Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
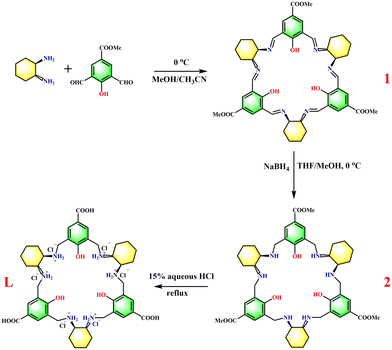
Synthesis and characterization of two metallo-hydrogen-bonded organic frameworks with diverse structures and properties†
Mi
Zhou
ab,
Yujiang
Wang
a,
Guoyuan
Yuan
a,
Zhanfeng
Ju
 b and
Daqiang
Yuan
b and
Daqiang
Yuan
 *b
*b
aCollege of Chemistry and Chemical Engineering, Chongqing University of Science and Technology, Chongqing 401331, China
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: ydq@fjirsm.ac.cn
First published on 23rd October 2023
Abstract
Two metallo-hydrogen-bonded organic frameworks (MHOFs) were synthesized by self-assembling a phosphate complex and a macrocyclic ligand with three peripheral carboxylic acids. The two MHOFs have different hydrogen-bond linkages and structures, depending on whether the phosphate participates in the hydrogen bonding. The various hydrogen-bonded networks result in different thermal stabilities, BET surface areas, and water vapor uptake capacities of the MHOFs. MHOF-PO4-2, which has more hydrogen bonds and higher porosity, shows better thermal stability and water vapor adsorption than MHOF-PO4-1. This study demonstrates the possibility of constructing diverse and functional MHOFs based on phosphate complexes.
Hydrogen-bonded organic frameworks (HOFs), an emerging type of crystalline porous material, have demonstrated promising applications in various fields such as catalysis,1–3 sensing,4–8 proton conductivity,9,10 and adsorption.11–17 The construction of HOFs relies on using functional groups with hydrogen-bonded acceptor and donor properties to create molecular building blocks. However, incorporating multiple different functional groups into a single molecule is challenging and limits the functional and structural diversity of HOFs.18–30 To overcome this limitation, a subclass of HOFs called metallo-hydrogen-bonded organic frameworks (MHOFs) has been developed.31–35 MHOFs are constructed from complexes that offer abundant geometric configurations, functional groups, and the possibility of incorporating open metal sites.36,37
The tetrahedral anion PO43− possesses four oxygen atoms on its vertices, thus providing each oxygen atom with the potential to act as a hydrogen bond acceptor. This anion has been utilized in forming supramolecular self-assemblies through hydrogen bonding owing to its highly symmetrical geometry.38,39 However, to the best of our knowledge, there have been no reports on HOFs based on PO43−. This is likely due to the high charge of PO43− and the absence of suitable hydrogen-bond donors.40,41 Therefore, it is crucial to introduce additional components to overcome these limitations. Notably, pioneering researchers have reported the synthesis of a macrocycle that contains a coordinated pocket capable of securely encapsulating three metal ions, particularly Cu(II) and Zn(II), due to macrocyclic and chelating effects.42–45
Additionally, charge-balanced anions are present around the resulting complex.46,47 Inspired by these studies, we synthesized a macrocyclic ligand (L) functionalized with three peripheral carboxylic acids using the synthetic pathway depicted in Scheme 1. In this work, we report MHOFs based on the complexation of the macrocycle, Cu(II) and phosphate. These MHOFs exhibit distinct hydrogen-bond linkages and structures, resulting in significant differences in their BET surface areas, thermal stabilities, and water vapor capacities.
The self-assembly of L, Cu(NO3)2, and K3PO4 in H2O results in the formation of MHOF-PO4-1, which is a dark green crystal with a chemical formula LCu3PO4·6H2O. Analysis using single-crystal X-ray diffraction reveals that MHOF-PO4-1 crystallizes in the space group F4132, with one-third of the formula unit present in the asymmetric unit (Fig. S1a†). Within this complex, three Cu(II) ions adopt a pseudo-square pyramid geometry and are enclosed within the central coordination pocket of L. The binding is facilitated by three deprotonated phenol hydroxyls and six secondary amines. Additionally, the three Cu(II) ions are associated with three O atoms from one PO43− anion, forming a neutral complex. It is important to note that the PO43− ion is located on one side of the complex, while the other remains unoccupied. As a result, the hydrogen atoms of the six secondary amines can be classified into cis-groups and trans-groups based on their position relative to the PO43− ion (Fig. 1a and b).
 | ||
| Fig. 1 (a) The structure of the complex; (b) the two kinds of N–H groups in the complex. Purple, sky blue, green, gray, blue, and red spheres represent the P, Cu, H, C, N, and O atoms, respectively. | ||
The bonding between the complex and water molecules leads to the formation of the framework structure of MHOF-PO4-1. Each complex is surrounded by six bridging water molecules and three terminal water molecules within this framework. Three of the bridging water molecules form three O–H⋯O hydrogen bonds with three peripheral carboxylic acids, with an average bond length of 1.847 Å and a bond angle of 162.75°. The other three bridging water molecules form three N–H⋯O hydrogen bonds with three trans-N–H groups, with a bond length of 2.455 Å and a bond angle of 144.94°. Additionally, the three terminal water molecules form three N–H⋯O hydrogen bonds with three cis-N–H groups, with a bond length of 2.252 Å and a bond angle of 160.98° (Fig. 2a). It is important to note that each bridging water molecule simultaneously connects to a carboxylic acid and a trans-N–H group from two complexes. In other words, each complex extends to three other complexes through six bridging water molecules, forming a 3-connected hydrogen-bonded network with an SRS topology (Fig. 2b and S1b†).
Furthermore, four of these networks interweave through weak intermolecular interactions to create a 4-fold interpenetrated framework (Fig. 2c and d). Within the MHOF-PO4-1 framework, a pseudo-cuboctahedral cavity is observed, with four complexes occupying four triangular faces (Fig. S1c and d†). MHOF-PO4-1 is nonporous when analyzed with Zeo++ software using a probe radius of 1.86 Å.48 However, after removing all the water molecules from the framework, a porosity of 19.5% is observed.
The analysis indicates that the cis-N–H groups, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of carboxylic acids, and O atoms of phosphate in MHOF-PO4-1 have minimal contribution to the hydrogen-bond framework. If all potential hydrogen-bond sites are utilized in the framework construction, an MHOF with a stronger hydrogen-bond linkage could be produced. As a result, MHOF-PO4-2, which crystallizes in the P63 space group, was obtained using MHOF-PO4-1 as the starting material. MHOF-PO4-2 has the formula LCu3PO4·3H2O, and the asymmetric unit consists of one-third of the formula unit (Fig. S2a†). Structural analysis reveals that the peripheral carboxylic acids of each complex extend outward to three adjacent complexes by forming six hydrogen bonds with three cis-N–H groups and three coordinated O atoms of the capped PO43−. The distances for O–H⋯O and N–H⋯O bonds are approximately 1.669 Å and 2.247 Å, respectively, with bond angles of about 171.68° and 168.53°. Additionally, the three cis-N–H groups and three coordinated phosphate O atoms of each complex form six hydrogen bonds with three carboxylic acids from another three complexes simultaneously (Fig. 3a and S2b†). Moreover, edge to face π⋯π interaction is observed between adjacent complexes (Fig. 3b). Furthermore, the capped PO43− forms six hydrogen bonds with three water molecules, with O–H⋯O distances of approximately 1.883 Å and 2.619 Å and bond angles of about 172.17° and 129.06°. The three hydrogen atoms of the trans-N–H groups form three hydrogen bonds with three water molecules, with an average N–H⋯O distance of 2.282 Å and a bond angle of 138.46°. Consequently, each ligand is connected to two other ligands along the c direction through six bridging water molecules, forming a hydrogen-bonded pillar along the c direction (Fig. 3c). When calculated using a probe radius of 1.86 Å, MHOF-PO4-2 exhibits 17.5% porosity and a one-dimensional channel along the c direction, with an average pore diameter of 4.96 Å (Fig. 3d and e and S3d†).‡
O groups of carboxylic acids, and O atoms of phosphate in MHOF-PO4-1 have minimal contribution to the hydrogen-bond framework. If all potential hydrogen-bond sites are utilized in the framework construction, an MHOF with a stronger hydrogen-bond linkage could be produced. As a result, MHOF-PO4-2, which crystallizes in the P63 space group, was obtained using MHOF-PO4-1 as the starting material. MHOF-PO4-2 has the formula LCu3PO4·3H2O, and the asymmetric unit consists of one-third of the formula unit (Fig. S2a†). Structural analysis reveals that the peripheral carboxylic acids of each complex extend outward to three adjacent complexes by forming six hydrogen bonds with three cis-N–H groups and three coordinated O atoms of the capped PO43−. The distances for O–H⋯O and N–H⋯O bonds are approximately 1.669 Å and 2.247 Å, respectively, with bond angles of about 171.68° and 168.53°. Additionally, the three cis-N–H groups and three coordinated phosphate O atoms of each complex form six hydrogen bonds with three carboxylic acids from another three complexes simultaneously (Fig. 3a and S2b†). Moreover, edge to face π⋯π interaction is observed between adjacent complexes (Fig. 3b). Furthermore, the capped PO43− forms six hydrogen bonds with three water molecules, with O–H⋯O distances of approximately 1.883 Å and 2.619 Å and bond angles of about 172.17° and 129.06°. The three hydrogen atoms of the trans-N–H groups form three hydrogen bonds with three water molecules, with an average N–H⋯O distance of 2.282 Å and a bond angle of 138.46°. Consequently, each ligand is connected to two other ligands along the c direction through six bridging water molecules, forming a hydrogen-bonded pillar along the c direction (Fig. 3c). When calculated using a probe radius of 1.86 Å, MHOF-PO4-2 exhibits 17.5% porosity and a one-dimensional channel along the c direction, with an average pore diameter of 4.96 Å (Fig. 3d and e and S3d†).‡
Based on the analysis above, it is evident that there is a significant variation in the number of hydrogen bonds between MHOF-PO4-1 and MHOF-PO4-2. MHOF-PO4-2 exhibits more hydrogen bonds, specifically twenty-one, whereas MHOF-PO4-1 only possesses nine hydrogen bonds (including three terminal bonds). This suggests that MHOF-PO4-2 maintains a more robust network of hydrogen bonds than MHOF-PO4-1. Furthermore, MHOF-PO4-2 exhibits a higher degree of porosity than MHOF-PO4-1, with a calculated probe radius of 1.86 Å, resulting in a porosity of 17.5% for MHOF-PO4-2. In contrast, MHOF-PO4-1 exhibits no porosity at this probe radius.
To analyze the porosity of MHOF-PO4-1 and MHOF-PO4-2, N2 (77 K) sorption analysis was conducted. Unexpectedly, MHOF-PO4-1 exhibited a type-I adsorption isotherm with an uptake amount of approximately 85 cm3 g−1 (Fig. 4a), which contradicts the findings of the structural analysis. Additionally, the crystallinity of MHOF-PO4-1 remained intact throughout the sorption analysis (Fig. 4b). These abnormal results may be attributed to the loss of select water molecules during activation, with the framework supported by residual hydrogen bonds. On the other hand, MHOF-PO4-2 exhibited a standard isotherm expected of a microporous material with an uptake amount of around 140 cm3 g−1. The BET surface area value of MHOF-PO4-2, calculated from the experimental N2 adsorption data, was approximately 520 m2 g−1.
 | ||
| Fig. 4 (a) N2 (77K) uptake of MHOF-PO4-1 (red) and MHOF-PO4-2 (blue); PXRD patterns of (b) MHOF-PO4-1 and (c) MHOF-PO4-2; (d) variable temperature PXRD patterns of MHOF-PO4-2. | ||
Furthermore, MHOF-PO4-2 displayed good crystallinity even after adsorption (Fig. 4c). These findings indicate that MHOF-PO4-2 possesses a higher BET surface area and N2 uptake capacity than MHOF-PO4-1. However, the two materials exhibited different behaviors when subjected to heat. Specifically, the framework of MHOF-PO4-1 collapsed when heated to 80 °C (Fig. 4b), whereas MHOF-PO4-2 maintained its crystallinity up to 120 °C based on variable temperature PXRD analysis (Fig. 4d). Upon heating to higher temperatures, new peaks were observed between 9 and 15°, indicating the formation of a new phase within the bulk sample. Finally, when heated to 200 °C, the sample completely lost its crystallinity, likely due to the breakage of the H-bond linkage. These results are consistent with the findings of the TGA analysis (Fig. S5b†).
Water adsorption is an increasingly popular application for porous materials.49,50 However, research on this topic is scarce regarding HOFs. Due to the excellent stability of MHOF-PO4-1 and MHOF-PO4-2 in water (Fig. 4b and c), water-vapor adsorption experiments were conducted at a temperature of 298 K. The results indicate that MHOF-PO4-1 only has a water vapor adsorption capacity of 0.003 g g−1 (0.176 mmol g−1) (Fig. 5a and S6a†), which can be attributed to its limited pore size and porosity. On the other hand, MHOF-PO4-2 exhibits a type-IV adsorption isotherm and a total water adsorption amount of 0.254 g g−1 (14.1 mmol g−1) (Fig. 5a). The rapid uptake observed at low relative pressures (0 to 0.98 mbar) can be attributed to monolayer adsorption in the channel of MHOF-PO4-2. Moreover, the isotherm shows a significant increase in water uptake from 12.92 to 16.94 mbar, suggesting pore-filling progression. Although a hysteresis cycle is observed in the isotherm, the water adsorption capacity of MHOF-PO4-2 remains reversible. Additionally, the uptake capacity remains almost unchanged after four adsorption and desorption cycles (Fig. 5b and S6†).
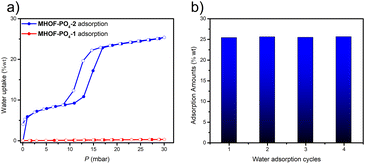 | ||
| Fig. 5 (a) Water vapor uptake (298 K) of MHOF-PO4-1 (red) and MHOF-PO4-2 (blue); (b) water adsorption–desorption (298 K) cycling of MHOF-PO4-2. | ||
Conclusions
We have successfully synthesized two MHOFs based on a phosphate complex and a macrocyclic ligand. The two MHOFs show different hydrogen-bond linkages and structures, which affect their thermal stabilities, BET surface areas, and water vapor uptake capacities. MHOF-PO4-2, which has more hydrogen bonds and higher porosity, performs better than MHOF-PO4-1. This study demonstrates the possibility of constructing diverse and functional MHOFs based on phosphate complexes. It may provide a new strategy for designing HOFs with tunable properties.Author contributions
M. Z. was responsible for the design, synthesis, and characterization of the compounds. Additionally, he analyzed the crystal structure and collaborated in writing the paper. Y. W. conducted the water vapor uptake experiments and contributed to the article's writing. G. Y. analyzed the data collected from the water-vapor uptake experiments. Z. J. designed the synthetic route for the ligand. D. Y. planned and supervised the research and collaborated in writing the paper. All authors participated in discussions regarding the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Chongqing (CSTB2022BSXM-JCX0138), the Research Foundation of Chongqing University of Science and Technology (ckrc2021028), and the Chongqing Postdoctoral Science Foundation (2010010005168734).Notes and references
- Q. Yin, E. V. Alexandrov, D. H. Si, Q. Q. Huang, Z. B. Fang, Y. Zhang, A. A. Zhang, W. K. Qin, Y. L. Li, T. F. Liu and D. M. Proserpio, Angew. Chem., Int. Ed., 2022, 61, e202115854 CrossRef CAS PubMed
.
- K. Ma, P. Li, J. H. Xin, Y. Chen, Z. Chen, S. Goswami, X. Liu, S. Kato, H. Chen, X. Zhang, J. Bai, M. C. Wasson, R. R. Maldonado, R. Q. Snurr and O. K. Farha, Cell Rep. Phys. Sci., 2020, 1, 100024 CrossRef
.
- R. Azizi, M. Shamsipur, A. Taherpour and A. Pashabadi, J. Mater. Chem. A, 2023, 11, 1491–1502 RSC
.
- Q. Huang, W. Li, Z. Mao, H. Zhang, Y. Li, D. Ma, H. Wu, J. Zhao, Z. Yang, Y. Zhang, L. Gong, M. P. Aldred and Z. Chi, Chem, 2021, 7, 1321–1332 CAS
.
- B. Wang, R. He, L.-H. Xie, Z.-J. Lin, X. Zhang, J. Wang, H. Huang, Z. Zhang, K. S. Schanze, J. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2020, 142, 12478–12485 CrossRef CAS PubMed
.
- W. Jiang, H. Zheng, Y. Wu, P. Wu, L. Jing, X. Yuan, X. Wang and J. Wang, Inorg. Chem. Front., 2023, 10, 5430–5438 RSC
.
- C. Yang, X. Xu and B. Yan, Inorg. Chem. Front., 2023, 10, 2951–2960 RSC
.
- Q. Huang, W. Li, Z. Yang, J. Zhao, Y. Li, Z. Mao, Z. Yang, S. Liu, Y. Zhang and Z. Chi, CCS Chem., 2022, 4, 1643–1653 CrossRef CAS
.
- E. A. Zhigileva, Y. Y. Enakieva, A. A. Sinelshchikova, V. V. Chernyshev, I. N. Senchikhin, K. A. Kovalenko, I. A. Stenina, A. B. Yaroslavtsev, Y. G. Gorbunova and A. Y. Tsivadze, Dalton Trans., 2023, 52, 8237–8246 RSC
.
- Q. Yang, X. Li, C. Xie, Z. Wang, Z. Kong, J. Yang, Z. Kang, R. Wang and D. Sun, J. Mater. Chem. C, 2023, 11, 12206–12212 RSC
.
- F. Hu, C. Liu, M. Wu, J. Pang, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2017, 56, 2101–2104 CrossRef CAS PubMed
.
- B. Yu, S. Geng, H. Wang, W. Zhou, Z. Zhang, B. Chen and J. Jiang, Angew. Chem., Int. Ed., 2021, 60, 25942–25948 CrossRef CAS PubMed
.
- B. Wang, R.-B. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2020, 142, 14399–14416 CrossRef CAS PubMed
.
- B. Wang, X.-L. Lv, J. Lv, L. Ma, R.-B. Lin, H. Cui, J. Zhang, Z. Zhang, S. Xiang and B. Chen, Chem. Commun., 2020, 56, 66–69 RSC
.
- D.-D. Zhou, Y.-T. Xu, R.-B. Lin, Z.-W. Mo, W.-X. Zhang and J.-P. Zhang, Chem. Commun., 2016, 52, 4991–4994 RSC
.
- Y. Zhou, L. Kan, J. F. Eubank, G. Li, L. Zhang and Y. Liu, Chem. Sci., 2019, 10, 6565–6571 RSC
.
- Y. Liu, J. Dai, L. Guo, Z. Zhang, Y. Yang, Q. Yang, Q. Ren and Z. Bao, CCS Chem., 2022, 4, 381–388 CrossRef CAS
.
- C.-F. Ng, H.-F. Chow and T. C. W. Mak, Cryst. Growth Des., 2022, 22, 3365–3375 CrossRef CAS
.
- F. Haase, G. A. Craig, M. Bonneau, K. Sugimoto and S. Furukawa, J. Am. Chem. Soc., 2020, 142, 13839–13845 CrossRef CAS PubMed
.
- Y. Zhao, C. Fan, C. Pei, X. Geng, G. Xing, T. Ben and S. Qiu, J. Am. Chem. Soc., 2020, 142, 3593–3599 CrossRef CAS PubMed
.
- T. Khadivjam, H. Che-Quang, T. Maris, Z. Ajoyan, A. J. Howarth and J. D. Wuest, Chem. – Eur. J., 2020, 26, 7026–7040 CrossRef CAS PubMed
.
- Y. Li, M. Handke, Y.-S. Chen, A. G. Shtukenberg, C. T. Hu and M. D. Ward, J. Am. Chem. Soc., 2018, 140, 12915–12921 CrossRef CAS PubMed
.
- T. Hashimoto, R. Oketani, A. Inoue, K. Okubo, K. Oka, N. Tohnai, K. Kamiya, S. Nakanishi and I. Hisaki, Chem. Commun., 2023, 59, 7224–7227 RSC
.
- J.-Q. Zhao, L.-L. Mao, G.-H. Zhang, S.-Z. Zhan, H. Xiao, S. Zhang, C.-H. Tung, L.-Z. Wu and H. Cong, J. Mater. Chem. A, 2023, 11, 4672–4678 RSC
.
- T. H. Chen, I. Popov, W. Kaveevivitchai, Y. C. Chuang, Y. S. Chen, O. Daugulis, A. J. Jacobson and O. S. Miljanic, Nat. Commun., 2014, 5, 5131 CrossRef CAS PubMed
.
- A. Pulido, L. Chen, T. Kaczorowski, D. Holden, M. A. Little, S. Y. Chong, B. J. Slater, D. P. McMahon, B. Bonillo, C. J. Stackhouse, A. Stephenson, C. M. Kane, R. Clowes, T. Hasell, A. I. Cooper and G. M. Day, Nature, 2017, 543, 657–664 CrossRef CAS PubMed
.
- G. Xing, I. Bassanetti, S. Bracco, M. Negroni, C. Bezuidenhout, T. Ben, P. Sozzani and A. Comotti, Chem. Sci., 2019, 10, 730–736 RSC
.
- J. Lü, C. Perez-Krap, F. Trousselet, Y. Yan, N. H. Alsmail, B. Karadeniz, N. M. Jacques, W. Lewis, A. J. Blake, F.-X. Coudert, R. Cao and M. Schröder, Cryst. Growth Des., 2018, 18, 2555–2562 CrossRef PubMed
.
- J. Sun, H.-X. Liu and T.-F. Liu, Chin. J. Struct. Chem., 2021, 40, 1082–1087 CAS
.
- P. Liu, A. Liu, P. Wang, Y. Chen and B. Li, Chin. J. Struct. Chem., 2023, 42, 100001 CrossRef
.
- H.-L. Wang, X.-F. Ma, Z.-H. Zhu, Y.-Q. Zhang, H.-H. Zou and F.-P. Liang, Inorg. Chem. Front., 2019, 6, 2906–2913 RSC
.
- S. Chand, S. C. Pal, A. Pal, Y. Ye, Q. Lin, Z. Zhang, S. Xiang and M. C. Das, Chem. – Eur. J., 2019, 25, 1691–1695 CrossRef CAS PubMed
.
- Z. Ju, G. Liu, Y. S. Chen, D. Yuan and B. Chen, Chem. – Eur. J., 2017, 23, 4774–4777 CrossRef CAS PubMed
.
- R. B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC
.
- Z. Bao, D. Xie, G. Chang, H. Wu, L. Li, W. Zhou, H. Wang, Z. Zhang, H. Xing, Q. Yang, M. J. Zaworotko, Q. Ren and B. Chen, J. Am. Chem. Soc., 2018, 140, 4596–4603 CrossRef CAS PubMed
.
- M. Zhou, G. Liu, Z. Ju, K. Su, S. Du, Y. Tan and D. Yuan, Cryst. Growth Des., 2020, 20, 4127–4134 CrossRef CAS
.
- U. Yamashita, N. Yoshinari, R. Sodkhomkhum, N. Meundaeng and T. Konno, CrystEngComm, 2020, 22, 2700–2704 RSC
.
- B. Li, B. Zheng, W. Zhang, D. Zhang, X.-J. Yang and B. Wu, J. Am. Chem. Soc., 2020, 142, 6304–6311 CrossRef CAS PubMed
.
- T. S. C. MacDonald, B. L. Feringa, W. S. Price, S. J. Wezenberg and J. E. Beves, J. Am. Chem. Soc., 2020, 142, 20014–20020 CrossRef CAS PubMed
.
- R. S. Voguri, S. Ranga, A. Dey and S. Ghosal, Cryst. Growth Des., 2020, 20, 7647–7657 CrossRef CAS
.
- W. Gong, D. Chu, H. Jiang, X. Chen, Y. Cui and Y. Liu, Nat. Commun., 2019, 10, 600 CrossRef CAS PubMed
.
- M. Petryk, A. Janiak, L. J. Barbour and M. Kwit, Eur. J. Org. Chem., 2018, 2018, 1916–1923 CrossRef CAS
.
- S. Y. Lin, Y. N. Guo, Y. Guo, L. Zhao, P. Zhang, H. Ke and J. Tang, Chem. Commun., 2012, 48, 6924–6926 RSC
.
- J. Gao, R. A. Zingaro, J. H. Reibenspies and A. E. Martell, Org. Lett., 2004, 6, 2453–2455 CrossRef CAS PubMed
.
- S. R. Korupoju, N. Mangayarkarasi, P. S. Zacharias, J. Mizuthani and H. Nishihara, Inorg. Chem., 2002, 41, 4099–4101 CrossRef CAS PubMed
.
- S. Y. Lin, C. Wang, L. Zhao, J. Wu and J. Tang, Dalton Trans., 2015, 44, 223–229 RSC
.
- Z. Chu, W. Huang, L. Wang and S. Gou, Polyhedron, 2008, 27, 1079–1092 CrossRef CAS
.
- T. F. Willems, C. H. Rycroft, M. Kazi, J. C. Meza and M. Haranczyk, Microporous Mesoporous Mater., 2012, 149, 134–141 CrossRef CAS
.
- Z. Zheng, N. Hanikel, H. Lyu and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144, 22669–22675 CrossRef CAS PubMed
.
- N. Hanikel, X. Pei, S. Chheda, H. Lyu, W. Jeong, J. Sauer, L. Gagliardi and O. M. Yaghi, Science, 2021, 374, 454–459 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and X-ray crystallography data in CIF format. CCDC numbers 2286962 and 2287221. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ce00917c |
‡ Crystal data for MHOF-PO4-1: C45H69Cu3N6O19P, M = 1219.65, cubic, space group F413, a = 37.4254(2) Å, V = 52420.3(8) Å3, Z = 32, T = 100.0(3) K, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 912 reflections collected, 4330 unique (Rint = 0.0387). Final GooF = 0.992, R1 = 0.0538, wR2 = 0.1547, R indices based on 3485 reflections with I > 2s(I) (refinement on F2), 236 parameters, 157 restraints. Lp and adsorption corrections applied, m = 1.904 mm−1. Absolute structure parameter = 0.025(19). Crystal data for MHOF-PO4-2: C45H63Cu3N6O16P, M = 1165.60, hexagonal, space group P63, a = b = 19.7940(2) Å, c = 9.02850(10) Å, V = 3063.47(7) Å3, Z = 2, T = 155(7) K, 22 912 reflections collected, 4330 unique (Rint = 0.0387). Final GooF = 0.992, R1 = 0.0538, wR2 = 0.1547, R indices based on 3485 reflections with I > 2s(I) (refinement on F2), 236 parameters, 157 restraints. Lp and adsorption corrections applied, m = 1.904 mm−1. Absolute structure parameter = 0.025(19). Crystal data for MHOF-PO4-2: C45H63Cu3N6O16P, M = 1165.60, hexagonal, space group P63, a = b = 19.7940(2) Å, c = 9.02850(10) Å, V = 3063.47(7) Å3, Z = 2, T = 155(7) K, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875 reflections collected, 3924 unique (Rint = 0.0228). Final GooF = 1.084, R1 = 0.0199, wR2 = 0.0536, R indices based on 3878 reflections with I > 2s(I) (refinement on F2), 226 parameters, 144 restraints. Lp and adsorption corrections applied, m = 1.977 mm−1. Absolute structure parameter = 0.004(7). 875 reflections collected, 3924 unique (Rint = 0.0228). Final GooF = 1.084, R1 = 0.0199, wR2 = 0.0536, R indices based on 3878 reflections with I > 2s(I) (refinement on F2), 226 parameters, 144 restraints. Lp and adsorption corrections applied, m = 1.977 mm−1. Absolute structure parameter = 0.004(7). |
| This journal is © The Royal Society of Chemistry 2023 |