Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On the microscopic origin of Soret coefficient minima in liquid mixtures†
Oliver R.
Gittus
 * and
Fernando
Bresme
* and
Fernando
Bresme

Department of Chemistry, Molecular Sciences Research Hub, Imperial College London, London W12 0BZ, UK. E-mail: o.gittus18@imperial.ac.uk; f.bresme@imperial.ac.uk
First published on 12th December 2022
Abstract
Temperature gradients induce mass separation in mixtures in a process called thermodiffusion and quantified by the Soret coefficient. The existence of minima in the Soret coefficient of aqueous solutions at specific salt concentrations was controversial until fairly recently, where a combination of experiments and simulations provided evidence for the existence of this physical phenomenon. However, the physical origin of the minima and more importantly its generality, e.g. in non-aqueous liquid mixtures, is still an outstanding question. Here, we report the existence of a minimum in liquid mixtures of non-polar liquids modelled as Lennard-Jones mixtures, demonstrating the generality of minima in the Soret coefficient. The minimum originates from a coincident minimum in the thermodynamic factor, and hence denotes a maximization of non-ideality mixing conditions. We rationalize the microscopic origin of this effect in terms of the atomic coordination structure of the mixtures.
Introduction
Thermal gradients induce the transport of colloids in suspension (thermophoresis) and concentration gradients in liquid mixtures and solutions (thermodiffusion). The Soret coefficient, ST, measures the mass separation of mixtures in thermal fields and is becoming a central property to characterise the non-equilibrium response of soft matter and fluids.1–8Experimental and computational studies have advanced significantly in recent years, but several outstanding questions remain. One such question is the microscopic origin of the forces driving the phenomenology observed in thermodiffusion measurements. Aqueous solutions feature a particularly rich phenomenology.9–11 Gaeta et al.10 reported minima in the Soret coefficients of NaCl(aq) and KCl(aq) as a function of concentration. These experiments were performed with thermogravitational columns, and the minima could not be reproduced using state-of-the-art thermal diffusion forced Rayleigh scattering techniques, which circumvent convection effects.11 Hence the ST minimum remained controversial for many years. However, this situation changed with recent experiments and computer simulations of LiCl(aq), which support the existence of a minimum in the Soret coefficient.12,13 Very recently, minima at high concentrations (∼2 M) were observed for thiocyanate (NaSCN(aq) and KSCN(aq)) and acetate (CH3COOK(aq)) salt solutions,14,15 giving further impetus to the investigation of the physical origin of the ST minima.
In addition to aqueous electrolyte solutions, minima in ST with composition were observed in mixtures of polar fluids: ethanol/water,5,16,17 dimethyl-sulfoxide/water18 and acetone/water.18,19 In all these systems (electrolyte solutions and polar fluid mixtures), one of the components is water. This observation might suggest that the ST minima are interlinked with water as a solvent and, therefore, its specific thermal transport properties. Indeed, molecular simulations of atomistic (non-polar) Lennard-Jones (LJ) binary mixtures at supercritical conditions do not offer evidence for the existence of minima in ST with composition.20 However, some experiments of non-polar or weakly polar liquid mixtures reported maxima/minima in ST (e.g. cyclohexane/cis-decaline) and in some cases, accounting for an extrapolation to infinite dilution, a weak extrema can be inferred (e.g. toluene/1,3-dichlorobenzene).21 That work made no attempt to explain the microscopic origin of the extrema in ST, but crucially highlights the importance of the thermodynamic factor, Γ, a key quantity determining the heat of transport.13,22
To investigate the existence of Soret coefficient minima in non-polar mixtures, and to probe the microscopic origin of such minima, we have performed computer simulations of the simplest liquid binary mixture, modelled with the Lennard-Jones model, which accounts for dispersion interactions. We show for the first time that a minimum in the Soret coefficient at a specific composition, and constant temperature T and pressure P, can be observed in simple non-polar liquid mixtures, hence showing that the minimum is a completely general physical phenomenon.
Methods
We consider binary LJ mixtures at constant T and P, and different mole fractions x1. Inter-particle interactions were modelled using the LJTS potential, which is the LJ potential |![[scr V, script letter V]](https://www.rsc.org/images/entities/char_e149.gif) LJij(r) = 4εij[(σij/r)12 − (σij/r)6] truncated and shifted at a cutoff radius of rc = 2.5σ, |
LJij(r) = 4εij[(σij/r)12 − (σij/r)6] truncated and shifted at a cutoff radius of rc = 2.5σ, |![[scr V, script letter V]](https://www.rsc.org/images/entities/char_e149.gif) LJTSij(r) = (
LJTSij(r) = (![[scr V, script letter V]](https://www.rsc.org/images/entities/char_e149.gif) LJij(r) −
LJij(r) − ![[scr V, script letter V]](https://www.rsc.org/images/entities/char_e149.gif) LJij(rc))θ(rc − r) with θ being the Heaviside step function. All the particles have the same diameter and mass, but the interactions between of particles of type “1” or “2” are different. The parameters ε = ε22 and σ = σ11 = σ22 together with the mass of each particle m1 = m2 = m define the usual LJ units. The energy scale was defined in terms of the high-boiling component, with ε11/ε22 = 0.6. The Lorentz–Berthelot combining rules were used for
LJij(rc))θ(rc − r) with θ being the Heaviside step function. All the particles have the same diameter and mass, but the interactions between of particles of type “1” or “2” are different. The parameters ε = ε22 and σ = σ11 = σ22 together with the mass of each particle m1 = m2 = m define the usual LJ units. The energy scale was defined in terms of the high-boiling component, with ε11/ε22 = 0.6. The Lorentz–Berthelot combining rules were used for  and σ12 = (σ11 + σ22)/2 = σ. Experiments of liquid–liquid mixtures have reported extrema in ST, therefore we target subcritical conditions for the LJ mixtures. Mixtures were modelled at T = 0.62εkB−1 (kB is the Boltzmann constant) and P = 0.46 εσ−3, below the critical point23 for both species. The entire 0 ≤ x1 ≤ 1 composition domain is therefore expected to be subcritical. We note that along this isobar-isotherm, the thermodynamically stable phase for x1 = 0 is a solid and we estimate the liquids to be at x1 ≈ 0.2 (see the ESI†). Simulations below this mole fraction correspond to a metastable liquid–liquid mixture. Nevertheless, as we will show below, the observed ST minimum is safely within the liquid–liquid mixture portion of the phase diagram.
and σ12 = (σ11 + σ22)/2 = σ. Experiments of liquid–liquid mixtures have reported extrema in ST, therefore we target subcritical conditions for the LJ mixtures. Mixtures were modelled at T = 0.62εkB−1 (kB is the Boltzmann constant) and P = 0.46 εσ−3, below the critical point23 for both species. The entire 0 ≤ x1 ≤ 1 composition domain is therefore expected to be subcritical. We note that along this isobar-isotherm, the thermodynamically stable phase for x1 = 0 is a solid and we estimate the liquids to be at x1 ≈ 0.2 (see the ESI†). Simulations below this mole fraction correspond to a metastable liquid–liquid mixture. Nevertheless, as we will show below, the observed ST minimum is safely within the liquid–liquid mixture portion of the phase diagram.
We performed a variety of equilibrium molecular simulations (EMS), molecular dynamics (MD) and Monte Carlo methods, as well as non-equilibrium molecular dynamics (NEMD) simulations to calculate ST and related quantities. From NEMD, ST was evaluated at the stationary state characterised by zero net mass flux as ST = − (w1w2)−1(∇w1/∇T) = −(x1x2)−1(∇x1/∇T) where xi and wi are the mole and mass fractions of species i. EMS methods calculate ST ≡ DT/D12 from24 the mutual diffusion coefficient D12 = L11(∂μs,1/∂w1)P,T/(ρw2T) and thermal diffusion coefficient 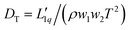 . Onsager's phenomenological coefficients Lαβ were calculated using the Green–Kubo (GK) integral formulas and taking into account the enthalpy terms for
. Onsager's phenomenological coefficients Lαβ were calculated using the Green–Kubo (GK) integral formulas and taking into account the enthalpy terms for  . The chemical potential μ1, and subsequently the specific chemical potential μs,1 = μ1/m1, was calculated using a free energy perturbation (FEP) method at constant T and P. (∂μ1/∂x1)P,T was then calculated from the numerical derivative of μ1. (∂μ1/∂x1)P,T was also obtained from Kirkwood–Buff solution theory via two different methods of evaluating the Kirkwood–Buff integrals (KBIs): (1) from particle number fluctuations in grand canonical Monte Carlo (GCMC) simulations and (2) from the extrapolation to infinite system size of finite-volume KBIs,25 which were in turn calculated using radial distribution functions (RDFs) from MD simulations in the NVT ensemble. Thus, three EMS methods were used to calculate (∂μ1/∂x1)P,T and subsequently the thermodynamic factor: FEP, KBI(RDF) and KBI(GCMC). Combining these with the GK calculations for L11 and
. The chemical potential μ1, and subsequently the specific chemical potential μs,1 = μ1/m1, was calculated using a free energy perturbation (FEP) method at constant T and P. (∂μ1/∂x1)P,T was then calculated from the numerical derivative of μ1. (∂μ1/∂x1)P,T was also obtained from Kirkwood–Buff solution theory via two different methods of evaluating the Kirkwood–Buff integrals (KBIs): (1) from particle number fluctuations in grand canonical Monte Carlo (GCMC) simulations and (2) from the extrapolation to infinite system size of finite-volume KBIs,25 which were in turn calculated using radial distribution functions (RDFs) from MD simulations in the NVT ensemble. Thus, three EMS methods were used to calculate (∂μ1/∂x1)P,T and subsequently the thermodynamic factor: FEP, KBI(RDF) and KBI(GCMC). Combining these with the GK calculations for L11 and  give three corresponding “equilibrium” routes to ST: GK + FEP, GK + KBI(RDF) and GK + KBI(GCMC). Other thermophysical properties were also calculated from MD simulations. Details about all these simulations and calculations are given in the ESI.† All simulations were performed using LAMMPS26 (v. 3 March 2020).
give three corresponding “equilibrium” routes to ST: GK + FEP, GK + KBI(RDF) and GK + KBI(GCMC). Other thermophysical properties were also calculated from MD simulations. Details about all these simulations and calculations are given in the ESI.† All simulations were performed using LAMMPS26 (v. 3 March 2020).
Results & discussion
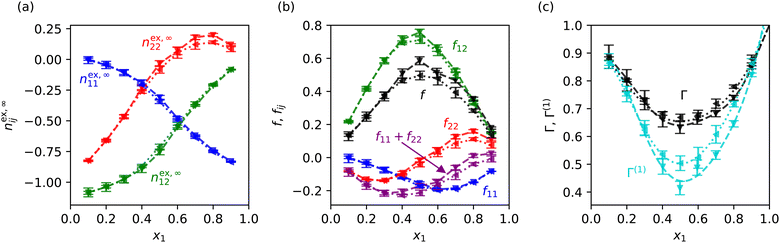
Fig. 1(a) contains the main result of this communication: ST features a minimum as a function of composition at . Fitting cubic functions to the data give
. Fitting cubic functions to the data give  for all four methods: NEMD, GK + FEP, GK + KBI(RDF) and GK + KBI(GCMC). The thermodiffusion response at the minimum is significantly enhanced with respect to diluted mixtures, by ∼30–40% relative to x1 = 0.1, 0.9, and by ∼60–80% when compared to the extrapolated value of ST at x1 = 1. For all compositions, ST < 0 which indicates that species 1 (the low-boiling component) is thermophilic and preferentially collects in the hot region. The ST values calculated from NEMD and all three EMS methods are in excellent agreement.
for all four methods: NEMD, GK + FEP, GK + KBI(RDF) and GK + KBI(GCMC). The thermodiffusion response at the minimum is significantly enhanced with respect to diluted mixtures, by ∼30–40% relative to x1 = 0.1, 0.9, and by ∼60–80% when compared to the extrapolated value of ST at x1 = 1. For all compositions, ST < 0 which indicates that species 1 (the low-boiling component) is thermophilic and preferentially collects in the hot region. The ST values calculated from NEMD and all three EMS methods are in excellent agreement.
S T ≡ DT/D12 is determined by D12 and DT, which monotonically increase and decrease with x1, respectively (Fig. 1(b)). Thus, the ST minimum arises from a balance of DT and D12, as opposed to being carried through only by one of the transport coefficients.
The Soret coefficient ST can be written in terms of the phenomenological coefficients and the thermodynamic factor Γ, as24
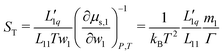 | (1) |
 | (2) |
 is essentially constant for all compositions (Fig. 1(d)). This suggests that the minimum arises from the w1(∂μ1/∂w1)P,T term. As shown in Fig. 1(c), Γ features a distinctive minimum at xmin(Γ)1 ∼ 0.5, with all three EMS methods predicting values in good agreement with each other. The minimum in STis connected to the minimum in the thermodynamic factor, and therefore the minimum signals the composition at which the mixture features the largest non-ideality, max|1 − Γ|.
is essentially constant for all compositions (Fig. 1(d)). This suggests that the minimum arises from the w1(∂μ1/∂w1)P,T term. As shown in Fig. 1(c), Γ features a distinctive minimum at xmin(Γ)1 ∼ 0.5, with all three EMS methods predicting values in good agreement with each other. The minimum in STis connected to the minimum in the thermodynamic factor, and therefore the minimum signals the composition at which the mixture features the largest non-ideality, max|1 − Γ|.
We note that in our work, the minimum in Γ leads to a minimum in ST since  (L11 ≥ 0,24 and a stable single-phase binary mixture requires Γ > 0). For
(L11 ≥ 0,24 and a stable single-phase binary mixture requires Γ > 0). For  , the minimum in Γ leads to a maximum in ST. Indeed, since ST and DT change sign under the permutation of components in a binary mixture (ST,1 = −ST,2 and DT,1 = −DT,2), the Γ minimum leads to a maximum in the Soret coefficient of species 2.
, the minimum in Γ leads to a maximum in ST. Indeed, since ST and DT change sign under the permutation of components in a binary mixture (ST,1 = −ST,2 and DT,1 = −DT,2), the Γ minimum leads to a maximum in the Soret coefficient of species 2.
The results presented above indicate that the non-ideal contribution dominates at conditions near the minimum. This can be visualised by splitting Γ into its ideal (id) and excess (ex) parts (see ESI,† Section S1.2), showing as expected that Γex, is responsible for the minimum in Γ. To gain insight into the microscopic origins of the minimum in Γ and ST, we turn to Kirkwood–Buff theory, which connects Γ to the structural properties of the binary mixture,
 | (3) |
 is the ideal coordination number. ρN,j is the number density of species j. Hence, the KBIs are given by
is the ideal coordination number. ρN,j is the number density of species j. Hence, the KBIs are given by  , and the thermodynamic factor by Γ = (1 + f)−1 where f = (1 − x1)nex,∞11 + x1nex,∞22 − 2x1nex,∞12 = f11 + f22 + f12.
, and the thermodynamic factor by Γ = (1 + f)−1 where f = (1 − x1)nex,∞11 + x1nex,∞22 − 2x1nex,∞12 = f11 + f22 + f12.
In order to disentangle the contributions from nex,∞11, nex,∞22 and nex,∞12 we take the first-order approximation to the thermodynamic factor, Γ(1) = 1 − f. As shown in Fig. 2(c), Γ(1) results in underestimations of 1–35% across the range of compositions, with larger errors for more non-ideal mixtures, but nevertheless provides insight into the relative importance of the fij terms. f12 features a maximum at x1 ∼ 0.5 (Fig. 2(b)), indicating that the cross-species contribution is responsible for the minimum in Γ(1) and Γ. |(f11 + f22)/f12| = 0–0.41 making the cross-species contribution much more significant, and ∼3–4 times larger in the region of the Γ(1) and Γ minima. Consequently, the phenomenology of Γ(1) and Γ are primarily determined by f12. Thus, the composition dependence of nex,∞12 (Fig. 2(a)), which represents a net depletion of species 2 around 1 relative to the ideal state, and increases monotonically with x1, is the primary microscopic origin of the minimum in Γ and therefore ST.
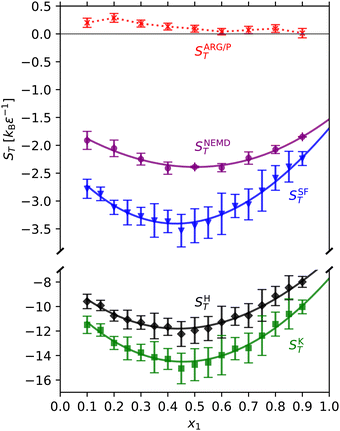
Now we examine the accuracy of theoretical approaches to predict the ST minimum reported above. We note that existing theoretical models do not accurately predict ST in general.27–33 In some cases, even the sign of ST is not predicted correctly.28–33 Especially in earlier works, the discrepancies can, at least in part, be attributed to inaccuracies in experimentally determined properties. For example the Haase30,34 and Kempers30 models are very sensitive to partial molar properties, and therefore the equation of state used.30,31 In computer simulations all the required quantities can be accurately calculated, and as shown here using an exact model, all the theories examined herein feature noticeable deviations from the ST values obtained by direct simulation. Previous simulations have shown that the theories are accurate only in a very limited number of cases, even for simple LJ mixtures and hard-sphere mixtures.20,32,33 We have tested the standard theoretical models against our simulation data. We calculate the Soret coefficient according to the models of Haase30,34 (SHT), Kempers30 (SKT), Shukla and Firoozabadi29 (SSFT), and Artola, Rousseau and Galliéro32/Prigogine35,36 (SARG/PT). Further details are given in the ESI.†
We show in Fig. 3 the Soret coefficients predicted by these models, alongside the NEMD values for reference. Out of the four models, SSFT is the most accurate: it overestimates |ST| by ∼20–50%. SKT and SHT overestimate |ST| by ∼400–500% and ∼300–400%, respectively. SARG/PT underestimates |ST| by ∼100–110%, predicting values ∼10−1kBε−1. Furthermore, the model predicts the wrong sign: SARG/PT > 0 or straddles 0 when accounting for the associated uncertainties.
S HT, SKT and SSFT all possess a minimum because they contain x1(∂μ1/∂x1)P,T = kBTΓ in the denominator (see the ESI†). The x1(∂μ1/∂x1)P,T term in SSFT originates directly from the phenomenological equations for thermodiffusion from linear non-equilibrium thermodynamics, which the model uses as a starting point for its derivation. For SKT, the x1(∂μ1/∂x1)P,T term arises naturally from the statistical thermodynamics approach employed by Kempers. Originally an educated guess, SHT can be derived more rigorously within the framework of Kempers.30 In contrast, SARG/PT does not predict a minimum: it features a weak concentration dependence, and generally decreases with increasing x1. SARG/PT contains kBT (as opposed to kBTΓ) in the denominator, which is only valid for ideal mixtures. Indeed the ARG/P model does not explicitly consider a concentration gradient along the reaction coordinate; doing so would result in a similar “non-ideality” term in the denominator.37
Closing remarks
We close this communication with a discussion of our results in the context of recent literature. LJ mixtures are representative of mixtures of approximately spherical non-polar molecules, in which intermolecular interactions are dominated by van der Waals forces. Comparing to mixtures of non-polar organic solvents, at 25 °C cyclohexane/cis-decalin possesses a ST maximum at x1 ≈ 0.2 but Γ ≈ 1.0 for the entire 0 ≤ x1 ≤ 1 range (there is a very shallow Γ minimum at x1 ≈ 0.8), indicative of a different origin compared to the LJ mixture. Also in contrast with the LJ mixture, cyclohexane/benzene features a monotonic increase of ST with x1 at 25 °C, but does have a strong minimum in Γ at x1 ≈ 0.5.21S T minima have been observed in mixtures of polar organic solvents with water. Ethanol/water and acetone/water mixtures have minima at x1 ∼ 0.6 and x1 ∼ 0.5, respectively, roughly coincident with minima in D12 and Γ for both mixtures.5,16–19,38,39 Comparing to aqueous solutions, while the ST minimum examined in this work originates from a coincident minimum in Γ, for LiCl(aq), Γ increases monotonically in the concentration range of the ST minimum.13 Furthermore, simulations have highlighted the importance of ion solvation structure on the existence of the minimum in LiCl(aq).40 In contrast, local structural changes in the LJ mixture are minor (see ESI,† Section S1.4). We note that many simple salts are thought to have a D12 minimum at low concentrations (∼10−1 mol dm−3) often attributed to ion-pair formation and solute–solvent association.14,41–43 Recent experiments observed ST minima in NaSCN(aq), KSCN(aq), K2CO3(aq) and CH3COOK(aq) that are carried through DT.14,15 Hence, the physical origin of the minima in aqueous solutions and mixtures might be quite different to the one reported here for non-polar liquids, since the LJ mixtures do not feature extrema in D12 or DT.
It is evident that the physical origins of ST minima/maxima must be considered on a case-by-case basis for different mixtures, even those belonging to the same class (aqueous solution, non-polar organic solvent mixtures, etc.). We provide here a proof of principle and demonstrate that ST minima can exist in even the simplest of mixtures, as exemplified by a binary LJ mixture in which the components differ by only the interaction parameter ε. The concentration dependence of the ST is, at least for simple mixtures, typically attributed to the cross-interactions between unlike particles20 – a notion sustained in recent reviews.7,44,45 For dense supercritical LJ mixtures with cross-interactions given by 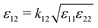 it was found that ST(x1) ≈ bx1 + c, with the slope b controlled by k12.20 Greater |1 − k12| values resulted in greater |b| values. In this work, we identify a mixture with k12 = 1 that nevertheless features a strong composition dependence and more complex phenomenology (the ST minimum). Extrapolating our results, we expect that the observation of Soret coefficient minima/maxima in LJ mixtures is strongly correlated with their degree of non-ideality; sufficiently non-ideal mixtures might be more difficult to achieve at e.g. supercritical conditions. Clearly, further work is required to explain the composition dependence of ST in liquid mixtures and different thermodynamic conditions.
it was found that ST(x1) ≈ bx1 + c, with the slope b controlled by k12.20 Greater |1 − k12| values resulted in greater |b| values. In this work, we identify a mixture with k12 = 1 that nevertheless features a strong composition dependence and more complex phenomenology (the ST minimum). Extrapolating our results, we expect that the observation of Soret coefficient minima/maxima in LJ mixtures is strongly correlated with their degree of non-ideality; sufficiently non-ideal mixtures might be more difficult to achieve at e.g. supercritical conditions. Clearly, further work is required to explain the composition dependence of ST in liquid mixtures and different thermodynamic conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Leverhulme Trust for Grant No. RPG-2018-384. We gratefully acknowledge a PhD studentship (Project Reference 2135626) for O. R. G. sponsored by ICL's Chemistry Doctoral Scholarship Award, funded by the EPSRC Doctoral Training Partnership Account (EP/N509486/1). We acknowledge the ICL RCS High Performance Computing facility and the UK Materials and Molecular Modelling Hub for computational resources, partially funded by the EPSRC (Grant No. EP/P020194/1 and EP/T022213/1).Notes and references
- S. Duhr and D. Braun, Phys. Rev. Lett., 2006, 96, 168301 CrossRef PubMed.
- S. Duhr and D. Braun, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 19678–19682 CrossRef CAS PubMed.
- S. N. Rasuli and R. Golestanian, Phys. Rev. Lett., 2008, 101, 108301 CrossRef PubMed.
- A. Würger, Phys. Rev. Lett., 2008, 101, 108302 CrossRef PubMed.
- S. Wiegand, J. Phys.: Condens. Matter, 2004, 16, R357–R379 CrossRef CAS.
- R. Piazza, Soft Matter, 2008, 4, 1740–1744 RSC.
- W. Köhler and K. I. Morozov, J. Non-Equilib. Thermodyn., 2016, 41, 151–197 Search PubMed.
- C. J. Wienken, P. Baaske, U. Rothbauer, D. Braun and S. Duhr, Nat. Commun., 2010, 1, 100 CrossRef PubMed.
- K. Alexander, Z. Phys. Chem., 1954, 203, 213–227 CrossRef.
- F. S. Gaeta, G. Perna, G. Scala and F. Bellucci, J. Phys. Chem., 1982, 86, 2967–2974 CrossRef CAS.
- F. Römer, Z. Wang, S. Wiegand and F. Bresme, J. Phys. Chem. B, 2013, 117, 8209–8222 CrossRef PubMed.
- J. Colombani, J. Bert and J. Dupuy-Philon, J. Chem. Phys., 1999, 110, 8622–8627 CrossRef CAS.
- S. Di Lecce, T. Albrecht and F. Bresme, Sci. Rep., 2017, 7, 44833 CrossRef CAS PubMed.
- S. Mohanakumar, J. Luettmer-Strathmann and S. Wiegand, J. Chem. Phys., 2021, 154, 084506 CrossRef CAS PubMed.
- S. Mohanakumar and S. Wiegand, Eur. Phys. J. E: Soft Matter Biol. Phys., 2022, 45, 10 CrossRef CAS PubMed.
- A. Königer, B. Meier and W. Köhler, Philos. Mag., 2009, 89, 907–923 CrossRef.
- L. Zhang, Q. Wang, Y.-C. Liu and L.-Z. Zhang, J. Chem. Phys., 2006, 125, 104502 CrossRef PubMed.
- H. Ning and S. Wiegand, J. Chem. Phys., 2006, 125, 221102 CrossRef PubMed.
- H. Cabrera, L. Martí-López, E. Sira, K. Rahn and M. García-Sucre, J. Chem. Phys., 2009, 131, 031106 CrossRef PubMed.
- P.-A. Artola and B. Rousseau, Phys. Rev. Lett., 2007, 98, 125901 CrossRef PubMed.
- S. Hartmann, G. Wittko, F. Schock, W. Groß, F. Lindner, W. Köhler and K. I. Morozov, J. Chem. Phys., 2014, 141, 134503 CrossRef CAS PubMed.
- J. N. Agar, C. Y. Mou and J. L. Lin, J. Phys. Chem., 1989, 93, 2079–2082 CrossRef CAS.
- M. Thol, G. Rutkai, R. Span, J. Vrabec and R. Lustig, Int. J. Thermophys., 2015, 36, 25–43 CrossRef CAS.
- S. R. de Groot and P. Mazur, Non-Equilibrium Thermodynamics, Dover, 1984 Search PubMed.
- P. Krüger, S. K. Schnell, D. Bedeaux, S. Kjelstrup, T. J. H. Vlugt and J.-M. Simon, J. Phys. Chem. Lett., 2013, 4, 235–238 CrossRef PubMed.
- A. P. Thompson, H. M. Aktulga, R. Berger, D. S. Bolintineanu, W. M. Brown, P. S. Crozier, P. J. in’t Veld, A. Kohlmeyer, S. G. Moore, T. D. Nguyen, R. Shan, M. J. Stevens, J. Tranchida, C. Trott and S. J. Plimpton, Comput. Phys. Commun., 2022, 271, 108171 CrossRef CAS.
- M. Eslamian and M. Z. Saghir, J. Non-Equilib. Thermodyn., 2009, 34, 97–131 CrossRef CAS.
- L. J. T. M. Kempers, J. Chem. Phys., 1989, 90, 6541–6548 CrossRef CAS.
- K. Shukla and A. Firoozabadi, Ind. Eng. Chem. Res., 1998, 37, 3331–3342 CrossRef CAS.
- L. J. T. M. Kempers, J. Chem. Phys., 2001, 115, 6330–6341 CrossRef CAS.
- M. G. Gonzalez-Bagnoli, A. A. Shapiro and E. H. Stenby, Philos. Mag., 2003, 83, 2171–2183 CrossRef CAS.
- P.-A. Artola, B. Rousseau and G. Galliéro, J. Am. Chem. Soc., 2008, 130, 10963–10969 CrossRef CAS PubMed.
- H. Hoang and G. Galliero, Eur. Phys. J. E: Soft Matter Biol. Phys., 2022, 45, 42 CrossRef CAS PubMed.
- R. Haase, Z. Phys., 1949, 127, 1–10 CAS.
- I. Prigogine, L. De Brouckere and R. Amand, Physica, 1950, 16, 577–598 CrossRef CAS.
- I. Prigogine, L. De Brouckere and M. R. Amand, Physica, 1950, 16, 851–860 CrossRef CAS.
- W. M. Rutherford and H. G. Drickamer, J. Chem. Phys., 1954, 22, 1157–1165 CrossRef CAS.
- M. T. Tyn and W. F. Calus, J. Chem. Eng. Data, 1975, 20, 310–316 CrossRef CAS.
- R. Taylor and H. A. Kooijman, Chem. Eng. Commun., 1991, 102, 87–106 CrossRef CAS.
- S. Di Lecce, T. Albrecht and F. Bresme, Phys. Chem. Chem. Phys., 2017, 19, 9575–9583 RSC.
- G.-H. Gao, H.-B. Shi and Y.-X. Yu, Fluid Phase Equilib., 2007, 256, 105–111 CrossRef CAS.
- J. P. Mitchell, J. B. Butler and J. G. Albright, J. Solution Chem., 1992, 21, 1115–1129 CrossRef CAS.
- A. Katz and S. Ben-Yaakov, Mar. Chem., 1980, 8, 263–280 CrossRef CAS.
- P.-A. Artola and B. Rousseau, Mol. Phys., 2013, 111, 3394–3403 CrossRef CAS.
- K. Harstad, Ind. Eng. Chem. Res., 2009, 48, 6907–6915 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cp04256h |
| This journal is © the Owner Societies 2023 |