Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A molecular sponge that exclusively adsorbs acetonitrile†
Yoichi
Habata
 *ab,
Ayumi
Wada
a,
Eunji
Lee
*ab,
Ayumi
Wada
a,
Eunji
Lee
 c,
Huieyong
Ju
c,
Huieyong
Ju
 d,
Yuki
Yoshiba
a,
Hiroki
Horita
d,
Yuki
Yoshiba
a,
Hiroki
Horita
 a,
Jun-ichi
Ishii
ab,
Mari
Ikeda
a,
Jun-ichi
Ishii
ab,
Mari
Ikeda
 e and
Shunsuke
Kuwahara
e and
Shunsuke
Kuwahara
 ab
ab
aDepartment of Chemistry, Faculty of Science, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan. E-mail: habata@chem.sci.toho-u.ac.jp
bResearch Center for Materials with Integrated Properties, Faculty of Science, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan
cDepartment of Chemistry, Gangneung-Wonju National University, Gangneung 25457, South Korea
dWestern Seoul Center, Korea Basic Science Institute, 150 Bugahyeon-ro, Seodaemun-gu, Seoul 03759, South Korea
eDepartment of chemistry, Education Center, Faculty of Engineering, Chiba Institute of Technology, Shobazono, Narashino, Chiba 275-0023, Japan
First published on 16th March 2023
Abstract
We report that a tetra-armed cyclen (L) with phenylethynylmethyl (Ph–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2–) groups as side-arms forms L·CH3CN (L·AN) inclusion crystals from acetonitrile–organic mixed solvents with high selectivity and acts as if it is an acetonitrile selective molecular sponge.
C–CH2–) groups as side-arms forms L·CH3CN (L·AN) inclusion crystals from acetonitrile–organic mixed solvents with high selectivity and acts as if it is an acetonitrile selective molecular sponge.
Acetonitrile is one of the most common organic solvents used as a reaction solvent in organic synthesis and as a mobile phase in HPLC. Because of its high dielectric constant, acetonitrile is also used in various situations, such as coordination with insoluble metal salts to make them soluble in organic solvents. Inclusion complexes of acetonitrile with cyclic host compounds such as crown ethers1 and calixarenes2 have been examined, and their X-ray crystal structures were reported. In crown ether–acetonitrile and calix[4]arene–acetonitrile complexes, hydrogen bonds between oxygen atoms in the crown ethers and hydrogen atoms in the methyl group of acetonitrile and CH⋯π interactions between phenyl groups in the calix[4]arene and hydrogen atoms in acetonitrile are the main driving force in forming the inclusion complexes, respectively.3 There are also host–guest systems using non-cyclic compounds, the so-called “organic inclusion crystals”.4 Toda et al. reported5 that tetraphenyl acetylene derivatives and aromatic diols form inclusion complexes with acetonitrile. However, there is no selectivity towards acetonitrile in these cyclic and non-cyclic host–guest systems.
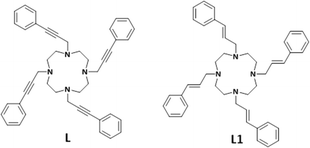
We have reported that a silver complex with a tetra-armed cyclen having styrylmethyl groups (Ph–CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH–CH2–) as side-arms (L1 in Fig. 1) incorporates organic solvents such as acetonitrile in a pseudo-cavity formed by the side-arms.6 In connection with this study, we prepared L with linear and rigid functional groups, phenylethylylmethyl (Ph–C
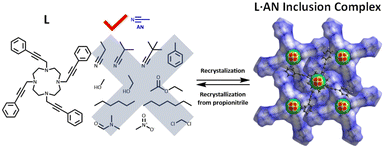
CH–CH2–) as side-arms (L1 in Fig. 1) incorporates organic solvents such as acetonitrile in a pseudo-cavity formed by the side-arms.6 In connection with this study, we prepared L with linear and rigid functional groups, phenylethylylmethyl (Ph–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2–) groups, as aromatic side-arms. During the purification of L, we found that L forms an inclusion complex with acetonitrile during the recrystallization of L from acetonitrile. Interestingly, L never forms organic inclusion crystals with other common organic solvents in a laboratory, and the crystallization rate is quite high. Recently, Torres-Huerta et al. reported7 a molecular sponge in which triphenylboroxine–piperazine self-assembled adducts assume different conformations depending on the type of solvent used. However, to the best of our knowledge, molecular sponges that crystallize by selectively absorbing a single organic solvent have not been reported. Here, we report the highly selective crystallization of L·CH3CN (L·AN) inclusion crystals in acetonitrile–organic solvent mixtures. Furthermore, the L·AN inclusion crystals can be dissolved in propionitrile and recrystallized to desorb acetonitrile and recover L again.
C–CH2–) groups, as aromatic side-arms. During the purification of L, we found that L forms an inclusion complex with acetonitrile during the recrystallization of L from acetonitrile. Interestingly, L never forms organic inclusion crystals with other common organic solvents in a laboratory, and the crystallization rate is quite high. Recently, Torres-Huerta et al. reported7 a molecular sponge in which triphenylboroxine–piperazine self-assembled adducts assume different conformations depending on the type of solvent used. However, to the best of our knowledge, molecular sponges that crystallize by selectively absorbing a single organic solvent have not been reported. Here, we report the highly selective crystallization of L·CH3CN (L·AN) inclusion crystals in acetonitrile–organic solvent mixtures. Furthermore, the L·AN inclusion crystals can be dissolved in propionitrile and recrystallized to desorb acetonitrile and recover L again.
The new tetra-armed cyclen, L, was prepared by the reaction of 3-phenyl-2-propynal (1 in the ESI†)8 and cyclen in the presence of NaBH(OAc)3 in THF in 78% yield. When L was recrystallized from acetonitrile, the L·AN inclusion complex was obtained as single crystals. As shown in ESI† Video S1, the L·AN inclusion crystals precipitate very quickly from the acetonitrile solution of L. Interestingly, crystals of L were obtained using propionitrile as a recrystallization solvent. Characterization of L and L·AN was performed by 1H and 13C NMR, FAB-MS, IR, X-ray crystallography, and elemental analysis (Fig. S1–S3†). In addition, the L·AN inclusion crystals were subjected to thermogravimetric-differential thermal analysis (TG-DTA). Fig. S4† shows that the weight loss at 100 °C was 6.2%, attributed to the one acetonitrile molecule. The DTA curve shows that the melting point of the L·AN inclusion complex is 99.5–100.0 °C. The TG-DTA curve shows a small endotherm at 100 °C, suggesting that the crystals start to melt and the acetonitrile molecules are released.
The structure of the tetra-armed cyclen L with four phenylethynylmethyl groups was characterized in the solid state by X-ray crystallography (Fig. 2a). Colorless single crystals of L were grown by slow evaporation from its propionitrile solution. In the crystal structure, the cyclen unit exhibits a chair-like conformation. In addition, the N–C–C–N conformation of the cyclen ring is a δδλλ conformation, and the ligand has two enantiomers of the same conformation, related by the crystal glide planes. The meso-conformation of L is the same as that of other tetra-armed cyclens previously reported.7,9 In the packing structure (Fig. 2b), intermolecular CH⋯π interactions exist between the hydrogen atoms (H5A) of the methylene group of the cyclen and ethynyl carbons (C15 and C16), and the hydrogen atoms (H22A) of the phenyl group and ethynyl carbons (C15 and C16), showing typical distances.
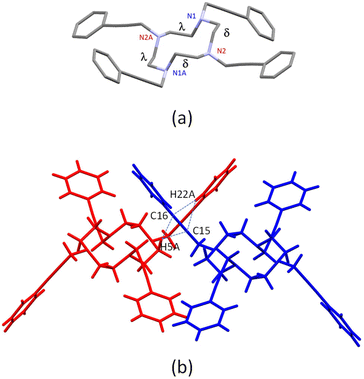 | ||
| Fig. 2 X-ray structure of L (a) and molecular interactions between L molecules (b). Dotted lines indicate interacting atoms. | ||
On the other hand, two conformations of the cyclen ring (δδδδ and λλλλ) exist in the L·AN inclusion crystals (Fig. S5 and S6†). An acetonitrile molecule is surrounded by one L molecule from the top and the bottom, and four from the equatorial plane. As shown in Fig. 3a, each acetonitrile forms hydrogen bonds with L as follows: N(acetonitrile)⋯H–C(cyclen ring) (H1A–N3, 2.653; H3A–N3, 2.644 Å) and N(cyclen ring)⋯H–C(acetonitrile) (N2–H24A, 2.577; N2–H24B, 2.738; N1–H24C, 2.783 Å). Furthermore, there are intermolecular CH⋯π interactions between L molecules (C8–H14A, 2.865; C13–H14A, 2.807; C11–H5B, 2.786 Å) (Fig. 3b). The distances of these hydrogen bonds and CH⋯π interactions are also typical. The X-ray crystallographic analysis of the L·AN complex revealed the strong interaction between acetonitrile and L and between L molecules.
 | ||
| Fig. 3 Molecular interactions between the ligand (L) and acetonitrile (a) and between L molecules (b). Dotted lines indicate interacting atoms. | ||
Hirshfeld surface analysis,10 performed using “CrystalExplorer”, a program developed by Spackman et al.,11 is a valuable tool for understanding intermolecular interactions. In the Hirshfeld surface analysis, areas for intermolecular contacts closer than the sum of their van der Waals radii are highlighted in red, longer contacts are blue, and contacts at around the sum of van der Waals radii are white. As shown in Fig. 4, the red and white areas are shown in methyl of acetonitrile and nitrogen, respectively. In addition, there are many white areas in the ligand (ESI† Videos S2 and S3). These results indicate that acetonitrile molecules are tightly encased from the top, bottom, back, left, and right by the ligands in the solid state, and there are robust CH⋯π interactions between the ligands.
 | ||
| Fig. 4 Hirshfeld surface analysis of the L·AN inclusion complex. (a) Top view, (b) back view, and top views of the Hirshfeld surface focusing on CH3CN (c) and L (d). | ||
Inclusion crystal formation of L·AN from acetonitrile–organic solvent mixtures was carried out to confirm whether L forms inclusion crystals only for acetonitrile (Fig. 5a). The structures of crystals obtained from each mixed solvent system were confirmed by X-ray crystallography. When alkyl nitriles (propionitrile (CH3CH2CN), isobutyronitrile ((CH3)2CHCN), and pivalonitrile ((CH3)3CCN)), alcohols (methanol (MeOH) and ethanol (EtOH)), and n-alkanes (hexane and heptane) were mixed with acetonitrile, L·AN inclusion crystals were obtained with more than a 92% yield. On the other hand, ethyl acetate (EtOAc), toluene, dichloromethane (DCM), and N,N-dimethylformamide (DMF) were employed, and the yield decreased owing to the relatively high solubility of the L·AN complex. When acetonitrile–acetone and acetonitrile–chloroform mixtures were used, crystals could not be obtained because the L·AN complex readily dissolved in these solvents. The yields of L·AN inclusion crystals were determined using methanol as the mixing solvent and varying the ratio of acetonitrile (Fig. 5b). As a result, L·AN inclusion crystals were obtained in more than a 93% yield when the ratio of acetonitrile in the acetonitrile–methanol mixture was more than 20%. These results indicate that L forms L·AN inclusion crystals from various mixed solvent systems. Furthermore, the crystallization is relatively rapid, and videos of the crystallization from acetonitrile and a mixture of acetonitrile/dichloromethane are shown in the ESI† (Video S4).
 | ||
Fig. 5 (a) Yields of L·AN inclusion crystals from CH3CN–organic solvent mixtures. [L] = 8.0 × 10−5 mol per 4 mL (CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) organic solvent = 2.0 mL/2.0 mL). The mean value is the average of three experiments at 298 K. (b) Yields of L·AN inclusion crystals from mixtures with different ratios of CH3CN–CH3OH. [L] = 8.0 × 10−5 mol per 4 mL (CH3CN organic solvent = 2.0 mL/2.0 mL). The mean value is the average of three experiments at 298 K. (b) Yields of L·AN inclusion crystals from mixtures with different ratios of CH3CN–CH3OH. [L] = 8.0 × 10−5 mol per 4 mL (CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH = 0.4 mL/3.6 mL to 4.0 mL/0.0 mL). The mean value is the average of three experiments at 298 K. The yields of L·AN were calculated by the equation noted below.‡ CH3OH = 0.4 mL/3.6 mL to 4.0 mL/0.0 mL). The mean value is the average of three experiments at 298 K. The yields of L·AN were calculated by the equation noted below.‡ | ||
To estimate the binding constant of L to acetonitrile, acetonitrile-induced 1H NMR spectral changes were monitored in CDCl3. The binding constant for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was calculated using “Bindfit”12 software. As shown in Fig. S12,† log
1 complex was calculated using “Bindfit”12 software. As shown in Fig. S12,† log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K is approximately 0.5. In a dilute solution, the interaction between L and acetonitrile is fragile, i.e., L binds to acetonitrile in the presence of a significant excess of acetonitrile.
K is approximately 0.5. In a dilute solution, the interaction between L and acetonitrile is fragile, i.e., L binds to acetonitrile in the presence of a significant excess of acetonitrile.
The AN selectivity is thought to be caused by the following process. The ligand L, obtained by recrystallization from propionitrile, forms a relatively asymmetric δδλλ conformation in the absence of an organic solvent, as shown in Fig. 2. However, to allow efficient hydrogen bonding from the methyl group of the guest molecule AN, the ligand must adopt a quadruple symmetric conformation (δδδδ or λλλλ). This ensures that all four nitrogen lone pairs are oriented in a direction suitable for hydrogen bonding. Once this conformation is achieved, another L molecule with the same conformation is positioned directly above the AN molecule. Together with the first L molecule, the C–H bonds of the cyclen are properly oriented and form hydrogen bonds with the electronegative N atoms of AN. This process is repeated to form a stack. Furthermore, the distance between two ligand molecules in the stack is the ideal distance at which the phenylethynyl groups of the neighboring stack can be inserted, resulting in a pseudo-body-centered structure. For this process to occur, the organic solvent used must have a methyl group attached, and its molecules must have linear, electronegative functional groups. Of the organic solvents tested, only AN satisfies these two conditions. Based on this hypothesis, it is suggested that L may also form organic inclusion crystals with CH3Br or CH3I due to their linear molecules containing electronegative functional and methyl groups. However, the boiling point of CH3Br is 3.6 °C, which may make it difficult to verify experimentally. In addition, CH3I easily forms quaternary ammonium salts with tertiary amines, further complicating experimental verification.
The L·AN inclusion crystals were added to propionitrile, heated to dissolution, and recrystallized. The resulting crystals were filtered by suction to obtain pure L with a recovery of 77%.
In summary, the tetra-armed cyclen with phenylethynylmethyl side-arms acts as a molecular sponge that can only encapsulate acetonitrile (Fig. 6). The acetonitrile-bound ligands can be reused by recrystallization from propionitrile. The high selectivity and simple adsorption/desorption of the ligand for acetonitrile may not be used as a practical method for the selective recovery of organic molecules because the ligand uses expensive starting materials, but it is expected to contribute to the development of a new method for separation and purification techniques.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Grant-in-Aid for Scientific Research from the MEXT of Japan (Grant No. 17K05844 and 20K05480). EL acknowledges the support from the JSPS International Research Fellow (Toho University, 18F18343). HJ was supported by fellowships from the Marubun Research Promotion Foundation (Japan).Notes and references
- G. W. Gokel, D. J. Cram, C. L. Liotta, H. P. Harris and F. L. Cook, J. Org. Chem., 1974, 39, 2445–2446 CrossRef CAS.
- W. Xu, R. J. Puddephatt, L. Manojlovic-Muir, K. W. Muir and C. S. Frampton, J. Inclusion Phenom. Mol. Recognit. Chem., 1994, 19, 277–290 CrossRef CAS.
- (a) R. S. Rogers, L. K. Kurihara and P. D. Richards, J. Chem. Soc., Chem. Commun., 1987, 604–606 RSC; (b) K. Panneerselvam, K. K. Chacko, E. Weber and H. J. Koehler, J. Inclusion Phenom. Mol. Recognit. Chem., 1990, 9, 337–347 CrossRef CAS.
- (a) M. R. Caira, A. Jacobs, L. R. Nassimbeni and F. Toda, Supramol. Chem., 2004, 16, 107–112 CrossRef CAS; (b) S. A. Bourne, K. L. Gifford, G. Nash and F. Toda, J. Inclusion Phenom. Mol. Recognit. Chem., 1998, 32, 91–102 CrossRef CAS.
- (a) F. Toda, A. Kai, R. Toyotaka, W.-H. Yip and T. C. W. Mak, Chem. Lett., 1989, 18, 1921–1924 CrossRef; (b) F. Toda, Top. Curr. Chem., 1987, 140, 43–69 CrossRef CAS.
- H. Ju, H. Tenma, M. Iwase, E. Lee, M. Ikeda, S. Kuwahara and Y. Habata, Dalton Trans., 2020, 49, 3112–3119 RSC.
- A. Torres-Huerta, M. D. J. Velásquez-Hernández, D. Martínez-Otero, H. Höpfl and V. Jancik, Cryst. Growth Des., 2017, 17, 2438–2452 CrossRef CAS.
- M. Journet, D. Cai, L. M. DiMichele and R. D. Larsen, Tetrahedron Lett., 1998, 39, 6427–6428 CrossRef CAS.
- Y. Habata, M. Ikeda, S. Yamada, H. Takahashi, S. Ueno, T. Suzuki and S. Kuwahara, Org. Lett., 2012, 14, 4576–4579 CrossRef CAS PubMed.
- P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, J. Appl. Crystallogr., 2021, 54, 1006–1011 CrossRef CAS PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- Bindfit software, https://supramolecular.org/ Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. Supporting figures and tables. CCDC 2233509 (L) and 2233510 (L·AN). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ce00169e |
‡  . . |
| This journal is © The Royal Society of Chemistry 2023 |