 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The structure of protic ionic liquids based on sulfuric acid, doped with excess of sulfuric acid or with water†
Anne
McGrogan
 a,
Emily L.
Byrne
a,
Emily L.
Byrne
 a,
Robert
Guiney
a,
Thomas F.
Headen
a,
Robert
Guiney
a,
Thomas F.
Headen
 b,
Tristan G. A.
Youngs
b,
Anna
Chrobok
b,
Tristan G. A.
Youngs
b,
Anna
Chrobok
 c,
John D.
Holbrey
c,
John D.
Holbrey
 *a and
Małgorzata
Swadźba-Kwaśny
*a and
Małgorzata
Swadźba-Kwaśny
 *a
*a
aThe QUILL Research Centre, School of Chemistry and Chemical Engineering, Queen's University Belfast, Belfast BT9 5AG, UK. E-mail: m.swadzba-kwasny@qub.ac.uk
bRutherford Appleton Laboratory, Chilton, Didcot, OX11 0QX, UK
cDepartment of Chemical Organic Technology and Petrochemistry, Silesian University of Technology, Krzywoustego 4, 44-100, Gilwice, Poland
First published on 10th January 2023
Abstract
Neutron scattering with isotopic substitution was used to study the structure of concentrated sulfuric acid, and two protic ionic liquids (PILs): a Brønsted-acidic PIL, synthesised using pyridine and excess of sulfuric acid, [Hpy][HSO4]·H2SO4, and a hydrated PIL, in which an equimolar mixture of sulfuric acid and pyridine has been doped with water, [Hpy][HSO4]·2H2O. Brønsted acidic PILs are excellent solvents/catalysts for esterifications, driving reaction to completion by phase-separating water and ester products. Water-doped PILs are efficient solvents/antisolvents in biomass fractionation. This study was carried out to provide an insight into the relationship between the performance of PILs in the two respective processes and their liquid structure. It was found that a persistent sulfate/sulfuric acid/water network structure was retained through the transition from sulfuric acid to PILs, even in the presence of 2 moles (∼17 wt%) of water. Hydrogen sulfate PILs have the propensity to incorporate water into hydrogen-bonded anionic chains, with strong and directional hydrogen bonds, which essentially form a new water-in-salt solvent system, with its own distinct structure and physico-chemical properties. It is the properties of this hydrated PIL that can be credited both for the good performance in esterification and beneficial solvent/antisolvent behaviour in biomass fractionation.
Introduction
Protic ionic liquids (PILs) derived from sulfuric acid and amines combine excellent performance across a number of chemical processes, with inherently lower costs than comparable aprotic ionic liquids. Even among PILs, these ionic liquids are amongst the least expensive since both sulfuric acid and triethylamine are cheap commodity chemicals. A technoeconomic assessment concluded that triethylammonium hydrogensulfate, [HN222][HSO4], could be produced for as little as US$0.78 kg−1, comparable to many organic solvents in commercial use.1–4 Ionic liquids formulated from di- and tri-alkylamines with excess sulfuric acid were postulated to be even cheaper,5 dispensing with the still-popular misconception that ionic liquids are, generically, too expensive for mainstream industrial use.6Brønsted acidic sulfuric acid based PILS can act as potent acidic catalysts.7–10 In esterification reactions, equilibria are strongly shifted towards the products because phase separation of esters from the acidic PIL phase is enhanced when compared to use of concentrated sulfuric acid.10 Hydrated PILs formulated from equimolar quantities of an amine and sulfuric acid, but with the addition of 10–40 wt% water, have been found to be excellent solvents for lignocellulosic biomass pre-treatment, enhancing the fractionation of cellulose, lignin and hemicellulose, leading to higher yields of sugars from subsequent saccharification of the carbohydrates.11–13
In both of these examples, enhanced performance arises from doping of the simple hydrogensulfate salt, [Hbase][HSO4], either with an excess of acid or with water, respectively, exemplifying a “4th generation” of ionic liquids,14 where molecular dopants are incorporated into the ionic liquid matrix, at quantities that preserve the key characteristics of an ionic liquid system (that is, without turning it into a concentrated solution of ions) while enhancing physico-chemical characteristics, viz. increased acidity, lowered viscosity or favourably modified phase behaviour. There are two characteristic features of these doped PILs: simplicity of the preparation, and potential complexity of the speciation/liquid structure of the resulting three-component mixtures that requires investigation and understanding.
There has been a history of debate surrounding the speciation in ionic liquids generated from proton transfer reactions, especially those with non-stoichiometric compositions, which could be described as network vs. cluster models. In 1981, Evans, Arnett and co-workers postulated that ethylammonium nitrate exhibited 3D hydrogen bonded network similar to that of water.15 The liquid network structure of ethylammonium nitrate was only confirmed, through far-infrared spectroscopy and DFT calculations, in 2009.16 This was followed by direct liquid structural investigations using neutron17–20 and X-ray21 scattering that identified nanostructured polar/non-polar domains generated by the extensive hydrogen bonding interactions in the polar regions. Similar structure is also present in PILs formed from other primary alkylamines bearing a Cn alkyl chain (n = 2–4) and anions including hydrogen sulfate, thiocyanate, nitrate and formate.22 Weaker acids add further complexity of incomplete proton transfer. For example, mixtures of pyridine and acetic acid were shown to feature a hydrogen-bonded network, which consisted of charge-neutral molecules of acetic acid, with free pyridine sitting in pockets of this network, unprotonated even with a large excess of acid.23
On the other hand, in PILs synthesised from amines and excess of hydrogen halides (χacid > 0.5, X = F−,24–29 Cl−,30 and Br−,31) discrete dimeric and oligomeric clusters, and chains of [X⋯H⋯X]− have been identified, with ample crystallographic evidence in addition to liquid-phase studies. Complex anionic clusters have been proposed in other Brønsted acidic PILs.10,32,33 For example, in mixtures of amines and trifluoroacetic acid (HTFA), where χHTFA > 0.5, was postulated to contain [TFA(HTFA)x]− clusters.34
Doping ethylammonium nitrate with water resulted in gradual altering of the liquid structure, but even upon the addition of as much as six moles of water, the structure of the hydrated PIL remained ionic liquid-like, distinctly different from homogenous solution.35 These results, along with numerous other contributions,36 suggest that hydrated PILs form their own class of water-in-salt solvents, a sub-class of a wide family of solvent-doped ionic liquids.14 Consequently, macroscopic properties of hydrated PILs, and their liquids structures, are distinct from both the parent PIL and simple aqueous solution.
Considering the structure of PILs derived from sulfuric acid, a 3D networked structure could be postulated based on the structure of sulfuric acid. Neutron and X-ray scattering studies of H2SO4 have shown that sulfate moieties have an ordered, dense network of hydrogen bonds in concentrated liquid acid,37–39 in the solid state,40–44 and in aqueous solution.45,46 With increasing dilution the structure trends to resemble water, albeit with nearest neighbour intermolecular rO⋯O bond distances ca. 0.2 Å shorter than in pure H2O.
On the other hand, discrete oligomeric hydrogen bonded anionic clusters have been reported to exist in the gas phase, in the Earth's stratosphere.47–50 The first paper by our group reporting PILs derived from sulfuric acid10 also postulated the existence of anionic clusters, in analogy to earlier works on trifluoroacetate ionic liquids25 and other strongly hydrogen bonding anions. FT-IR and NMR spectroscopic studies have been used to justify these assignments, but whereas these early results do show strong interactions between molecules of H2SO4 and [HSO4]−, they could admittedly occur in both the cluster and the 3D network arrangement.
Here, neutron scattering has been used to compare the liquid structures of concentrated sulfuric acid and two pyridine/sulfuric acid PILs containing either excess of the acid or doped with water. The objective was to elucidate potential relationships between the local liquid structure, the effectiveness of these PILs as catalysts and solvents for esterification reactions8–10 and for treatment of lignocellulosic biomass.11–13
Experimental
Materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, [Hpy][HSO4]·H2SO4 and a hydrated PIL, formed from concentrated sulfuric acid, pyridine and water in 1
1 molar ratio, [Hpy][HSO4]·H2SO4 and a hydrated PIL, formed from concentrated sulfuric acid, pyridine and water in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio [Hpy][HSO4]·2H2O. For each composition, isotopologues containing protiated (H), deuteriated (D) or equimolar mixture of protiated and deuteriated components (H/D) were prepared. A detailed synthetic procedure can be found in the ESI.†
2 molar ratio [Hpy][HSO4]·2H2O. For each composition, isotopologues containing protiated (H), deuteriated (D) or equimolar mixture of protiated and deuteriated components (H/D) were prepared. A detailed synthetic procedure can be found in the ESI.†
All samples (Table 1) were homogeneous liquids at ambient temperature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyridine
pyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, their compositions and corresponding levels of protiation and/or deuteration
water, their compositions and corresponding levels of protiation and/or deuteration
| Sample number | Theoretical formula (actual H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) py py![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) H2O) |
Sulfuric acid | py | water |
|---|---|---|---|---|
| 1 | Concentrated sulfuric acid H2SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) 0.2) |
H | H | |
| 2 | H/D | H/D | ||
| 3 | D | D | ||
| 4 | Brønsted acidic PIL [Hpy][HSO4]·H2SO4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) 0.4) |
H | D | H |
| 5 | H/D | D | H/D | |
| 6 | D | D | D | |
| 7 | D | H | D | |
| 8 | D | H/D | D | |
| 9 | H | H | H | |
| 10 | H | H/D | H | |
| 11 | Hydrated PIL | H | D | H |
| 12 | [Hpy][HSO4]·2H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2) 2.2) |
H/D | D | H/D |
| 13 | D | D | D | |
| 14 | D | H | D | |
| 15 | D | H/D | D | |
| 16 | H | H | H | |
| 17 | H | H/D | H |
Total scattering data were reduced into a differential scattering cross section using the GUDRUN package.51 Data collected on a 3.1 mm vanadium-niobium alloy plate standard was used for calibration, while data recorded on the empty SANDALS instrument and an empty 1 mm quartz cell were used for background subtraction.52
Reduced data were analysed using the Empirical Potential Structure Refinement (EPSR)53,54 software to examine the time-averaged liquid structure. EPSR uses a Monte Carlo simulation approach coupled with Lennard-Jones potentials with atom-centred point charges, combined with basic structural information about the atoms or molecules and the total atomic densities present of the system.53,54 Differences between the experimental and simulated data sets in Q space (i.e. the empirical perturbation potential) are determined, enabling iterative refinement to generate a self-consistent fit to the scattering cross sections obtained from isotopically distinct samples. Simulations were equilibrated over ca. 2000–3000 cycles before accumulating and averaging data. The EPSR refinements, in each case, were initialised using an equilibrated Monte Carlo simulation containing 500 or 1000 molecular moieties (pyridine, hydrogen sulfate, molecular sulfuric acid and water, with atomic sites labelled as shown in Fig. 1) depending on the sample.
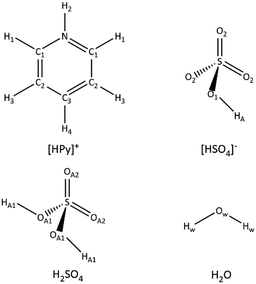 | ||
| Fig. 1 Atom types used in the EPSR simulation models for the pyridinium cation, hydrogen sulfate anion, molecular sulfuric acid and water. | ||
The full set of reference potential parameters and constraints used in the EPSR simulation model, and the total number of pyridine, hydrogen sulfate and/or sulfuric acid and water and size of each simulation box, corresponding to the experimentally determined molecular densities of the fully protiated mixtures are shown in ESI.† Charges were scaled to ±0.8 e, in line with results from neutron diffraction and MD simulations of ILs.55 Here reduced charges have been shown to reproduce experimental data more effectively. This better simulates effects of electronic polarisability captured when using more expensive polarisable force fields.56
Results and discussion
Selection of samples
The experimental design included the study of concentrated sulfuric acid, a Brønsted acidic PIL formulated with two moles of H2SO4 per one mol of pyridine, [Hpy][HSO4]·H2SO4 and a hydrated ionic liquid, [Hpy][HSO4]·2H2O. Adjusting for the actual water content from these idealised compositions, concentrated sulfuric acid (98%) was assumed to contain 0.2 M of either H2O (in H2SO4) or D2O (in D2SO4). This was confirmed by examination of the respective measured neutron scattering levels. As such, actual compositions of the examined samples used to model the neutron scattering data were: concentrated sulfuric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, acid
0.2, acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water), Brønsted acidic PIL (2
water), Brønsted acidic PIL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, acid
0.4, acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyridine
pyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) and hydrated PIL (1
water) and hydrated PIL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2, acid
2.2, acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyridine
pyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water), as summarised in Table 1.
water), as summarised in Table 1.
The structure of concentrated sulfuric acid was used as a baseline to compare with the structures of both PILs. The composition of the Brønsted acidic PIL, [Hpy][HSO4]·H2SO4, was one of the two standard acidic compositions used in our earlier catalytic studies.5,8–10 The aim of this work was to elucidate its structure (3D network vs. discrete anionic clusters) and understand how differences in the liquid structure of this PIL and sulfuric acid account for their different miscibilities with the ester product. Finally, the hydrated PIL, [Hpy][HSO4]·2H2O, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2, acid
2.2, acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyridine
pyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio (ca. 17 wt% of water), was close in composition to the 20 wt% water content, cited as the optimum composition for delignification of cellulosic biomass.4,11–13,57 It was anticipated that particularly efficient biomass fractionation reported for this aqueous PIL composition could be tied to the speciation of water in the 1
water ratio (ca. 17 wt% of water), was close in composition to the 20 wt% water content, cited as the optimum composition for delignification of cellulosic biomass.4,11–13,57 It was anticipated that particularly efficient biomass fractionation reported for this aqueous PIL composition could be tied to the speciation of water in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 mixture.
2.2 mixture.
EPSR modelling and fit to experimental data
The data from sulfuric acid (samples 1–3) was modelled using molecular descriptions of the species present (H2SO4 and H2O in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 molar ratio) – see Fig. 1. Although several more complex water species (H3O+, H5O2+) have been reported as constituents of sulfuric acid hydrates,40–44 descriptors were limited to the main moieties, enabling the examination of key associations in these already complex mixtures. This approach is aligned with EPSR analysis of neutron scattering data recorded for other ‘neat’ acids (acetic acid, formic acid),58 in which molecular (undissociated) descriptions were used. The same strategy has been used in MD simulations of sulfuric acid
0.2 molar ratio) – see Fig. 1. Although several more complex water species (H3O+, H5O2+) have been reported as constituents of sulfuric acid hydrates,40–44 descriptors were limited to the main moieties, enabling the examination of key associations in these already complex mixtures. This approach is aligned with EPSR analysis of neutron scattering data recorded for other ‘neat’ acids (acetic acid, formic acid),58 in which molecular (undissociated) descriptions were used. The same strategy has been used in MD simulations of sulfuric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures59 and other ‘neat’ acids, small molecules and mixtures.52,58–66
water mixtures59 and other ‘neat’ acids, small molecules and mixtures.52,58–66
The Brønsted acidic PIL (samples 4–10) and the hydrated PIL (samples 11–17) were modelled using fully protonated pyridinium cations [Py-H]+, anionic [HSO4]− and molecular H2O. Additionally, the model of samples 4–10 contained molecular H2SO4.
Although pyridine in the presence of sulfuric acid can be considered fully protonated, based on both FT-IR spectroscopy67 and crystallographic data for pyridinium hydrogen sulfate,68 an alternative ‘free proton’ model,23 (with discrete H+, labelled as HF), [HSO4]− and H2O components in the simulation box) was also examined. However, the degree of pyridine protonation found was significantly underestimated with an average N⋯HF coordination number of only 0.2 ± 0.4 and with the maximum in the N⋯HF correlation (corresponding to N–H bonds in pyridinium cations) overestimated at 1.6 Å. In consequence, the fully-protonated cationic pyridinium descriptor was used.
Comparisons of experimental and simulated total structure factors, F(R), and the corresponding Fourier transforms to real space G(r) for each of the isotopically distinct experimental mixtures (Fig. 2) show the quality of fit to the experimental data. With the exception of the region at Q ≤ 1 Å−1, which is most susceptible to inconsistencies due to inelastic scattering contributions from hydrogen in the data, the fitted data aligns well with experiment. For the neat sulfuric acid systems (1–3, Table 1), a larger degree of scattering at low Q is present in the experimental data than is captured within the EPSR model which may reflect either incomplete subtraction of the inelastic hydrogen background from the data, or additional long range order. Similar profiles are also apparent for the fully deuteriated PILs (samples 6 and 13) suggesting that some hydrogen is present, generating inelastic scattering from the sample in this region.
 | ||
Fig. 2 Total structure factors F(Q) (top), and the corresponding Fourier transform to real space G(r) radial distribution functions (bottom) showing experimental data (red symbols) and EPSR modelled (blue solid line) for left: sulfuric acid (H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2); middle: Brønsted acidic PIL (H2SO4 0.2); middle: Brønsted acidic PIL (H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) py py![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 2 H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) and right: hydrated PIL (H2SO4 0.4) and right: hydrated PIL (H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) py py![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2). Labels represent the experimental compositions shown in Table 1 and the curves have been offset for clarity. 2.2). Labels represent the experimental compositions shown in Table 1 and the curves have been offset for clarity. | ||
Centre of mass radial distribution functions
Centre of mass (COM) radial distribution functions (RDFs) were calculated using the SHARM routines within EPSR, for each of the components in the three systems (Fig. 3). The RDFs reveal a remarkable persistence of structure, similar between the three systems: H2SO4, [Hpy][HSO4]·H2SO4 and [Hpy][HSO4]·2H2O.In concentrated sulfuric acid, the {SO4}–{SO4} RDF shows a first shell correlation with a maximum at ca. 4.6 Å, with a broad second shell correlation between 6.6–11.5 Å (Fig. 3, top, blue dotted line). The corresponding water–{SO4} first shell correlation (Fig. 3, bottom, blue dotted line) is centred around 4 Å (minimum 5.5 Å), and the water–water RDF correlation (Fig. 3, bottom, dotted green) at 3.2 Å. This is significantly longer than water–water distances in bulk water (2.6 Å), confirming that water molecules are confined within sulfuric acid as hydrates. Further supporting this conclusion, the water–water RDF lacks a second shell peak between 4–6 Å, that would have been indicative of ‘free’ water.
In both PIL systems, the cation–{SO4} RDFs are characteristic of strong cation–anion association, typical of ionic liquids.69 The cation–anion RDFs (Fig. 3, top, green curves) exhibit a first correlation peak at 5.0–5.2 Å with a shoulder at 4.2 Å, and a second broader correlation peak, indicative of the second shell, at ca. 9 Å. The shoulder at 4.2 Å reflects the oblate topology of the pyridinium cations, allowing two distinct routes to approach its centre of mass. The cation–cation first correlations (Fig. 3, top, red curves) are present at larger separations (maxima at ∼6 Å), followed by a second shell at 9–10 Å, overlapping with the second shell of cation–anion correlations. Again, a small shoulder at ca. 4 Å in the cation–cation RDFs may be due to a small number of face-to-face correlations, typical of the π–π interactions observed in liquid pyridine at distances below 5 Å.70 Similar features are observed in aprotic ILs containing N-alkylpyridinium cations, arising from anions associating equatorially around pyridinium cations.71–73
In contrast to typical ionic liquid structure, there is very close anion–anion interaction in both PILs. The corresponding {SO4}–{SO4} associations (Fig. 3, top, blue dashed and solid curves) closely resemble sulfate associations in ‘neat’ sulfuric acid (first shell at 4.6 Å, second one at 6.6–11 Å). In PILs, the first correlation lengthens slightly (4.8 Å), which can be attributed to the decreasing number of acidic hydrogens in the H2SO4/[HSO4]− components, therefore reducing the number of available S–O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bonding motifs: from 2 per {SO4} unit in H2SO4, through 1.5 per {SO4} unit in [Hpy][HSO4]·H2SO4, to 1 per {SO4} unit in [Hpy][HSO4]·2H2O (assuming this is terminal and not bifurcated).
S hydrogen bonding motifs: from 2 per {SO4} unit in H2SO4, through 1.5 per {SO4} unit in [Hpy][HSO4]·H2SO4, to 1 per {SO4} unit in [Hpy][HSO4]·2H2O (assuming this is terminal and not bifurcated).
This close anion–anion interaction in both PILs results in the unusual presence of both cations and anions in the first coordination shell of {SO4}. Their liquid structure combines characteristics of ionic liquid (close cation–anion correlations) and of the parent sulfuric acid (sulfate–sulfate organisation).
The water⋯{SO4} and water⋯[H-Py]+ RDFs (Fig. 3, bottom, blue and red lines, respectively) have their corresponding first peak correlations of 4.0 and 4.8 Å. There is no significant difference in the water association with either {SO4} or [H-Py]+ on changing the water content of the PILs.
In contrast, water–water RDFs show a marked difference. In H2SO4 and in [Hpy][HSO4]·H2SO4, that is with low water content, H2O⋯H2O correlation is found at 3.2 Å, slightly more intense in [Hpy][HSO4]·H2SO4. In [Hpy][HSO4]·2H2O, this distance decreases to 2.7 Å, showing both greater self-association of water molecules and a larger number of correlations, as indicated by the increase in intensity. This distance is close to H2O⋯H2O correlation in bulk water (2.6 Å). However, lack of significant second shell correlation (4–6 Å) indicates that there are no large-size water clusters and [Hpy][HSO4]·2H2O retains water-in-IL rather than IL-in-water characteristics.
The similarity of RDF correlation profiles (Fig. 3), aside from the water-water correlation, suggests essentially similar liquid structure and character of both PILs, and that the presence of the water (up to 2 moles, ca. 17 wt%) does not perturb the ion-ion structure significantly. Moreover, the hydrogen-bonding network of sulfuric acid appears to be retained as a core structural motif in the PILs, in addition to the typical Coulombic charge screening structure usually observed in ionic liquids.
In the context of applications of Brønsted acidic PILs in esterification, it was initially assumed that phase-separation of the organic phase, that is much more efficient in Brønsted acidic PILs than in concentrated sulfuric acid, may be related to marked differences between the structures of the two liquids: H2SO4vs. [Hpy][HSO4]·H2SO4. However, the results here suggest that there are greater similarities between the structure-defining associations within these liquids rather than significant differences that would explain the distinctly different phase behaviours of H2SO4 and PILs in esterification reactions.
In contrast, the water-in-salt structure of the hydrated PIL, [Hpy][HSO4]·2H2O, with negatively-charged hydrogen-bonded network of hydrogen sulfate and water (with or without H2SO4 present) can be expected to have distinctly different properties when compared to anhydrous [Hpy][HSO4], as described in the literature.11,74,75 Furthermore, in esterification reactions, where water and ester are generated, the Brønsted acidic PIL can be envisaged to gradually bind water to form a similar hydrogen sulfate – sulfuric acid – water anionic network, in which water is bound as a hydrate and less likely to hydrolyse the ester product.
Detailed, comparative structural analysis of the three liquid systems studied in this work are provided below.
Correlation and association around {SO4} groups
Detailed site–site analysis of contributions to the scattering were made. The positions of first peaks within the partial RDFs of selected site–site correlations and corresponding coordination numbers, calculated to the first minima after the peak, are included within the ESI.†
Fig. 4 shows oxygen⋯oxygen correlations between {SO4} groups, originating both from [HSO4]− and H2SO4, were determined from the first peak in the site–site pRDFs. In all three systems, the ‘hetero’ S–OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S mode of correlation is dominant, appearing as a strongly defined peak in the RDF at 2.6–2.7 Å (Fig. 4, green line). In contrast, first contact correlations of S–OH⋯HO–S and S
S mode of correlation is dominant, appearing as a strongly defined peak in the RDF at 2.6–2.7 Å (Fig. 4, green line). In contrast, first contact correlations of S–OH⋯HO–S and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯O
O⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S only occur around 3.0 Å, with a peak at ca. 5 Å, corresponding to the separation in the COM RDF. It is therefore evident that the primary mode of association between {SO4} groups is S–OH⋯O
S only occur around 3.0 Å, with a peak at ca. 5 Å, corresponding to the separation in the COM RDF. It is therefore evident that the primary mode of association between {SO4} groups is S–OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bonding, retained from sulfuric acid in both PILs.
S hydrogen bonding, retained from sulfuric acid in both PILs.
Correlation distances for concentrated H2SO4 are broadly consistent with the literature.37,46,59 Andreani et al. first reported data derived from direct Fourier transform of experimental X-ray and neutron scattering data,37 with S⋯S and O⋯O separation distances of 5.3 Å and 2.42 Å. However, the S⋯S separation, extrapolated from summation of intermolecular S–O and intramolecular O–O distances, appears to be over-estimated. Kameda et al.46 subsequently reported a shorter S⋯S correlation of 4.8 Å which is more consistent with simulation between contact pairs in concentrated H2SO4/H2O (4.6 ± 0.1 Å),76 and small clusters of bulk H2SO4 (4.8 Å)62 where an O⋯O distance of 3.1 Å was also reported. Here, the first shell S⋯S separation distance was determined as 4.6 Å, with O⋯O correlations between OA1 and OA2 sites (S–OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) at 2.6 Å, and minima after this first correlation peak at 3 Å. The {SO4}⋯{SO4} coordination number (Ncoord = 11 ± 1) between neighbouring moieties is also in agreement with the literature (Ncoord = 12),37,59 and confirms the formation of an extended, tetrahedral hydrogen bonded network linked through S–OH⋯O
S) at 2.6 Å, and minima after this first correlation peak at 3 Å. The {SO4}⋯{SO4} coordination number (Ncoord = 11 ± 1) between neighbouring moieties is also in agreement with the literature (Ncoord = 12),37,59 and confirms the formation of an extended, tetrahedral hydrogen bonded network linked through S–OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S interactions.77
S interactions.77
In both PILs, interatomic distances for the first shell O⋯O correlations, as well as corresponding Ncoord values, are very close to sulfuric acid, suggesting hydrogen bonding of similar strength across the three samples. However, the {SO4}⋯{SO4} first shell coordination environment (determined from S⋯S correlations) decreases: from 11 in H2SO4, to 7.5 in [Hpy][HSO4]·H2SO4, and to 4.8 in [Hpy][HSO4]·2H2O, commensurate with the reduction in the number of available S–OH groups to act as hydrogen-bond donors.
Site–site RDFs between water (Ow/Hw) and sulfate units are shown in Fig. 5. For the concentrated acid and anhydrous acidic PIL, the presence of ca. 0.2 mole fraction of water leads to a broad peak in the Ow⋯O1/O2 RDFs between ∼2.6–3.0 Å (Fig. 5, dashed lines). The Hw⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S correlation at 1.8 Å (Fig. 5, solid blue lines) indicates directional hydrogen-bonding retained between the acid and PILs.
S correlation at 1.8 Å (Fig. 5, solid blue lines) indicates directional hydrogen-bonding retained between the acid and PILs.
In the hydrated PIL, [Hpy][HSO4]·2H2O, the magnitude of these correlations increases, as there are more water molecules available, and less S–OH sites. Associated Ow⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S correlation at 2.8 Å (Fig. 5, dashed blue line) also becomes sharper. Interestingly, the corresponding Ow⋯O(H)–S correlation at 2.8 Å (Fig. 5, dashed red line) is dramatically decreased. This indicates that the remaining available S–OH sites hydrogen bond preferentially to O
S correlation at 2.8 Å (Fig. 5, dashed blue line) also becomes sharper. Interestingly, the corresponding Ow⋯O(H)–S correlation at 2.8 Å (Fig. 5, dashed red line) is dramatically decreased. This indicates that the remaining available S–OH sites hydrogen bond preferentially to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, rather than water. In short, water in [Hpy][HSO4]·2H2O (absence of strong Brønsted acid) binds preferentially as Hw⋯O
S, rather than water. In short, water in [Hpy][HSO4]·2H2O (absence of strong Brønsted acid) binds preferentially as Hw⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, not as Ow⋯HO–S, acting as hydrogen bond donor, rather than hydrogen bond acceptor.
S, not as Ow⋯HO–S, acting as hydrogen bond donor, rather than hydrogen bond acceptor.
Water–water association
In the low-water samples, H2SO4 and [Hpy][HSO4]·H2SO4, the Ow⋯Ow distance is longer than in ‘bulk’ water, and the corresponding coordination number is low (Ncoord≈ 0.5), which is indicative of isolated water molecules, strongly associated with the sulfate structure. In contrast, the Ow⋯Ow correlation in [Hpy][HSO4]·2H2O (Fig. 6, green line) has a first peak at 2.8 Å, comparable to that in pure water, and the coordination number (Ncoord = 4.1 ± 2) slightly lower than that of bulk water (Ncoord = 4.7).78 However, the Ow⋯Ow RDF does not have the distinctive second peak around 4.5 Å, which would have been indicative of a long-range tetrahedral order seen in bulk water, or its larger clusters.The combination of water-like first shell and unlike-water second shell has been reported for ‘bound’ water in inorganic molten salt hydrates,79 and is consistent with the [Hpy][HSO4]·2H2O PIL having a water-in-ionic liquid structure,14 but approaching the transition to a concentrated salt solution.
Correlation and association around [H-Py]+
RDFs between [H-Py]+ and {SO4}, and between [H-Py]+ and water Ow, are shown in Fig. 7. In both PILs, the hydrogen-bond donating N–H site of [H-Py]+ has equally close contact with hydrogen bond-accepting sites in {SO4} and in water. The N1⋯O2 and N1⋯Ow correlations occur at 2.7 Å in [Hpy][HSO4]·H2SO4 (Fig. 7, black solid lines) and at 2.8 Å in [Hpy][HSO4]·2H2O (Fig. 7, black dashed lines). Carbon atoms of pyridine are separated by 3.4–3.6 Å from both O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and Ow sites; these are consistent with weak contacts at the van der Waals separation distances.
S and Ow sites; these are consistent with weak contacts at the van der Waals separation distances.
Hydrogen bond donation from S–OH sites (O1/OA1) to the pyridinium cation is not observed. Consequently, we can see water molecules acting as hydrogen-bond donors to [HSO4]− anions through Hw⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S interaction, and as hydrogen-bond acceptors from [H-Py]+ cations through, Ow⋯H-Py interaction. The presence and directionality of water molecules within the first shells of both {SO4} and [H-Py]+ species demonstrates the structure-forming nature of water molecules, which contribute to the overall hydrogen bond network, reinforcing the sulfate/sulfuric acid network and bridging cations and anions.14,80
S interaction, and as hydrogen-bond acceptors from [H-Py]+ cations through, Ow⋯H-Py interaction. The presence and directionality of water molecules within the first shells of both {SO4} and [H-Py]+ species demonstrates the structure-forming nature of water molecules, which contribute to the overall hydrogen bond network, reinforcing the sulfate/sulfuric acid network and bridging cations and anions.14,80
Spatial association
There is a remarkable retention of the correlation pattern across the three systems examined, as shown in the pRDFs, and it is interesting to explore whether this translates to the retention of the network structure present in sulfuric acid to PILs, despite the reduced number of acidic S–OH sites and the introduction of [H-Py]+ cations.Spatial distribution functions (SDFs) in Fig. 8 are plotting the top 15% probability for correlations within the first coordination shell.
 | ||
| Fig. 8 Spatial distribution functions (SDFs) showing the first shell environments around H2SO4 (a), [Hpy][HSO4]·H2SO4 (b) and (c), and [Hpy][HSO4]·2H2O (d) and (e), presenting the {SO4} (a), (b), (d) and [H-Py]+ (c), (e) environments, with the nitrogen-site of [H-Py]+ pointing up. All SDFs are plotted to show the top 15% probability for correlation within the first shell, determined from the first minimum in the corresponding COM RDFs (Fig. 3). The [HSO4]−/H2SO4 moieties are shown in yellow, H2O in green and [H-Py]+ in blue. | ||
For sulfuric acid, {SO4}⋯{SO4} correlations (Fig. 8(a), yellow surface) show a pronounced tetrahedral symmetry with four high probability nodes, each sitting over a triangular face of the {SO4} tetrahedron, bridged by six bands bisecting O–S–O edges. The SDF map shows holes around each oxygen atom, presumably occupied by hydrogen atoms participating in S–OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonding from adjacent acids in the first coordination shell. The weakly correlated association of water molecules with sulfuric acid, shown in Fig. 5 RDFs, is also evident in the SDF, (Fig. 8(a), green surface), lacking a distinct spatial ordering. Plotting water–sulfuric acid SDF at 50% probability (ESI†) shows a non-defined spherical distribution.
S bonding from adjacent acids in the first coordination shell. The weakly correlated association of water molecules with sulfuric acid, shown in Fig. 5 RDFs, is also evident in the SDF, (Fig. 8(a), green surface), lacking a distinct spatial ordering. Plotting water–sulfuric acid SDF at 50% probability (ESI†) shows a non-defined spherical distribution.
Structure refinement for [Hpy][HSO4]·H2SO4 was made with two different {SO4} moieties: [HSO4]− and [H2SO4]. Comparable distributions of both species with a central reference [HSO4]− were found, and are plotted as merged functions, forming the same sulfuric acid-like tetrahedral distribution (Fig. 8(b), yellow surface). The equivalent SDFs around H2SO4 are included in the ESI.† Compared to H2SO4, the symmetry of the distribution of {SO4}⋯{SO4} association was somewhat reduced, with the S–OH site in [HSO4]− showing a greater probability density than the corresponding S–O oxygens. This is a consequence of the change in relative numbers of S–OH and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S oxygens in the system, from 1
S oxygens in the system, from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, reducing the relative proportion of hydrogen-bond donor to acceptor sites. In contrast, distribution of H2O molecules around [HSO4]− (Fig. 8(b), green surface) was more structured in [Hpy][HSO4]·H2SO4 than it was in H2SO4, tracking to the positions of {SO4} moieties in the first shell.
4, reducing the relative proportion of hydrogen-bond donor to acceptor sites. In contrast, distribution of H2O molecules around [HSO4]− (Fig. 8(b), green surface) was more structured in [Hpy][HSO4]·H2SO4 than it was in H2SO4, tracking to the positions of {SO4} moieties in the first shell.
Likewise, [H-Py]+ cations (Fig. 8(b), green surface) show correlations in these positions, in an approximately tetrahedral distribution with maxima associated with each triangular face of {SO4} reflecting the association through hydrogen-bond donation from [H-Py]+ cations to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S sites (see Fig. 7). Both the water and [H-Py]+ SDFs around [HSO4]− are truncated over the acidic S–OH group, as a result of the predominance of this site as a hydrogen-bond donor to other {SO4} units, rather than a hydrogen-bond acceptor.
S sites (see Fig. 7). Both the water and [H-Py]+ SDFs around [HSO4]− are truncated over the acidic S–OH group, as a result of the predominance of this site as a hydrogen-bond donor to other {SO4} units, rather than a hydrogen-bond acceptor.
In the hydrated PIL, [Hpy][HSO4]·2H2O, the S–OH to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S ratio is reduced to 1
S ratio is reduced to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, which results in closer {SO4}⋯{SO4} association through the remaining S–OH site in [HSO4]− (Fig. 8(d), yellow surface). The reduced number of the S–OH hydrogen bond donors leads to increased correlation of water with the O
3, which results in closer {SO4}⋯{SO4} association through the remaining S–OH site in [HSO4]− (Fig. 8(d), yellow surface). The reduced number of the S–OH hydrogen bond donors leads to increased correlation of water with the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S sites (Fig. 8(d), green surface), consistent with the increased magnitude and definition of the Hw⋯O2 pRDF (Fig. 5).
S sites (Fig. 8(d), green surface), consistent with the increased magnitude and definition of the Hw⋯O2 pRDF (Fig. 5).
Solvation environments around [H-Py]+ in both PILs are characterised by very similar spatial distributions of {SO4}, in a band above/below the pyridinium ring, straddling the N–H site (Fig. 8(c) and (e), yellow surface). Water occupies similar positions, and also arranges more broadly within the equatorial plane of the [H-Py]+ ring, especially in the hydrated PIL (Fig. 8(c) and (e), yellow surface). It is in alignment with water acting as a hydrogen bond acceptor via Ow⋯H–N and to a lesser extent Ow⋯H–C hydrogens of the pyridine ring (Fig. 7).
Finally, the [H-Py]+⋯[H-Py]+ SDFs (Fig. 8(c) and (e), blue surface) show a broad correlation around the C2/C3 positions of pyridinium rings, that even plotting the top 15% probability is highly diffuse and non-specific.
Overall, from H2SO4, through the Brønsted acidic PIL, and to the hydrated PIL, the first shell environment of the {SO4} moiety shows a transition from a highly symmetric tetrahedral distribution of acid {SO4} groups towards an increased probability density for {SO4}⋯{SO4} association at the S–OH position, as a function of the increase in anionic O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S oxygens relative to S–OH acid groups. This is evident in [Hpy][HSO4]·H2SO4 through the larger node at the S–OH site, with water–{SO4} and [H-Py]+⋯{SO4} correlations to sites associated with the O
S oxygens relative to S–OH acid groups. This is evident in [Hpy][HSO4]·H2SO4 through the larger node at the S–OH site, with water–{SO4} and [H-Py]+⋯{SO4} correlations to sites associated with the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S oxygens. For [Hpy][HSO4]·2H2O, the highest probability SDF correlations between [HSO4]− anions are with the S–OH site, with hydrogen-bonding between water and O
S oxygens. For [Hpy][HSO4]·2H2O, the highest probability SDF correlations between [HSO4]− anions are with the S–OH site, with hydrogen-bonding between water and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S sites becoming more dominant, substituting for S–OH⋯O
S sites becoming more dominant, substituting for S–OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S interactions in the first shell. However these changes in the first shell correlations around {SO4}, induced by the changes in availability of different hydrogen-bond donors, have remarkably little effect on the longer range order in the liquids. The second shell {SO4}⋯{SO4} correlations between {SO4} units in the three systems are shown in Fig. 9, plotting the top 15% probability surfaces for the first shell (3–6.5 Å) and second shell (6.5–11 Å). Remarkably, across all three systems a strong tetrahedral structure is retained, irrespective of the changes in relative {SO4} and water distributions in the first correlation shell. All systems show the same tetrahedral pattern, despite differences in the first shell. This gives strong evidence that water (and to a lesser extent pyridinium cations) act as surrogates for S–OH hydrogen-bond donors retaining the structure of the parent acid within the PILs.
S interactions in the first shell. However these changes in the first shell correlations around {SO4}, induced by the changes in availability of different hydrogen-bond donors, have remarkably little effect on the longer range order in the liquids. The second shell {SO4}⋯{SO4} correlations between {SO4} units in the three systems are shown in Fig. 9, plotting the top 15% probability surfaces for the first shell (3–6.5 Å) and second shell (6.5–11 Å). Remarkably, across all three systems a strong tetrahedral structure is retained, irrespective of the changes in relative {SO4} and water distributions in the first correlation shell. All systems show the same tetrahedral pattern, despite differences in the first shell. This gives strong evidence that water (and to a lesser extent pyridinium cations) act as surrogates for S–OH hydrogen-bond donors retaining the structure of the parent acid within the PILs.
Conclusions
The RDFs indicate that anion–cation correlations between protonated [H-py]+ cations and [HSO4]− anions typical of ionic liquids (and molten salts)69 are formed in both the ‘anhydrous’ acidic PIL and ‘hydrated' PIL with charge screening between alternate oppositely charged ions. However, in addition to the formation of this typical cation–anion ionic liquid structure, the overall tetrahedral network structure, directed by hydrogen-bonding around the {SO4} groups in ‘neat’ sulfuric acid is persistent and retained in the PILs. This is complemented by interactions with the [H-Py]+ cations (as can be seen in the presence of correlation nodes between [H-Py+] and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S sites in 2
S sites in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 ‘anhydrous’ acidic PIL) and increasingly, by replacement of acidic S–OH hydrogen bond donors by water molecules in the 1
0.4 ‘anhydrous’ acidic PIL) and increasingly, by replacement of acidic S–OH hydrogen bond donors by water molecules in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 ‘hydrated’ PIL to retain the supramolecular {SO4}-network present in the parent sulfuric acid.
2.2 ‘hydrated’ PIL to retain the supramolecular {SO4}-network present in the parent sulfuric acid.
As such, it is clear that the ‘anhydrous’ acidic 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 PIL retains many features of concentrated sulfuric acid in terms of both acidity10 and network structure, while simultaneously adopting the anticipated anion–cation correlation pattern characteristic of ionic liquids. Consequently, difference in phase behaviour between sulfuric acid and PILs, which result, for example, in different performances as solvents for biomass fractionation or esterification reactions, cannot be attributed directly to differences in the liquid structures as the ‘anhydrous’ acidic PIL strongly resembles ‘neat’ sulfuric acid. The addition of 2 moles of water (ca. 17 wt%) provides additional hydrogen bond donation capacity to complement and replace the diminished number of S–OH hydrogen-bond donors present in the parent acid. This results in a change in the nature of the first shell hydrogen-bond donors around [HSO4]− anions, but not in the overall pattern of hydrogen bonding. This ‘hydrated’ sulfuric acid
0.2 PIL retains many features of concentrated sulfuric acid in terms of both acidity10 and network structure, while simultaneously adopting the anticipated anion–cation correlation pattern characteristic of ionic liquids. Consequently, difference in phase behaviour between sulfuric acid and PILs, which result, for example, in different performances as solvents for biomass fractionation or esterification reactions, cannot be attributed directly to differences in the liquid structures as the ‘anhydrous’ acidic PIL strongly resembles ‘neat’ sulfuric acid. The addition of 2 moles of water (ca. 17 wt%) provides additional hydrogen bond donation capacity to complement and replace the diminished number of S–OH hydrogen-bond donors present in the parent acid. This results in a change in the nature of the first shell hydrogen-bond donors around [HSO4]− anions, but not in the overall pattern of hydrogen bonding. This ‘hydrated’ sulfuric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyridine
pyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2) system has an equivalent water content to that of the aqueous alkylammonium PILs reported by Hallett et al.4,11,12,57,81–86 as media for delignification for cellulosic biomass, with ca. 15 wt% water in the IL. It is clear that the water molecules here are present as ‘bound’ water participating in the ionic liquid solvation structure and not as ‘free’ water. That is, the system can be viewed as one with water-in-IL rather than as a concentrated IL-in-water environment.
2.2) system has an equivalent water content to that of the aqueous alkylammonium PILs reported by Hallett et al.4,11,12,57,81–86 as media for delignification for cellulosic biomass, with ca. 15 wt% water in the IL. It is clear that the water molecules here are present as ‘bound’ water participating in the ionic liquid solvation structure and not as ‘free’ water. That is, the system can be viewed as one with water-in-IL rather than as a concentrated IL-in-water environment.
This finding allows for certain speculations in terms of phase behaviour. In esterification reactions, where water and ester are generated, the ionic liquid gradually binds water incorporated in the hydrogen sulfate network, without the formation of ‘bulk’ water that can contribute to the reverse reaction of ester hydrolysis. The resulting hydrated PIL is more hydrophilic, which contributes to a lower affinity to the ester products and, in consequence, enhanced phase separation. Likewise, it appears that the “composite” anionic structure of water-doped PIL has a higher propensity to dissolve lignin, its structure being different from that of anhydrous IL, but unlikely to contain “bulk” water at the optimised ratios of 10–40% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 composition amounts to ca. 20% water by weight).
2 composition amounts to ca. 20% water by weight).
These conclusions are aligned with the available experimental evidence, but nevertheless remain speculative. Further evidence can be provided by the study of solvation of model compounds in wet and dry PILs by neutron scattering, more advanced computational approaches, or thermodynamic studies of the energy of solvation.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
DfE (EB) and EPSRC (AMcG) are kindly acknowledged for PhD studentship funding and the Science and Technology Facilities Research Council thanked for use of SANDALS spectrometer during beam time (RB2010681).10.5286/ISIS.E.RB2010681.References
- F. J. V. Gschwend, L. M. Hennequin, A. Brandt-Talbot, F. Bedoya-lora, G. H. Kelsall, K. Polizzi, P. S. Fennell and J. P. Hallett, Green Chem., 2020, 22, 5032 RSC.
- H. Baaqel, I. Díaz, V. Tulus, B. Chachuat, G. Guillén-gosálbez and J. P. Hallett, Green Chem., 2020, 22, 3132 RSC.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098 RSC.
- A. George, A. Brandt, K. Tran, S. M. S. N. S. Zahari, D. Klein-Marcuschamer, N. Sun, N. Sathitsuksanoh, J. Shi, V. Stavila, R. Parthasarathi, S. Singh, B. M. Holmes, T. Welton, B. A. Simmons and J. P. Hallett, Green Chem., 2015, 17, 1728 RSC.
- (a) A. Brzęczek-Szafran, J. Więcławik, N. Barteczko, A. Szelwicka, E. Byrne, A. Kolanowska, M. Swadźba Kwaśny and A. Chrobok, Green Chem., 2021, 23, 4421 RSC; (b) A. Brzęczek-Szafran, K. Erfurt, M. Swadźba-Kwaśny, T. Piotrowski and A. Chrobok, ACS Sustainable Chem. Eng., 2022, 10, 13568 CrossRef.
- A. A. Quintana, A. M. Sztapka, V. de C. Santos Ebinuma and C. Agatemor, Angew. Chem., Int. Ed., 2022, 37, 61 Search PubMed.
- M. P. Atkins, M. J. Earle, K. R. Seddon, M. Swadźba-Kwaśny and L. Vanoye, Chem. Commun., 2010, 46, 1745 RSC.
- M. Przypis, K. Matuszek, A. Chrobok, M. Swadźba-Kwaśny and D. Gillner, J. Mol. Liq., 2020, 308, 113166 CrossRef CAS.
- K. Matuszek, A. Brzecek-Szafran, D. Kobus, D. R. MacFarlane, M. Swadźba-Kwaśny and A. Chrobok, Aust. J. Chem., 2019, 72, 130 CrossRef CAS.
- K. Matuszek, A. Chrobok, F. Coleman, K. R. Seddon and M. Swadźba-Kwaśny, Green Chem., 2014, 16, 3463 RSC.
- A. Brandt-Talbot, F. J. V. Gschwend, P. S. Fennell, T. M. Lammens, B. Tan, J. Weale and J. P. Hallett, Green Chem., 2017, 19, 3078 RSC.
- P. Verdía, A. Brandt, J. P. Hallett, M. J. Ray and T. Welton, Green Chem., 2014, 16, 1617 RSC.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489 RSC.
- D. R. MacFarlane, A. L. Chong, M. Forsyth, M. Kar, R. Vijayaraghavan, A. Somers and J. M. Pringle, Faraday Discuss., 2018, 206, 9 RSC.
- D. F. Evans, S. H. Chen, G. W. Schriver and E. M. Arnett, J. Am. Chem. Soc., 1981, 103, 481 CrossRef CAS.
- K. Fumino, A. Wulf and R. Ludwig, Angew. Chem., Int. Ed., 2009, 48, 3184 CrossRef CAS PubMed.
- R. Atkin and G. G. Warr, J. Phys. Chem. B, 2008, 112, 4164 CrossRef CAS.
- R. Hayes, S. Imberti, G. G. Warr and R. Atkin, Phys. Chem. Chem. Phys., 2011, 13, 3237 RSC.
- R. Hayes, G. G. Warr and R. Atkin, Chem. Rev., 2015, 115, 6357 CrossRef CAS PubMed.
- R. Hayes, S. Imberti, G. G. Warr and R. Atkin, Angew. Chem., Int. Ed., 2013, 125, 4721 CrossRef.
- Y. Umebayashi, W. Chung, T. Mitsugi, S. Fukuda, M. Takeuchi, K. Fujii, T. Takamuku, R. Kanzaki and S. Ishiguro, J. Comput. Chem., Jpn., 2008, 7, 125 CrossRef CAS.
- R. Hayes, S. Imberti, G. G. Warr and R. Atkin, Angew. Chem., Int. Ed., 2013, 52, 4623 CrossRef CAS PubMed.
- J. A. Mccune, A. H. Turner, F. Coleman, C. M. White, S. K. Callear, T. G. A. Youngs, M. Swadźba-Kwaśny and J. D. Holbrey, Phys. Chem. Chem. Phys., 2015, 17, 6767 RSC.
- R. Hagiwara, K. Matsumoto, Y. Nakamori, T. Tsuda, Y. Ito, H. Matsumoto and K. Momota, J. Electrochem. Soc., 2003, 150, D195 CrossRef CAS.
- R. Hagiwara and Y. Ito, J. Fluorine Chem., 2000, 105, 221 CrossRef CAS.
- K. Matsumoto and R. Hagiwara, J. Fluorine Chem., 2007, 128, 317 CrossRef CAS.
- R. Hagiwara, Y. Nakamori, K. Matsumoto and Y. Ito, J. Phys. Chem. B, 2005, 109, 5445 CrossRef CAS PubMed.
- R. Hagiwara, T. Hirashige, T. Tsuda and Y. Ito, J. Fluorine Chem., 1999, 99, 1 CrossRef CAS.
- Y. Saito, K. Hirai, K. Matsumoto, R. Hagiwara and Y. Minamizaki, J. Phys. Chem. B, 2005, 109, 2942 CrossRef CAS PubMed.
- J. W. Shuppert and C. A. Angell, J. Chem. Phys., 1977, 67, 3050 CrossRef CAS.
- G. Driver and K. E. Johnson, Green Chem., 2003, 5, 163 RSC.
- K. M. Johansson, E. I. Izgorodina, M. Forsyth, D. R. MacFarlane and K. R. Seddon, Phys. Chem. Chem. Phys., 2008, 10, 2972 RSC.
- G. A. Olah, T. Mathew, A. Goeppert, B. Török, I. Bucsi, X.-Y. Li, Q. Wang, E. R. Marinez, P. Batamack, R. Aniszfeld and G. K. Surya Prakash, J. Am. Chem. Soc., 2005, 127, 5964 CrossRef PubMed.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411 CrossRef PubMed.
- R. Hayes, S. Imberti, G. G. Warr and R. Atkin, Angew. Chem., Int. Ed., 2012, 51, 7468 CrossRef PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2015, 115, 11379 CrossRef PubMed.
- C. Andreani, C. Petrillo and F. Sacchetti, Mol. Phys., 1986, 58, 299 CrossRef.
- A. R. Moodenbaugh, J. E. Hartt, J. J. Hurst, W. Youngblood, D. E. Cox and B. C. Frazer, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 28, 3501 CrossRef.
- P. C. Pascard-Billy, Acta Crystallogr., 1965, 18, 827 CrossRef.
- A. D. Fortes, I. G. Wood and K. S. Knight, J. Chem. Phys., 2006, 125, 1 CrossRef PubMed.
- H. E. Maynard-Casely, H. E. A. Brand and K. S. Wallwork, J. Appl. Crystallogr., 2012, 45, 1198 CrossRef CAS.
- T. K. Hirsch and L. Ojamae, Acta Crystallogr., 2004, B60, 179 CAS.
- D. Mootz and A. Merschenz-Quack, Z. Naturforsch., B: J. Chem. Sci., 1987, 42, 1231 CrossRef CAS.
- A. D. Fortes, I. G. Wood, L. Vočadlo, L. Chapon, K. S. Knight and R. I. Smith, J. Chem. Phys., 2008, 5, 128 Search PubMed.
- A. K. Soper and M. G. Phillips, Chem. Phys., 1986, 107, 47 CrossRef CAS.
- Y. Kameda, K. Hosoya, S. Sakamoto, H. Suzuki, T. Usuki and O. Uemura, Stud. Phys. Theor. Chem., 1995, 83, 305 Search PubMed.
- J. Curtius, K. D. Froyd and E. R. Lovejoy, J. Phys. Chem. A, 2001, 105, 10867 CrossRef CAS.
- E. R. Lovejoy and J. Curtius, J. Phys. Chem. A, 2001, 105, 10874 CrossRef CAS.
- G.-L. Hou, W. Lin, S. H. M. Deng, J. Zhang, W.-J. Zheng, F. Paesani and X.-B. Wang, J. Phys. Chem. Lett., 2013, 4, 779 CrossRef CAS PubMed.
- T. I. Yacovitch, N. Heine, C. Brieger, T. Wende, C. Hock, D. M. Neumark and K. R. Asmis, J. Phys. Chem. A, 2013, 117, 7081 CrossRef CAS PubMed.
- A. K. Soper, GudrunN and GudrunX: Programs for correcting raw neutron and X-ray diffraction data to differential scattering cross section, 2011.
- J. A. Mccune, A. H. Turner, F. Coleman, C. M. White, S. K. Callear, T. G. A. Youngs, M. Swadźba-Kwaśny and J. D. Holbrey, Phys. Chem. Chem. Phys., 2015, 17, 6767 RSC.
- A. K. Soper, C. Phys., 1996, 202, 295 CAS.
- A. K. Soper, M. Phys., 2001, 99, 1503 CrossRef CAS.
- T. G. A. Youngs, J. D. Holbrey, C. L. Mullan, S. E. Norman, M. C. Lagunas, C. D’Agostino, M. D. Mantle, L. F. Gladden, D. T. Bowron and C. Hardacre, Chem. Sci., 2011, 2, 1594 RSC.
- D. Bedrov, J.-P. Piquemal, O. Borodin, A. D. Mackerell Jr., B. Roux and C. Schröder, Chem. Rev., 2019, 119, 7940 CrossRef CAS PubMed.
- W. C. Tu, L. Weigand, M. Hummel, H. Sixta, A. Brandt-Talbot and J. P. Hallett, Cellulose, 2020, 27, 4745 CrossRef CAS.
- S. Imberti and D. T. Bowron, J. Phys.: Consens. Matter., 2010, 22, 404212 Search PubMed.
- M. Canales and E. Guàrdia, J. Mol. Liq., 2016, 224, 1064 CrossRef CAS.
- L. Saiz, J. A. Padro and E. Guardia, Mol. Phys., 1999, 97, 897 CrossRef CAS.
- T. F. Headen, C. A. Howard, N. T. Skipper, M. A. Wilkinson, D. T. Bowron and A. K. Soper, J. Am. Chem. Soc., 2010, 132, 5735 CrossRef CAS PubMed.
- J. J. Towey, A. K. Soper and L. Dougan, Faraday Discuss., 2013, 167, 159 RSC.
- B. D. T. Bowron, J. L. Finney and A. K. Soper, Mol. Phys., 1998, 93, 531 CrossRef.
- M. Falkowska, D. T. Bowron, H. G. Manyar, C. Hardacre and T. G. A. Youngs, ChemPhysChem, 2016, 17, 2043 CrossRef CAS PubMed.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Green Chem., 2016, 18, 2736 RSC.
- O. S. Hammond, K. J. Edler, D. T. Bowron and L. Torrente-murciano, Nat. Commun., 2017, 8, 14150 CrossRef CAS PubMed.
- I. Tankov, R. Yankova, S. Genieva, M. Mitkova and D. Stratiev, J. Mol. Struct., 2017, 1139, 400 CrossRef CAS.
- R. D. Rogers and C. B. Bauer, J. Chem. Crystallogr., 1994, 24, 285 CrossRef CAS.
- C. Hardacre, J. D. Holbrey, M. Nieuwenhuyzen and T. G. A. Youngs, Acc. Chem. Res., 2007, 40, 1146 CrossRef CAS PubMed.
- T. F. Headen, P. L. Cullen, R. Patel, A. Taylor and N. T. Skipper, Phys. Chem. Chem. Phys., 2018, 20, 2704 RSC.
- A. H. Turner, S. Imberti, M. Swadźba-Kwaśny and J. D. Holbrey, Faraday Discuss., 2018, 206, 247 RSC.
- A. H. Turner, E. L. Byrne, T. Pereira and J. D. Holbrey, Phys. Chem. Chem. Phys., 2020, 22, 10219 RSC.
- A. H. Turner and J. D. Holbrey, J. Solution Chem., 2015, 44, 621 CrossRef CAS.
- M. M. Seitkalieva, A. v Vavina, A. v Posvyatenko, K. S. Egorova, A. S. Kashin, E. G. Gordeev, E. N. Strukova, L. v Romashov and V. P. Ananikov, ACS Sustainable Chem. Eng., 2021, 9, 3552 CrossRef CAS.
- A. R. Abouelela, A. Al Ghatta, P. Verdía, M. Shan Koo, J. Lemus and J. P. Hallett, ACS Sustainable Chem. Eng., 2021, 9, 10524 CrossRef CAS.
- C.-G. Ding, T. Taskila, K. Laasonen and A. Laaksonen, Chem. Phys., 2003, 287, 7 CrossRef CAS.
- G. F. King and M. Mobli, in Comprehensive Natural Products II, ed. H.-W. Liu and L. Mander, 2010, p.314 Search PubMed.
- A. K. Soper, ISRN Phys. Chem., 2013, 1 Search PubMed.
- S. Lenton, N. H. Rhys, J. J. Towey, A. K. Soper and L. Dougan, Nat. Commun., 2017, 8, 919 CrossRef PubMed.
- H. K. Stassen, R. Ludwig, A. Wulf and J. Dupont, Chem. – Eur. J., 2015, 21, 1 CrossRef PubMed.
- F. J. V. Gschwend, F. Malaret, S. Shinde, A. Brandt-Talbot and J. P. Hallett, Green Chem., 2018, 20, 3486 RSC.
- F. Malaret, F. J. V. Gschwend, J. M. Lopes, W. C. Tu and J. P. Hallett, RSC Adv., 2020, 10, 16050 RSC.
- C. L. Chambon, P. Verdía, P. S. Fennell and J. P. Hallett, Sci. Rep., 2021, 11, 15383 CrossRef PubMed.
- C. L. Chambon, T. Y. Mkhize, P. Reddy, A. Brandt-Talbot, N. Deenadayalu, P. S. Fennell and J. P. Hallett, Biotechnol. Biofuels, 2018, 11, 1 CrossRef PubMed.
- R. Muazzam, A. M. Asim, M. Uroos, N. Muhammad and J. P. Hallett, RSC Adv., 2021, 11, 19095 RSC.
- A. R. Abouelela and J. P. Hallett, ACS Sustainable Chem. Eng., 2021, 9, 704 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cp04292d |
| This journal is © the Owner Societies 2023 |






