Open Access Article
Open Access ArticleElectron-decoupled MAS DNP with N@C60
Nicholas
Alaniva
 *ab,
Edward P.
Saliba†
bc,
Patrick T.
Judge†
b,
Erika L.
Sesti†
b,
Wolfgang
Harneit
d,
Björn
Corzilius
*ab,
Edward P.
Saliba†
bc,
Patrick T.
Judge†
b,
Erika L.
Sesti†
b,
Wolfgang
Harneit
d,
Björn
Corzilius
 e and
Alexander B.
Barnes
e and
Alexander B.
Barnes
 a
a
aLaboratory of Physical Chemistry, ETH Zürich, Zürich 8093, Switzerland. E-mail: nalaniva@ethz.ch; Tel: +41 44 633 43 81
bWashington University in St. Louis, St. Louis 63130, MO, USA
cMassachusetts Institute of Technology, Cambridge 02139, MA, USA
dDepartment of Physics, Universität Osnabrück, Osnabrück 49076, Germany
eInstitute of Chemistry, Department Life, Light & Matter, Universität Rostock, 18059 Rostock, Germany
First published on 3rd February 2023
Abstract
Frequency-chirped microwaves decouple electron- and 13C-spins in magic-angle spinning N@C60:C60 powder, improving DNP-enhanced 13C NMR signal intensity by 12% for 7 s polarization, and 5% for 30 s polarization. This electron decoupling demonstration is a step toward utilizing N@C60 as a controllable electron-spin source for magic-angle spinning magnetic resonance experiments.
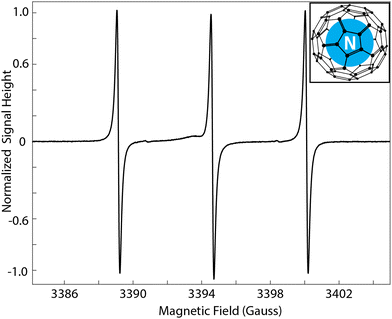
Electron decoupling improves nuclear magnetic resonance (NMR) signal that has been enhanced by dynamic nuclear polarization (DNP).1,2 Techniques for DNP enhancement often utilize doped radical electrons as a source of relatively high polarization and microwave irradiation to facilitate transfer of polarization to nuclear spins in dipolar contact with the electron spins, with spin diffusion sending the polarization to nuclear spins not coupled to the electron spin.3–5 Following polarization transfer, this dipolar contact reduces NMR signal intensity and resolution. The improvement afforded by electron decoupling comes from the attenuation of the electron-nuclear dipolar contact, by way of on-resonance, frequency-chirped microwave pulses. Combined with advances in cryogenic magic-angle spinning (MAS) NMR instrumentation, electron decoupling has been an effective tool for in-cell DNP NMR spectroscopy and a necessary component for “direct DNP” and MAS electron spin relaxation experiments.6–8 To date, these experiments have employed only trityl, (Finland radical) and trityl derivatives as a polarizing agent.9,10 Here, the utility of electron decoupling is expanded to the electron spin-3/2 of a nitrogen atom trapped in C60 fullerene (hereon referred to as N@C60, and represented in Fig. 1, in a corner call-out).11–14 Decoupling the electron spin in N@C60 improves 13C signal intensity by 5% and 12% following a DNP-transfer period of 30 and 7 seconds, respectively, at 4.0 kHz MAS and 90 K.
The symmetry of the C60 cage (g-tensor value = 2.0024) and a sparse spin-interaction network in 160 parts-per-million (ppm) N@C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C60 (160
C60 (160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106) poly-crystalline powder result in one of the narrowest electron paramagnetic relaxation (EPR) signals in solid state (Fig. 1).15,16 The spin-spin relaxation of the N@C60 electron spin is tens of microseconds (inhomogeneous contribution in this sample due to crystalline imperfections), up to an order of magnitude greater than that of other narrow-line radicals, such as trityl and BDPA, used for DNP.17–20 Additionally, the symmetric environment, even below the polycrystalline phase transition temperature, and the near absence of g-anisotropy give rise to a zero-field splitting of 0.52 MHz.16 Even considering the proportionally greater dipolar coupling due to the electron spin quantum number of 3/2, the stability and electron relaxation of N@C60 make it a sensible target for electron decoupling, and DNP experiments with electron spin control.
106) poly-crystalline powder result in one of the narrowest electron paramagnetic relaxation (EPR) signals in solid state (Fig. 1).15,16 The spin-spin relaxation of the N@C60 electron spin is tens of microseconds (inhomogeneous contribution in this sample due to crystalline imperfections), up to an order of magnitude greater than that of other narrow-line radicals, such as trityl and BDPA, used for DNP.17–20 Additionally, the symmetric environment, even below the polycrystalline phase transition temperature, and the near absence of g-anisotropy give rise to a zero-field splitting of 0.52 MHz.16 Even considering the proportionally greater dipolar coupling due to the electron spin quantum number of 3/2, the stability and electron relaxation of N@C60 make it a sensible target for electron decoupling, and DNP experiments with electron spin control.
While higher-power microwave devices are being developed for MAS DNP application (gyrotrons, gyro-amplifiers), current frequency-agile gyrotrons are able to produce shaped microwave pulses (chirps, sweeps) that cover the frequency range of the targeted electron spin resonance and exert control over the spin.21–23 A narrow electron spin resonance reduces the required frequency range of this chirped pulse to control the spin. The EPR signal from N@C60 is so narrow that electron spin control with hard pulses will be possible with existing frequency-agile gyrotron technology.24 The gyrotron used for these experiments generates an approximate microwave Rabi frequency of 0.38 MHz at the sample, which is on the order of the electron-13C (local 13C, on the N@C60 cage, itself) hyperfine dipolar coupling (predicted at 0.44 MHz; 1.3 MHz for ±3/2 spin - measured to be 0.30 MHz, however).16,25 Although certain pulsed-DNP mechanisms require currently-inaccessible microwave Rabi frequencies (at high magnetic field), there are some that become possible with such a “narrow-line” radical.2,26–29 Demonstration of electron decoupling on this 160 ppm N@C60:C60 poly-crystalline powder is the first step toward pulsed DNP MAS and pulsed EPR MAS using the N@C60 electron spin.
The experiments here are performed on a relatively dilute collection of electron spins. For effective use of N@C60 as a polarizing agent, 100% pure N@C60 will be required. The dipolar linewidth at 100% approximates 60 Gauss, but when diluted to lower concentration in a sample of interest, this interaction will be reduced and the corresponding linewidths narrow.30 Current limitations regarding the methods of endofullerene generation (ion implementation, gas-discharge, and radio-frequency plasma discharge) and purification, using high-performance liquid chromatography, severely restrict access to milligram-amounts of pure N@C60.31–37 Dissemination of endofullerene production and optimization of formation methods is a path toward production of realistic amounts of pure N@C60 for experiments. However, currently-accessible quantities of pure N@C60 would be sufficient for DNP NMR MAS experiments that utilize small sample volumes (1 μL and lower), which have been demonstrated using 2 mm MAS spherical rotors. However accomplished, the protection and symmetry afforded the electron spin by the C60 cage, the affinity of C60 for plasma membrane incorporation, and the capability of C60-functionalization make a variety of endofullerenes promising agents for powerful magnetic resonance experiments in burgeoning areas of science, such as in-cell DNP/EPR, inorganic/surface-DNP/EPR, and quantum computing.38,39
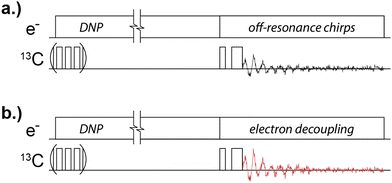
The electron-decoupled MAS DNP experiments presented here use a cryogenic-MAS radio-frequency probe for excitation and detection of nuclear spins, and a frequency-agile gyrotron for control of electron spins and transfer of polarization (DNP).22,40Fig. 2 shows the pulse sequences used to probe the electron decoupling effect. On the “electron channel” line, there are 3 different conditions: DNP, electron decoupling, and off-resonance chirps. The microwave power for each event is constant, and each event is characterized by different microwave frequency/frequencies. During DNP, the transfer of electron polarization to 13C spins is facilitated by microwave irradiation at 197.484 GHz. This satisfies the double-quantum solid effect condition (νe,Larmor − ν13C,Larmor). The solid effect is the “active” DNP mechanism since the inhomogeneous linewidth of the N@C60 electron resonance is less than the nuclear Larmor frequency. During electron decoupling (Fig. 2b), the microwave frequency is time-dependent, changing according to a chirped triangle-waveform that covers a 60 MHz range, centered over the electron resonance frequency (197.559 GHz), in 6.63 μs.1 This range is large enough to ensure that the chirp covers the entirety of the N@C60 electron spin resonance. The off-resonance chirps shown in the Fig. 2a sequence describe a frequency-chirped waveform of the same magnitude and shape, but is centered at 197.409 GHz (75 MHz below the DNP condition) so that electron spins remain unaffected. This control experiment provides a DNP-enhanced NMR signal to which the electron-decoupled signal is compared.
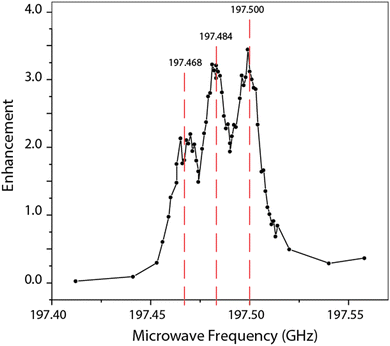
The exact microwave frequencies required to meet these conditions are verified experimentally with the construction of a “DNP profile” (Fig. 3). Each point on the profile indicates the intensity of 13C NMR-signal with microwave irradiation of the frequency corresponding to its place along the horizontal axis. The spacing between the enhancement local maxima correspond to the 15.9 MHz hyperfine splitting, as observed in the N@C60 EPR spectrum (Fig. 1). The difference in resolution between the EPR spectrum and the DNP profile is attributed to frequency instability of the gyrotron. Using these frequencies, the 13C Larmor frequency, and the condition for the double-quantum solid effect condition, the frequency-agile gyrotron is then calibrated so that electron decoupling chirps effectively cover the N@C60 electron spin resonance.
For electron decoupling and off-resonance/control experiments, a period of DNP transfer from electron to the 13C spins occurs between a 13C-spin “saturation train” and a 13C excitation pulse (π/2), which is followed by a rotor-synchronized refocusing pulse (Hahn echo, π). All 13C pulses applied have a nutation frequency of 83 kHz (3 μs pulse = π/2). These experiments are performed at a magnetic field of 7.05 T (75.49 MHz 13C Larmor frequency) and at a temperature of 90 K, with a 3.2 mm cylindrical rotor spinning at 4.0 kHz (±5 Hz). A vacuum-jacketed cryogenic transfer/recovery system allows for stable MAS DNP operation at 90 K.40,41 The frequency-agile gyrotron is controlled by a spectrometer-integrated arbitrary waveform generator (Tecmag Inc.). This, in series with a high voltage amplifier, alters the gyrotron anode potential, changing the microwave frequency.
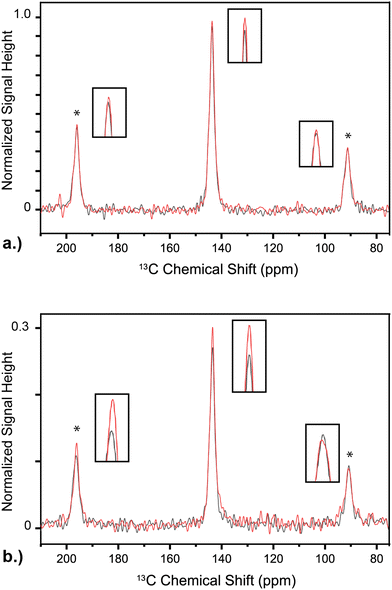
Fig. 4a and b show the comparisons of electron-decoupled, DNP-enhanced NMR signal (red) to DNP-enhanced signal acquired without electron decoupling (black) for experiments with 30 s and 7 s DNP-transfer periods, respectively. The 30 s spectra are summations of 320 transients each, and the 7 s spectra are 2048 transients. After a 30 s DNP transfer, the only significant improvement is observed on the isotropic peak at 143.6 ppm.42,43 Here, electron decoupling improves signal intensity by 5%. The effect of electron decoupling is greater for a 7 s DNP transfer, with a 12% improvement in signal intensity for the isotropic, central peak, and a 15% improvement for the spinning sideband at 196.6 ppm. The spinning sideband at 90.6 ppm shows no significant improvement in signal intensity with electron decoupling. All spectra are processed using tNMR software, and signal integration and linewidths are obtained using DMfit.44
The induced broadening from the electron-nuclear hyperfine interaction has been shown to completely attenuate 13C NMR signal at high N@C60 concentrations (N@C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C60 at a 3
C60 at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio).45 Shorter DNP-transfer periods enhance nuclear spins that are closer to the polarizing electron spins, effectively increasing the ratio of paramagnetic endofullerenes to 13C spins that contribute to observed, DNP-enhanced NMR signal. In this sample, the DNP build-up time is characterized by a time constant of 31 s, thus the difference in number of enhanced nuclear spins between 7 s and 30 s DNP transfer periods is noticeable (see relative vertical scales, signal-to-noise ratios in Fig. 4).46 As the DNP-transfer period is increased, spin diffusion spreads the improved polarization to 13C nuclei with weaker dipolar contact to the polarizing electron spins. The signal from these 13C spins will not be improved by electron decoupling; this same effect is observed when electron decoupling trityl-based radicals.1,6,7 Here a shorter DNP period results in greater electron decoupling effect, but the same trend is expected with higher concentration of N@C60.
2 ratio).45 Shorter DNP-transfer periods enhance nuclear spins that are closer to the polarizing electron spins, effectively increasing the ratio of paramagnetic endofullerenes to 13C spins that contribute to observed, DNP-enhanced NMR signal. In this sample, the DNP build-up time is characterized by a time constant of 31 s, thus the difference in number of enhanced nuclear spins between 7 s and 30 s DNP transfer periods is noticeable (see relative vertical scales, signal-to-noise ratios in Fig. 4).46 As the DNP-transfer period is increased, spin diffusion spreads the improved polarization to 13C nuclei with weaker dipolar contact to the polarizing electron spins. The signal from these 13C spins will not be improved by electron decoupling; this same effect is observed when electron decoupling trityl-based radicals.1,6,7 Here a shorter DNP period results in greater electron decoupling effect, but the same trend is expected with higher concentration of N@C60.
An interesting feature of the 7 s DNP experiments is the dissimilarity in the recovery of intensity between the first-order spinning sidebands upon electron decoupling (Fig. 4b). This difference suggests a change in asymmetry parameter associated with the chemical shift anisotropy (CSA) of the 13C spins between the decoupled and non-decoupled spectra.47,48 As this effect is not observed with electron decoupling following 30 s DNP, a possible explanation is that the 13C CSA of spins local or near to N@C60 is non-identical to that of bulk 13C (those not close to N@C60). Similar to the efficacy of electron decoupling, this effect is also highlighted at shorter DNP periods, when N@C60-proximal 13C spins are enhanced to a greater extent than the bulk, N@C60-distal spins.
Electron-decoupled MAS DNP using N@C60 as a polarizing agent is a step toward utilizing the unique properties of N@C60 for powerful experiments in MAS NMR/EPR. N@C60 and other endofullerene species are the focus of innovations in quantum bit engineering and small molecule study, as well as novel DNP techniques and use of the electron spin as an environmental sensor.49–58 Localized DNP with N@C60 can be extended to more significant systems, such as the interior of a lipid bilayer membrane where surrounding proton spins from the lipid tails should afford an improved rate of polarization transfer, or to catalyst surfaces where a functionalized C60 cage may be exploited.59,60 Combined with advances in endofullerene generation, frequency-agile gyrotrons can expand the application of MAS DNP using endofullerenes, and establish the practice of integrated NMR/EPR with MAS and at high magnetic field.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Daniel Klose for his assistance with EPR experiments, and the reviewers for their insightful comments. Funding for this work is provided through the Swiss National Science Foundation (SNSF 201070).Notes and references
- E. P. Saliba, E. L. Sesti, F. J. Scott, B. J. Albert, E. J. Choi, N. Alaniva, C. Gao and A. B. Barnes, J. Am. Chem. Soc., 2017, 139, 6310–6313 CrossRef CAS PubMed.
- E. P. Saliba, E. L. Sesti, N. Alaniva and A. B. Barnes, J. Phys. Chem. Lett., 2018, 9, 5539–5547 CrossRef CAS PubMed.
- L. R. Becerra, G. J. Gerfen, R. J. Temkin, D. J. Singel and R. G. Griffin, Phys. Rev. Lett., 1993, 71, 3561–3564 CrossRef CAS PubMed.
- M. Afeworki, R. A. McKay and J. Schaefer, Macromolecules, 1992, 25, 4084–4091 CrossRef CAS.
- B. Corzilius, Phys. Chem. Chem. Phys., 2016, 18, 27190–27204 RSC.
- N. Alaniva, E. P. Saliba, E. L. Sesti, P. T. Judge and A. B. Barnes, Angew. Chem., 2019, 58, 7259–7262 CrossRef CAS PubMed.
- E. L. Sesti, E. P. Saliba, N. Alaniva and A. B. Barnes, J. Magn. Reson., 2018, 295, 1–5 CrossRef CAS PubMed.
- P. T. Judge, E. L. Sesti, L. E. Price, B. J. Albert, N. Alaniva, E. P. Saliba, T. Halbritter, S. T. Sigurdsson, G. B. Kyei and A. B. Barnes, J. Phys. Chem. B, 2020, 124, 2323–2330 CrossRef CAS PubMed.
- T. J. Reddy, T. Iwama, H. J. Halpern and V. H. Rawal, J. Org. Chem., 2002, 67, 4635–4639 CrossRef CAS PubMed.
- P. T. Judge, E. L. Sesti, E. P. Saliba, N. Alaniva, T. Halbritter, S. T. Sigurdsson and A. B. Barnes, J. Magn. Reson., 2019, 305, 51–57 CrossRef CAS PubMed.
- T. A. Murphy, T. Pawlik, A. Weidinger, M. Höhne, R. Alcala and J.-M. Spaeth, Phys. Rev. Lett., 1996, 1075–1078 CrossRef CAS PubMed.
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- N. Weiden, H. Käss and K. P. Dinse, J. Phys. Chem. B, 1999, 103, 9826–9830 CrossRef CAS.
- A. E. Douglas, Nature, 1977, 269, 130–132 CrossRef CAS.
- J. J. Wittmann, T. V. Can, M. Eckardt, W. Harneit, R. G. Griffin and B. Corzilius, J. Magn. Reson., 2018, 290, 12–17 CrossRef CAS PubMed.
- K.-P. Dinse, H. Käβ, C. Knapp and N. Weiden, Carbon, 2000, 38, 1635–1640 CrossRef CAS.
- S. Knorr, A. Grupp, M. Mehring, M. Waiblinger and A. Weidinger, AIP Conf. Proc., 2001, 544, 191–194 CrossRef.
- C. Knapp, N. Weiden and K. P. Dinse, Magn. Reson. Chem., 2005, 43, 199–204 CrossRef PubMed.
- A. A. Smith, B. Corzilius, A. B. Barnes, T. Maly and R. G. Griffin, J. Chem. Phys., 2012, 136, 1–16 Search PubMed.
- G. Menzildjian, M. Y. A. Lund, D. Gajan, L. Niccoli, G. Karthikeyan, G. Casano, G. Jeschke, O. Ouari, M. Lelli and A. Lesage, J. Phys. Chem. B, 2021, 125, 13329–13338 CrossRef CAS PubMed.
- A. C. Torrezan, S. T. Han, I. Mastovsky, M. A. Shapiro, J. R. Sirigiri, R. J. Temkin, A. B. Barnes and R. G. Griffin, IEEE Trans. Plasma Sci., 2010, 38, 1150–1159 Search PubMed.
- F. J. Scott, E. P. Saliba, B. J. Albert, N. Alaniva, E. L. Sesti, C. Gao, N. C. Golota, E. J. Choi, A. P. Jagtap, J. J. Wittmann, M. Eckardt, W. Harneit, B. Corzilius, S. T. Sigurdsson and A. B. Barnes, J. Magn. Reson., 2018, 289, 45–54 CrossRef CAS PubMed.
- A. Equbal, K. Tagami and S. Han, J. Phys. Chem. Lett., 2019, 10, 7781–7788 CrossRef CAS PubMed.
- C. Gao, N. Alaniva, E. P. Saliba, E. L. Sesti, P. T. Judge, F. J. Scott, T. Halbritter, S. T. Sigurdsson and A. B. Barnes, J. Magn. Reson., 2019, 308, 1–10 CrossRef PubMed.
- D. E. Hoff, B. J. Albert, E. P. Saliba, F. J. Scott, E. J. Choi, M. Mardini and A. B. Barnes, Solid State Nucl. Magn. Reson., 2015, 72, 79–89 CrossRef CAS PubMed.
- T. V. Can, K. O. Tan, C. Yang, R. T. Weber and R. G. Griffin, J. Magn. Reson., 2021, 329, 1–7 CrossRef PubMed.
- G. Mathies, S. Jain, M. Reese and R. G. Griffin, J. Phys. Chem. Lett., 2016, 7, 111–116 CrossRef CAS PubMed.
- S. K. Jain, G. Mathies and R. G. Griffin, J. Chem. Phys., 2017, 147, 1–13 CrossRef PubMed.
- T. V. Can, R. T. Weber, J. J. Walish, T. M. Swager and R. G. Griffin, Angew. Chem., 2017, 56, 6744–6748 CrossRef CAS PubMed.
- P. Jakes, K. P. Dinse, C. Meyer, W. Harneit and A. Weidinger, Phys. Chem. Chem. Phys., 2003, 5, 4080–4083 RSC.
- S. Abe, G. Sato, T. Kaneko, T. Hirata, R. Hatakeyama, K. Yokoo, S. Ono, K. Omote and Y. Kasama, Jpn. J. Appl. Phys., 2006, 45, 8340–8343 CrossRef CAS.
- A. Weidinger, M. Waiblinger, B. Pietzak and T. A. Murphy, Appl. Phys. A, 1998, 66, 287–292 CrossRef CAS.
- H. Huang, M. Ata and M. Ramm, Chem. Commun., 2002, 2076–2077 RSC.
- T. Akasaka, N. Shigeru, B. Pietzak, A. Weidinger, K.-P. Dinse, A. Hirsch and Y. Kubozono, Dev. Fullerene Sci., 2002, 13–65 Search PubMed.
- T. Kaneko, S. Abe, H. Ishida and R. Hatakeyama, Phys. Plasmas, 2007, 14, 1–3 CrossRef.
- S. C. Cho, T. Kaneko, H. Ishida and R. Hatakeyama, Trans. Mat. Res. Soc. Japan, 2012, 37, 169–172 CrossRef CAS.
- S. C. Cho, T. Kaneko, H. Ishida and R. Hatakeyama, J. Appl. Phys., 2015, 117, 1–5 CrossRef.
- K. Furukawa, S. Okubo, H. Kato, H. Shinohara and T. Kato, J. Phys. Chem. A, 2003, 107, 10933–10937 CrossRef CAS.
- W. Harneit, K. Huebener, B. Naydenov, S. Schaefer and M. Scheloske, Phys. Status Solidi B, 2007, 244, 3879–3884 CrossRef CAS.
- F. J. Scott, N. Alaniva, N. C. Golota, E. L. Sesti, E. P. Saliba, L. E. Price, B. J. Albert, P. Chen, R. D. O’Connor and A. B. Barnes, J. Magn. Reson., 2018, 297, 23–32 CrossRef CAS PubMed.
- B. J. Albert, S. H. Pahng, N. Alaniva, E. L. Sesti, P. W. Rand, E. P. Saliba, F. J. Scott, E. J. Choi and A. B. Barnes, J. Magn. Reson., 2017, 283, 71–78 CrossRef CAS PubMed.
- H. He, J. T. Dias, J. Foulkes and J. Klinowski, Phys. Chem. Chem. Phys., 2000, 2, 2651–2654 RSC.
- R. Johnson, D. S. Bethune and C. S. Yannoni, Acc. Chem. Res., 1992, 25, 169–175 CrossRef CAS.
- D. Massiot, F. Fayon, M. Capron, I. King, S. L. Calvé, B. Alonso, J. O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS.
- H. Nikawa, Y. Araki, Z. Slanina, T. Tsuchiya, T. Akasaka, T. Wada, O. Ito, K. P. Dinse, M. Ata, T. Kato and S. Nagase, Chem. Commun., 2010, 46, 631–633 RSC.
- J. J. Wittmann, M. Eckardt, W. Harneit and B. Corzilius, Phys. Chem. Chem. Phys., 2018, 20, 11418–11429 RSC.
- R. Tycko, R. C. Haddon, G. Dabbagh, S. H. Glarum, D. C. Douglass and A. M. Mujsce, J. Phys. Chem., 1991, 95, 518–520 CrossRef CAS.
- W. I. F. David, R. M. Ibberson, T. J. S. Dennis, J. P. Hare and K. Prassides, Europhys. Lett., 1992, 18, 219–225 CrossRef CAS.
- W. Harneit, Endohedral Fullerenes: Electron Transfer and Spin, 2017, pp. 297–324 Search PubMed.
- J. E. Grose, E. S. Tam, C. Timm, M. Scheloske, B. Ulgut, J. J. Parks, H. D. Abruña, W. Harneit and D. C. Ralph, Nat. Mater., 2008, 7, 884–889 CrossRef CAS PubMed.
- N. Roch, R. Vincent, F. Elste, W. Harneit, W. Wernsdorfer, C. Timm and F. Balestro, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 1–4 CrossRef.
- W. Harneit, C. Boehme, S. Schaefer, K. Huebener, K. Fostiropoulos and K. Lips, Phys. Rev. Lett., 2007, 98, 1–4 CrossRef PubMed.
- S. Schaefer, K. Huebener, W. Harneit, C. Boehme, K. Fostiropoulos, H. Angermann, J. Rappich, J. Behrends and K. Lips, Solid State Sci., 2008, 10, 1314–1321 CrossRef CAS.
- S. Zhou, M. Yamamoto, G. A. D. Briggs, H. Imahori and K. Porfyrakis, J. Am. Chem. Soc., 2016, 138, 1313–1319 CrossRef CAS PubMed.
- J. J. Morton, A. M. Tyryshkin, A. Ardavan, S. C. Benjamin, K. Porfyrakis, S. A. Lyon and G. A. D. Briggs, Nat. Phys., 2006, 243, 3028–3031 Search PubMed.
- J. J. Morton, A. M. Tyryshkin, A. Ardavan, K. Porfyrakis, S. A. Lyon and G. A. D. Briggs, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 1–6 CrossRef.
- B. Meier, S. Mamone, M. Concistrè, J. Alonso-Valdesueiro, A. Krachmalnicoff, R. J. Whitby and M. H. Levitt, Nat. Commun., 2015, 6, 1–4 Search PubMed.
- D. Pinto, D. Paone, B. Kern, T. Dierker, R. Wieczorek, A. Singha, D. Dasari, A. Finkler, W. Harneit, J. Wrachtrup and K. Kern, Nat. Commun., 2020, 11, 1–6 CrossRef PubMed.
- K. A. Russ, P. Elvati, T. L. Parsonage, A. Dews, J. A. Jarvis, M. Ray, B. Schneider, P. J. Smith, P. T. Williamson, A. Violi and M. A. Philbert, Nanoscale, 2016, 8, 4134–4144 RSC.
- R. S. D’Rozario, C. L. Wee, E. J. Wallace and M. S. Sansom, Nanotechnology, 2009, 20, 1–7 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Owner Societies 2023 |