vienna Ab initio Simulation Package (VASP).58,59 In particular, the projected augmented wave (PAW) method60,61 was employed for the Kohn–Sham equations62,63 with a plane-wave energy cutoff of 400 eV. The generalized gradient approximated Perdew–Burke–Ernzerhof (GGA-PBE) functional64 was employed to describe the electron exchange-correlation. The Brillouin zone sampling was restricted to the Γ point (convergence tests of cutoff energy and k-point mesh are shown in Table S1 and S2, ESI†).65 van der Waals interactions were considered using the dispersion-corrected DFT-D3 (BJ) method.66,67 The periodic model of the CHA zeolite, utilized as the model zeolite for reducing the computational cost for the reaction mapping, was obtained from the International Zeolite Association (IZA) database (a = b = 13.7 Å, c = 14.8 Å, α = β = 90.0°, and γ = 120°).68 The lattice constants were fixed at the initial values during the calculations (comparison before/after the optimization of the lattice constants is shown in Table S3, ESI†). CHA zeolite is composed of only one crystallographically inequivalent T site,69 and the third-nearest-neighbor in a six-membered ring site (6MR3NN site) in CHA was considered a model paired Al site in this study (Fig. 1).26,70,71 Reaction route mapping was performed using the single-component artificial force induced reaction (SC-AFIR) method, as implemented in the GRRM17 program.16 In this method, a structural deformation is induced by pushing or pulling a pair of target atoms with the applied artificial force using the following AFIR function.16E(Q) represents the potential energy surface (PES) of geometrical parameters Q. In the second term, the artificial force is applied to the system, and the strength of the artificial force is controlled by α, where ρ is set to either 1 or −1. rij represents the interatomic distance of atoms i and j in fragments A and B, respectively. The weight ωij is described as,
where Ri and Rj represent each covalent radius.
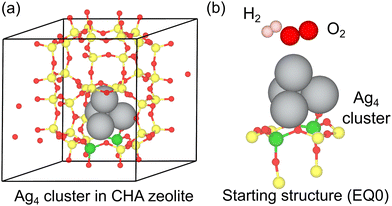 | ||
| Fig. 1 (a) Utilized periodic model of CHA zeolite including the Ag4 cluster and (b) the starting structure (EQ0) for the reaction route mapping with the SC-AFIR method. | ||
A model collision energy parameter of 1000 kJ mol−1 was used for all calculations. The obtained AFIR path was subsequently optimized by the locally updated plane (LUP) method to obtain the approximate equilibrium structures (EQs) and path top (PT) points, which were subsequently re-optimized by the following intrinsic reaction coordinate (IRC) calculation to determine the transition state (TS) structures and their connectivity.72 The structures are treated to be optimized when the difference in their electronic energies, the RMS error in interatomic distances, and the maximum error in interatomic distances are all smaller than 6.0 kJ mol−1, 3%, and 6%, respectively. Only a positive force was applied to the SC-AFIR calculations. Reactants in the gas phase (H2, O2, and NO molecules) of the initial structures were considered the target atoms of the SC-AFIR algorithm. During the calculations, the positions of the atoms in the zeolite framework were fixed at the initial positions, except for the Al atoms, the Si atoms around the Al atoms, and the O atoms connected to the Al and Si atoms (Fig. 1b).73 All the structures predicted in this study can be found in the ESI.†
3. Results and discussion
3.1 Reaction route mapping of the H2 + O2 reaction to produce an OH group over the Ag4 cluster in CHA zeolite
Our group previously reported the mechanism of NH3–SCR, wherein the OH species bound to transition metal cations oxidizes NO into HONO species (Mn+(OH) + NO → M(n−1)+ + HONO; Mn+ describes metal cations) that then migrate into Brønsted acid sites (BAS) to form H2O and NO+ species (HONO + H+Oz− → NO+Oz− + H2O; H+Oz− represents BAS) that easily react with NH3 to produce N2 and H2O (NO+Oz− + NH3 → H+Oz− + N2 + H2O).57,74–77 Thus, the generation of OH groups on active metal cations is a crucial step in the SCR reaction. Here, the H2 + O2 reaction was explored to assess the feasibility of generating OH groups on the Agnδ+ cationic cluster. Shibata et al. reported that in the presence of H2, Ag42+ clusters were generated within the zeolite framework and acted as active sites for the SCR reaction.54 Encouraged by their study, the Ag42+ cluster at the 2Al site was used as the initial structure. Fig. 1a shows the periodic model of CHA zeolites obtained from the IZA database. In this study, two T sites in the six-membered ring of CHA were replaced by Al.70 As a starting structure, O2 and H2 molecules were added to the periodic model, including the Ag42+ cluster (Fig. 1b), and the reaction route was explored by the SC-AFIR method. Fig. 2a shows the results of the reaction route mapping, and the relative energies along the main reaction coordinates are shown in Fig. 2b. 57 of the structures, which showed different shapes of Ag42+ clusters, and 57 of their connections (TS and PT) were found by the aid of the SC-AFIR method. In the first step, H2 molecules were dissociated to give OOH species and H atoms on the Ag4 cluster via an activation barrier (Ea) of 127 kJ mol−1. Sawabe et al. also performed DFT calculations to reveal that OOH species on the Ag42+ cluster are potential active species for the SCR reaction while H2 dissociation took place as the first step in their reaction path (activation barrier of OOH formation was not shown).53 Next, the formed OOH species dissociated into OH and O species on the cluster with an Ea of 125 kJ mol−1. Subsequently, the O atom combined with the H atom to give another OH species via low Eas (4, 29, and 3 kJ mol−1) with an exothermicity of 371 kJ mol−1. The dissociation of H2O molecules to form OH species on the Ag4 cluster was also examined (Fig. 3). The results revealed that the formation of OH species from H2 + O2 required a lower Ea than the H2O dissociation (127 kJ mol−1vs. 179 kJ mol−1). These results indicated that the H2 + O2 mixture more facilely dissociates on the Ag4 cluster than H2O dissociation. The following section discusses the reactivity of OOH and OH species with NO molecules over the Ag4 cluster.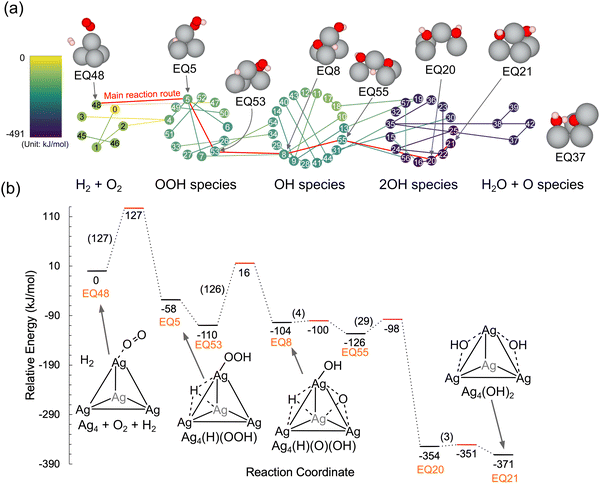 | ||
| Fig. 2 (a) Reaction route network for H2 + O2 on a Ag4 cluster in CHA zeolite. Nodes and edges describe obtained structures and paths connecting them (TS and PT, determined by GRRM program16). The color of the nodes and edges represents the relative energy (the color bar is shown on the left side). The representative structures are displayed together (only the Ag4 cluster and reactants are shown for clarity). (b) Energy profile for the main reaction route toward EQ21. The relative energies of each structure and the activation barriers are shown below the bar and in brackets, respectively. Schematic views of the structures are displayed together. | ||
3.2 Reactivity of OOH and OH species with a NO molecule to produce a HONO intermediate on a Ag4 cluster
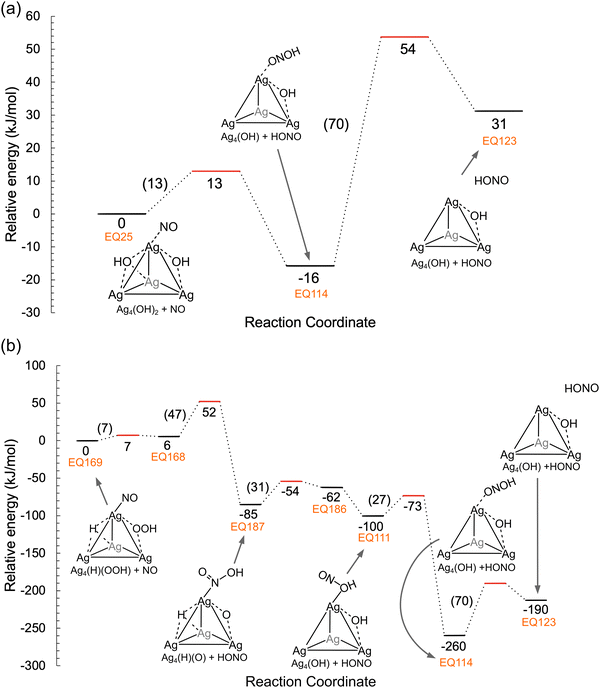
The reactivity of OOH and OH species previously formed on the Ag4 cluster with an NO molecule was examined by the SC-AFIR method. One NO molecule was added to the structure of EQ21, as shown in Fig. 2a (Ag4(OH)2 species). Fig. 4 shows the results of the reaction route mapping, and Fig. 5a shows the relative energy along the main reaction route of the OH and NO molecules on the Ag4 cluster. The adsorbed NO on the Ag4 cluster reacted with OH species to form HONO species as a facile process (Ea = 13 kJ mol−1). Subsequently, the formed HONO species was desorbed from the cluster via an Ea of 70 kJ mol−1. With the same starting structure (EQ21 in Fig. 2a), the reaction of the OOH species and NO molecules was also explored. Fig. 5b shows the relative energies along the main reaction route for OOH + NO. To form the HONO species, the O–O bond in the OOH species was cleaved to provide an OH fragment, which subsequently reacted with adsorbed NO via an Ea of 47 kJ mol−1. The formed HONO achieved the same structure as the case of OH species (EQ114) through relatively small Eas (31 and 27 kJ mol−1) before detaching through an Ea of 70 kJ mol−1 (EQ123). The formation of NO2 species on the Ag4 cluster was predicted as another reaction route from OOH + NO, and the evaluated Ea was low (17 kJ mol−1). Our previous study demonstrated that N2O4 species, formed via the dimerization of NO2, also produced NO+ species at the Al site in the zeolite.57 Besides, Bader charge analysis78 was performed to evaluate the charge of the Ag4 cluster before/after NO adsorption (Fig. S1, ESI†), and the result showed that the total charge of the cluster was not changed by NO adsorption while that of the NO molecule slightly decreased. | ||
| Fig. 4 Reaction route network for a NO molecule and Ag4(OH)2 in CHA zeolite (EQ21 in Fig. 2a). Nodes and edges describe obtained structures and paths connecting them (TS and PT, determined by GRRM program16). The color of the nodes and edges describes the relative energy (color bar is shown on the left side). The representative structures are displayed together (only Ag4 cluster and reactants are shown for clarity). | ||
In summary, OH and OOH species, formed via the H2 + O2 reaction, easily oxidize NO to HONO or NO2 species. Thus, H2 addition promotes the formation of the highly active species OH and OOH on the Ag4 cluster.
3.3 Reaction of NO + H2 on the Ag4 cluster toward NH3 formation.
Three H2 and one NO molecules were added to the periodic system, including the Ag4 cluster (Fig. 1a), and reaction route mapping was carried out using the SC-AFIR method. Fig. 6 shows the reaction route for the 3H2 + NO reaction; the adsorbed NO was exothermically hydrogenated in a step-by-step manner to yield NH3 and H2O. Fig. 7 shows the energy profile along the main reaction route toward NH3 and H2O. First, the N atom of the adsorbed NO molecule is hydrogenated by the adjacent adsorbed H2 molecule toward NH2O species. This step has an exothermicity of 126 kJ mol−1 through a relatively high Ea (138 kJ mol−1). Subsequently, the O atom in the formed NH2O species was hydrogenated to give hydroxylamine (NH2OH) and adsorbed H atoms with an Ea of 90 kJ mol−1. Finally, the N–O bond in the formed NH2OH is cleaved by the addition of H2 molecules to give molecular NH3 and H2O with a high exothermicity (329 kJ mol−1) through an Ea of 111 kJ mol−1. The first process in the entire reaction pathway to give the NH2O species exhibited the highest Ea. Considering the Ea for the formation of OH and OOH species (127 kJ mol−1), the direct hydrogenation of NO with H2 toward NH3 is less plausible for the SCR reaction.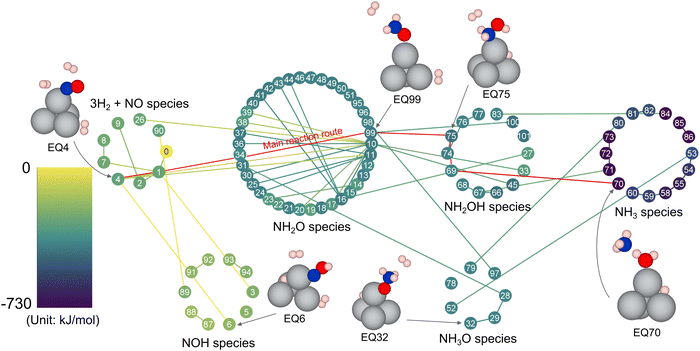 | ||
| Fig. 6 Reaction route network for one NO and three H2 molecules and the Ag4 cluster in CHA zeolite. Nodes and edges describe obtained structures and paths connecting them (TS and path top (PT), determined by GRRM program16). The color of the nodes and edges describes the relative energy (color bar is shown on the left side). The representative structures are displayed together (only the Ag4 cluster and reactants are shown for clarity). | ||
Fig. 8 shows the proposed reaction routes in this study, combined with previous reports.56,57 In the presence of H2 and O2, OH and OOH species were generated on the Ag4 cluster with an Ea of 127 kJ mol−1. Moreover, the generation of OH species from adsorbed H2O molecules required an Ea of 179 kJ mol−1. The active species formed on the Ag4 cluster, OH, and OOH species, easily reacted with the NO molecule to yield the HONO intermediate. As another possible reaction, the direct hydrogenation of NO with H2 was assessed; the Ea for NH3 formation was evaluated to be 138 kJ mol−1, which is higher than that for the H2 + O2 reaction. The proposed contribution of H2 addition to NH3–SCR by zeolite-based catalysts is to boost the formation of active OH and OOH species on the Ag4 cluster. Previous research on H2-assisted SCR over Ag-loaded Al2O3 proposes that the main contribution of H2 addition is stabilizing nitrate (NO3−) on the Al2O3 surface to prevent poisoning active Ag species, which is different from our result on zeolite-based catalysts.40,42,45,79 For HC-SCR, the previous study using in situ spectroscopy proposed that C3H8 reacted with active species (e.g. OOH species) to form NH3 (by the reaction with NO2) through CH3COO−, CH3NO2, and NCO− intermediates on the Ag4 cluster.52,55 According to this result, the formed HONO species is probably decomposed into N2 and H2O at Brønsted acid sites by the formed NH3. Thus, the present study demonstrated the effective utilization of automated reaction route mapping to explore the reactions over multinuclear clusters confined in zeolites.
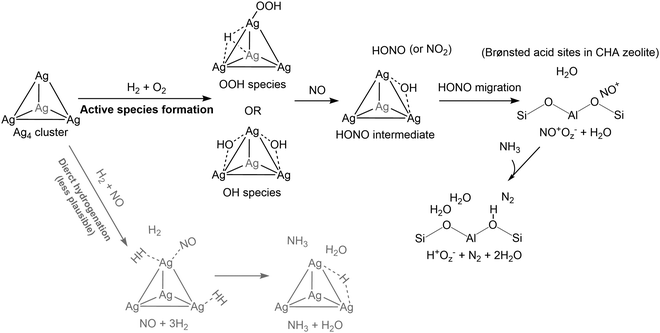 | ||
| Fig. 8 Whole view of H2-assisted NH3–SCR over the Ag4 cluster in CHA zeolite proposed in this study, combined with the reactions on Brønsted acid sites of CHA zeolite described in our previous reports.57,75 | ||
4. Conclusion
Automated reaction route mapping was carried out to explore the catalytic reaction over the Ag42+ cluster confined in the zeolite. The H2 + O2 reaction over the Ag42+ cluster indicated that the generation of active species, OH and OOH species, required lower activation energy than that for the formation of OH species from the dissociative adsorption of H2O (127 kJ mol−1vs. 179 kJ mol−1). The formed OH species were highly reactive with NO molecules to yield HONO species (Ea = 13 kJ mol−1), whereas the reaction of NO and OOH species gave HONO or NO2 species via Eas of 47 and 17 kJ mol−1, respectively. Additionally, the reactivity of H2 with NO over the Ag4 clusters was examined. This result revealed that the formation of NH3 from NO + H2 required an Ea as high as 138 kJ mol−1, thus indicating that the NO molecule preferentially reacted with OH or OOH species formed on the Ag4 cluster to give HONO. The present study demonstrated that the automated reaction route-searching method is suitable for investigating the catalytic reaction over multinuclear clusters with structural changes during the reaction.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was financially supported by the JST-CREST (JPMJCR17J3) and JSPS KAKENHI (21H04626). Some of the calculations were conducted employing the supercomputing resources at the Cyberscience Center of Tohoku University. This project was supported by the Joint Usage/Research Center for Catalysis. S.Y. is grateful to the MANABIYA system of the Institute for Chemical Reaction Design and Discovery (ICReDD) of Hokkaido University, which was established by the World Premier International Research Initiative (WPI), MEXT, Japan, to support the learning of the GRRM program techniques for DFT calculations. S.Y. acknowledges a Grant-in-Aid for JSPS Fellows (21J11744 (DC2)).References
- S. Stocker, G. Csányi, K. Reuter and J. T. Margraf, Nat. Commun., 2020, 11, 5505 CrossRef CAS PubMed.
- B. Kreitz, K. Sargsyan, K. Blöndal, E. J. Mazeau, R. H. West, G. D. Wehinger, T. Turek and C. F. Goldsmith, JACS Au, 2021, 1, 1656–1673 CrossRef CAS PubMed.
- T. Lan and Q. An, J. Am. Chem. Soc., 2021, 143, 16804–16812 CrossRef CAS PubMed.
- J. Xu, X. M. Cao and P. Hu, Phys. Chem. Chem. Phys., 2021, 23, 11155–11179 RSC.
- X. Fu, J. Li, J. Long, C. Guo and J. Xiao, ACS Catal., 2021, 11, 12264–12273 CrossRef CAS.
- R. T. Hannagan, G. Giannakakis, R. Réocreux, J. Schumann, J. Finale, Y. Wang, A. Michaelides, P. Deshlahra, P. Christopher, M. Flytzani-Stephanopoulous, M. Stamatakis and E. C. H. Sykes, Science, 2021, 372, 1444–1447 CrossRef CAS.
- Z. W. Ulissi, A. J. Medford, T. Bligaard and J. K. Nørskov, Nat. Commun., 2017, 8, 14621 CrossRef PubMed.
- A. A. Latimer, A. R. Kulkarni, H. Aljama, J. H. Montoya, J. S. Yoo, C. Tsai, F. Abild-Pedersen, F. Studt and J. K. Nørskov, Nat. Mater., 2017, 16, 225–229 CrossRef CAS PubMed.
- J.-M. Schweitzer, J. Rey, C. Bignaud, T. Bučko, P. Raybaud, M. Moscovici-Mirande, F. Portejoie, C. James, C. Bouchy and C. Chizallet, ACS Catal., 2022, 12, 1068–1081 CrossRef CAS.
- A. Bruix, J. T. Margraf, M. Andersen and K. Reuter, Nat. Catal., 2019, 2, 659–670 CrossRef CAS.
- K. Ohno and S. Maeda, J. Phys. Chem. A, 2006, 110, 8933–8941 CrossRef CAS PubMed.
- S. Maeda, K. Ohno and K. Morokuma, Phys. Chem. Chem. Phys., 2013, 15, 3683–3701 RSC.
- S. Maeda, T. Taketsugu, K. Ohno and K. Morokuma, J. Am. Chem. Soc., 2015, 137, 3433–3445 CrossRef CAS PubMed.
- S. Maeda, S. Komagawa, M. Uchiyama and K. Morokuma, Angew. Chem., Int. Ed., 2011, 50, 644–649 CrossRef CAS PubMed.
- S. Maeda, Y. Harabuchi, Y. Ono, T. Taketsugu and K. Morokuma, Int. J. Quantum. Chem., 2015, 115, 258–269 CrossRef CAS.
- S. Maeda, Y. Harabuchi, M. Takagi, K. Saita, K. Suzuki, T. Ichino, Y. Sumiya, K. Sugiyama and Y. Ono, J. Comput. Chem., 2018, 39, 233–250 CrossRef CAS PubMed.
- K. Sugiyama, Y. Sumiya, M. Takagi, K. Saita and S. Maeda, Phys. Chem. Chem. Phys., 2019, 21, 14366–14375 RSC.
- S. Maeda, K. Sugiyama, Y. Sumiya, M. Takagi and K. Saita, Chem. Lett., 2018, 47, 396–399 CrossRef CAS.
- K. Sugiyama, K. Saita and S. Maeda, J. Comput. Chem., 2021, 42, 2163–2169 CrossRef CAS PubMed.
- M. Gao, A. Lyalin, M. Takagi, S. Maeda and T. Taketsugu, J. Phys. Chem. C, 2015, 119, 11120–11130 CrossRef CAS.
- M. Gao, A. Lyalin, S. Maeda and T. Taketsugu, J. Chem. Theory Comput., 2014, 10, 1623–1630 CrossRef CAS PubMed.
- L. Liu, M. Lopez-Haro, J. A. Perez-Omil, M. Boronat, J. J. Calvino and A. Corma, Nat. Commun., 2022, 13, 821 CrossRef CAS PubMed.
- T. Yumura, A. Oda, H. Torigoe, A. Itadani, Y. Kuroda, T. Wakasugi and H. Kobayashi, J. Phys. Chem. C, 2014, 118, 23874–23887 CrossRef CAS.
- Y. Chai, W. Shang, W. Li, G. Wu, W. Dai, N. Guan and L. Li, Adv. Sci., 2019, 6, 1900299 CrossRef PubMed.
- S. Wu, X. Yang and C. Janiak, Angew. Chem., 2019, 131, 12468–12482 CrossRef.
- S. Yasumura, M. Huang, X. Wu, C. Liu, T. Toyao, Z. Maeno and K. Shimizu, Catal. Today, 2020, 352, 118–126 CrossRef.
- Z. Maeno, S. Yasumura, C. Liu, T. Toyao, K. Kon, A. Nakayama, J. Hasegawa and K. Shimizu, Phys. Chem. Chem. Phys., 2019, 21, 13415–13427 RSC.
- G. Li and E. A. Pidko, ChemCatChem, 2019, 11, 134–156 CrossRef CAS.
- S. Grundner, M. A. C. Markovits, G. Li, M. Tromp, E. A. Pidko, E. J. M. Hensen, A. Jentys, M. Sanchez-Sanchez and J. A. Lercher, Nat. Commun., 2015, 6, 7546 CrossRef.
- G. Li, P. Vassilev, M. Sanchez-Sanchez, J. A. Lercher, E. J. M. Hensen and E. A. Pidko, J. Catal., 2016, 338, 305–312 CrossRef CAS.
- M. H. Mahyuddin, A. Staykov, A. G. Saputro, M. K. Agusta, H. K. Dipojono and K. Yoshizawa, J. Phys. Chem. C, 2020, 124, 18112–18125 CrossRef CAS.
- M. H. Mahyuddin, Y. Shiota, A. Staykov and K. Yoshizawa, Acc. Chem. Res., 2018, 51, 2382–2390 CrossRef CAS PubMed.
- M. H. Mahyuddin and K. Yoshizawa, Catal. Sci. Technol., 2018, 8, 5875–5885 RSC.
- M. Gao, D. Horita, Y. Ono, A. Lyalin, S. Maeda and T. Taketsugu, J. Phys. Chem. C, 2017, 121, 2661–2668 CrossRef CAS.
- T. Iwasa, T. Sato, M. Takagi, M. Gao, A. Lyalin, M. Kobayashi, K. Shimizu, S. Maeda and T. Taketsugu, J. Phys. Chem. A, 2019, 123, 210–217 CrossRef CAS PubMed.
- J. P. Breen and R. Burch, Top. Catal., 2006, 39, 53–58 CrossRef CAS.
- K. Theinnoi, S. Sitshebo, V. Houel, R. R. Rajaram and A. Tsolakis, Energy Fuels, 2008, 22, 4109–4114 CrossRef CAS.
- R. Bartolomeu, A. N. Mendes, A. Fernandes, C. Henriques, P. da Costa and M. F. Ribeiro, Catal. Sci. Technol., 2016, 6, 3038–3048 RSC.
- F. Schuricht and W. Reschetilowski, Microporous Mesoporous Mater., 2012, 164, 135–144 CrossRef CAS.
- Y. Yu, H. He, X. Zhang and H. Deng, Catal. Sci. Technol., 2014, 4, 1239–1245 RSC.
- F. Wang, G. He, B. Zhang, M. Chen, X. Chen, C. Zhang and H. He, ACS Catal., 2019, 9, 1437–1445 CrossRef CAS.
- G. Xu, H. Wang, Y. Yu and H. He, J. Catal., 2021, 395, 1–9 CrossRef CAS.
- P. S. Kim, M. K. Kim, B. K. Cho, I. S. Nam and S. H. Oh, J. Catal., 2013, 301, 65–76 CrossRef CAS.
- J. P. Breen, R. Burch, C. Hardacre, C. J. Hill, B. Krutzsch, B. Bandl-Konrad, E. Jobson, L. Cider, P. G. Blakeman, L. J. Peace, M. V. Twigg, M. Preis and M. Gottschling, Appl. Catal. B, 2007, 70, 36–44 CrossRef CAS.
- S. Tamm, N. Vallim, M. Skoglundh and L. Olsson, J. Catal., 2013, 307, 153–161 CrossRef CAS.
- S. T. Korhonen, A. M. Beale, M. A. Newton and B. M. Weckhuysen, J. Phys. Chem. C, 2011, 115, 885–896 CrossRef CAS.
- H. Kubota, S. Mine, T. Toyao, Z. Maeno and K. Shimizu, ACS Catal., 2021, 3, 544–559 Search PubMed.
- J. Shibata, K. Shimizu, S. Satokawa, A. Satsuma and T. Hattori, Phys. Chem. Chem. Phys., 2003, 5, 2154–2160 RSC.
- J. Shibata, Y. Takada, A. Shichi, S. Satokawa, A. Satsuma and T. Hattori, J. Catal., 2004, 222, 368–376 CrossRef CAS.
- A. Satsuma, J. Shibata, K. Shimizu and T. Hattori, Catal. Surv. Asia, 2005, 9, 75–85 CrossRef CAS.
- K. Shimizu, K. Sugino, K. Kato, S. Yokota, K. Okumura and A. Satsuma, J. Phys. Chem. C, 2007, 111, 1683–1688 CrossRef CAS.
- K. Shimizu, K. Sawabe and A. Satsuma, Catal. Sci. Technol., 2011, 1, 331–341 RSC.
- K. Sawabe, T. Hiro, K. Shimizu and A. Satsuma, Catal. Today, 2010, 153, 90–94 CrossRef CAS.
- J. Shibata, K. Shimizu, Y. Takada, A. Shichi, H. Yoshida, S. Satokawa, A. Satsuma and T. Hattori, J. Catal., 2004, 227, 367–374 CrossRef CAS.
- K. Shimizu, K. Sugino, K. Kato, S. Yokota, K. Okumura and A. Satsuma, J. Phys. Chem. C, 2007, 111, 6481–6487 CrossRef CAS.
- K. Shimizu and A. Satsuma, Appl. Catal., B, 2007, 77, 202–205 CrossRef CAS.
- S. Yasumura, C. Liu, T. Toyao, Z. Maeno and K. Shimizu, J. Phys. Chem. C, 2021, 125, 1913–1922 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- D. Kresse, G. Joubert, G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef.
- P. Honhenberg and W. Kohn, Phys. Rev. [Sect.] B, 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Phys Rev A, 1965, 140, A1133–A1138 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- C. Baerlocher; L. B. McCusker; and H. van KoningsveldDatabase of Zeolite Structures; http://www.iza-structure.org/databases/(accessed Jun. 2021).
- Z. Zhao, Y. Xing, S. Li, X. Meng, F. S. Xiao, R. McGuire, A. N. Parvulescu, U. Müller and W. Zhang, J. Phys. Chem. C, 2018, 122, 9973–9979 CrossRef CAS.
- S. Yasumura, T. Ueda, H. Ide, K. Otsubo, C. Liu, N. Tsunoji, T. Toyao, Z. Maeno and K. Shimizu, Phys. Chem. Chem. Phys., 2021, 23, 22273–22282 RSC.
- C. Paolucci, A. A. Parekh, I. Khurana, J. R. di Iorio, H. Li, J. D. Albarracin Caballero, A. J. Shih, T. Anggara, W. N. Delgass, J. T. Miller, F. H. Ribeiro, R. Gounder and W. F. Schneider, J. Am. Chem. Soc., 2016, 138, 6028–6048 CrossRef CAS PubMed.
- K. Fukui, Acc. Chem. Res., 1981, 14, 363 CrossRef CAS.
- S. Yasumura, K. Saita, T. Miyakage, T. Toyao, Z. Maeno, T. Taketsugu and K. Shimizu, ChemRxiv, 2022, preprint DOI:10.26434/chemrxiv-2022-w9fjz.
- S. Yasumura, Y. Qian, T. Toyao, Z. Maeno and K. Shimizu, J. Phys. Chem. C, 2022, 126, 11082–11090 CrossRef CAS.
- S. Yasumura, Y. Qian, T. Kato, S. Mine, T. Toyao, Z. Maeno and K. Shimizu, ACS Catal., 2022, 12, 9983–9993 CrossRef CAS.
- H. Kubota, T. Toyao, Z. Maeno, Y. Inomata, T. Murayama, N. Nakazawa, S. Inagaki, Y. Kubota and K. Shimizu, ACS Catal., 2021, 11, 11180–11192 CrossRef CAS.
- C. Liu, S. Yasumura, T. Toyao, Z. Maeno and K. Shimizu, J. Phys. Chem. C, 2022, 126, 11594–11601 CrossRef CAS.
- W. Tang, E. Sanville and G. Henkelman, J. Phys.: Condens. Matter, 2009, 21, 084204 CrossRef CAS PubMed.
- G. Xu, J. Ma, L. Wang, Z. Lv, S. Wang, Y. Yu and H. He, ACS Catal., 2019, 9, 10489–10498 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cp04761f |
| This journal is © the Owner Societies 2023 |