 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comment on “Theoretical study of the NO3 radical reaction with CH2ClBr, CH2ICl, CH2BrI, CHCl2Br, and CHClBr2” by I. Alkorta, J. M. C. Plane, J. Elguero, J. Z. Dávalos, A. U. Acuña and A. Saiz-Lopez, Phys. Chem. Chem. Phys. 2022, 24, 14365
Claus Jørgen
Nielsen
 *a and
Yizhen
Tang
*a and
Yizhen
Tang
 b
b
aSection for Environmental Sciences, Department of Chemistry, University of Oslo, P.O. Box. 1033 Blindern, NO-0315 Oslo, Norway. E-mail: c.j.nielsen@kjemi.uio.no
bSchool of Environmental and Municipal Engineering, Qingdao University of Technology, Fushun Road 11, Qingdao, 266033, China
First published on 19th January 2023
Abstract
This comment addresses a systematic error in the potential energy surfaces of the title reactions presented in the original article by Alkorta et al. The NO3 radical has D3h symmetry in the electronic ground state while the M08HX functional employed in the original article predicts an incorrect C2v geometry and energy. By combining thermodynamic data for the OH + HNO3 → H2O + NO3 reaction with spectroscopic data and results from M08HX calculations on HNO3, H2O and the OH radical, the ground state NO3 radical energy is estimated to be 37 kJ mol−1 lower than reported for the C2v geometry.
The authors of the title paper1 presented theoretical results based on Kohn-Sham density functional theory2 calculations employing the M08HX functional3 and single point couple cluster calculations.
The NO3 radical presents a computational challenge. The electronic ground state has D3h symmetry  ;4,5 the experimental NO distance is 1.238 Å (r0-structure),6 and the fundamental modes of vibration (in cm−1) are
;4,5 the experimental NO distance is 1.238 Å (r0-structure),6 and the fundamental modes of vibration (in cm−1) are 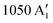 ,
,  , 1492.4 E′ and 360 E′.7
, 1492.4 E′ and 360 E′.7
It is not possible to calculate the electronic structure of the NO3 radical correctly using any standard size extensive UHF wave function based method that is also applicable to larger systems.8 HF calculations locate 3 distinct minimum energy structures: one of D3h symmetry, and two of C2v symmetry having lower energies and respectively 2 short and 1 long NO distance (2s,l), and 1 short and 2 long NO distances (2l,s).8 In contrast, MP2 calculations place the D3h structure lower in energy than the two C2v structures; even CCSD(T) cannot completely overcome the symmetry breaking of the reference function and still three solutions with slightly different energies are found.8
There is a plethora of functionals developed for use in Kohn–Sham density functional theory calculations. Most of the commonly used “pure” functionals locate a single minimum energy structure of D3h symmetry. Many hybrid functionals also predict the D3h symmetry structure as the global energy minimum, but there are also many showing symmetry breaking – the M08HX hybrid meta-GGA exchange–correlation functional being among those locating the D3h-structure as a saddle point.
An early study on the performance of DFT for symmetry breaking problems concluded that the exchange functional appears to be more important than the correlation functional in providing resistance to symmetry breaking, and that hybrid functionals mixing in large fractions of Hartree–Fock exchange exhibit symmetry breaking.9
The NO3 radical structures and the vibrational frequencies, obtained in M08HX/6-311+G(2df,2p) calculations, deviate substantially from the experimental data. The title study does not report the NO3 radical structure explicitly. However, the vibrational frequencies (in cm−1) reported in Table S10 – 1705 A1, 1162 A1, 544 A1, 770 B2 and 520 B2 – indicate that the C2l,s2v structure, having NO-distances of 1.2471 and 1.1805 Å, is selected.
While the calculated electronic energy differences between the two local C2v minimum energy structures and the D3h saddle point structure are <10 kJ mol−1 at the M08HX/6-311+G(2df,2p) level (C2s,l2v: −280.17982, C2l,s2v: −280.18006 and D3h: −280.17641 Hartree), the error in the calculated energy of the NO3 radical may well be quite different from the above variance.
Assuming that the quantum chemistry method harmonises with the thermochemistry data for HNO3, H2O and OH, one may estimate the error in the calculated ground state NO3 radical energy by combining the theoretical method results for the OH + HNO3 → H2O + NO3 reaction, the standard enthalpies of formation from the NIST-JANAF Thermochemical Tables for OH (38.99 ± 1.21 kJ mol−1), H2O (−241.826 ± 0.042 kJ mol−1) and HNO3 (−134.31 ± 0.42 kJ mol−1),10 the newest NO3 photodissociation results (73.72 ± 1.38 kJ mol−1),11 and the experimental fundamental modes of vibration for NO3.7
The abovementioned experimental enthalpies of formation give ΔrH298 = −72.79 ± 1.88 kJ mol−1 for the OH + HNO3 → H2O + NO3 reaction, which, combined with the M08HX/6-311+G(2df,2p) electronic energies and calculated thermal corrections to the Enthalpies (HNO3: −280.836573, 0.03187; H2O: −76.42803, 0.02551; OH: −75.72716, 0.01197) results in a ground state electronic energy of the NO3 radical of −280.19415 ± 0.00072 Hartree at the UM08HX/6-311+G(2df,2p) level of theory, which is 37 kJ mol−1 lower than the incorrect C2l,s2v energy used in the title study.
The electronic energy profiles presented in Fig. 5 in the title study are obviously not correct. The NO3 radical H-abstraction reaction occurs on a path starting with a ground state NO3 radical having D3h-symmetry, proceeding via a saddle point in which the NO3 radical distorts towards a (2s,l)-like structure, and terminating with HNO3. The saddle point region of the potential energy surface and the product region can presumably be characterized reasonably well in M08HX calculations. The path connecting the electronic ground state of the NO3 radical and the C2v-like distorted pre-reaction NO3 radical cannot. The pre-reaction complexes presented in the title study are, at least in part, artefacts of the methodology employed. Further, the barriers to the reactions are most likely not around 13 kJ mol−1 but rather around 50 kJ mol−1 above the entrance energy of the reactants. This does not change the main conclusion of the title study: the atmospheric chemical lifetimes of the alkyl halides investigated are not substantially affected by nitrate radical reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge support from the Research Council of Norway through its Centre of Excellence scheme, project number 262695, and from the VISTA-programme, project 6157.References
- I. Alkorta, J. M. C. Plane, J. Elguero, J. Z. Dávalos, A. U. Acuña and A. Saiz-Lopez, Phys. Chem. Chem. Phys., 2022, 24, 14365–14374 RSC
.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133–A1138 CrossRef
.
- Y. Zhao and D. G. Truhlar, J. Chem. Theory Comput., 2008, 4, 1849–1868 CrossRef CAS PubMed
.
- T. Ishiwata, I. Tanaka, K. Kawaguchi and E. Hirota, J. Chem. Phys., 1985, 82, 2196–2205 CrossRef CAS
.
- K. Kawaguchi, E. Hirota, T. Ishiwata and I. Tanaka, J. Chem. Phys., 1990, 93, 951–956 CrossRef CAS
.
- K. Kawaguchi, T. Ishiwata, E. Hirota and I. Tanaka, Chem. Phys., 1998, 231, 193–198 CrossRef CAS
.
- M. E. Jacox, J. Phys. Chem. Ref. Data, 1994, 1–461 CAS
.
- W. Eisfeld and K. Morokuma, J. Chem. Phys., 2000, 113, 5587–5597 CrossRef CAS
.
- C. D. Sherrill, M. S. Lee and M. Head-Gordon, Chem. Phys. Lett., 1999, 302, 425–430 CrossRef CAS
.
-
M. W. Chase, NIST-JANAF thermochemical tables, National Institute of Standards and Technology, Washington, D.C., Woodbury, N.Y., 1998 Search PubMed
.
- H. F. Davis, B. Kim, H. S. Johnston and Y. T. Lee, J. Phys. Chem., 1993, 97, 2172–2180 CrossRef CAS
.
| This journal is © the Owner Societies 2023 |
