Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The introduction of a base component to porous organic salts and their CO2 storage capability†
Takahiro
Ami‡
a,
Kouki
Oka‡
a,
Keiho
Tsuchiya
a,
Wataru
Kosaka
 bc,
Hitoshi
Miyasaka
bc,
Hitoshi
Miyasaka
 bc and
Norimitsu
Tohnai
bc and
Norimitsu
Tohnai
 *a
*a
aDepartment of Applied Chemistry and Center for Future Innovation (CFi), Graduate School of Engineering, Osaka University, Osaka 565-0871, Japan. E-mail: tohnai@chem.eng.osaka-u.ac.jp
bInstitute for Materials Research, Tohoku University, Sendai 980-8577, Japan
cDepartment of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan
First published on 21st March 2023
Abstract
Porous organic salts (POSs) are constructed by charge-assisted hydrogen bonding between amino and sulfonic groups, and can be used to design a variety of porous structures based on molecular design. In particular, triphenylmethylamine (TPMA) and aromatic sulfonic acids form robust POSs with a rigid diamond structure (d-POSs). In this study, by replacing one of the three phenyl rings of TPMA with a pyrimidine ring, we succeeded in constructing a d-POS with high porosity (43.8%) and with a base component (pyrimidine) on the void surface. In addition, the weak basicity of the pyrimidine did not interfere with the formation of d-POSs. This d-POS adsorbed CO2 over the primary air components (N2 and O2) and also exhibited CO2 storage capability: It retained CO2 at a relatively low pressure of Pe/P0 = 0.05, and readily desorbed CO2 below Pe/P0 = 0.05.
Introduction
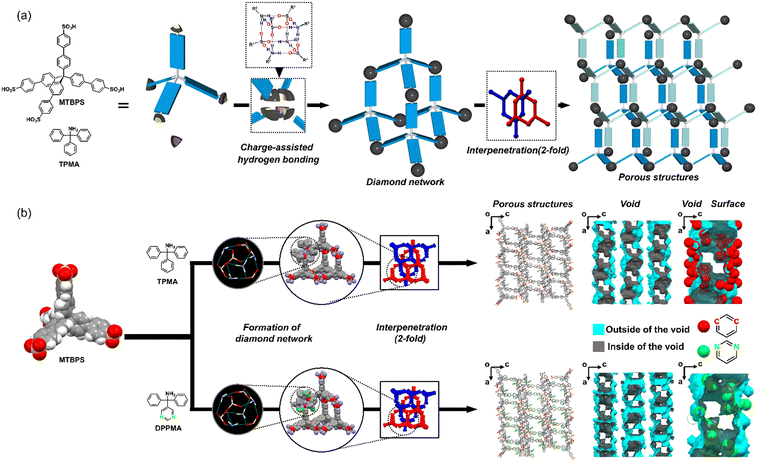
Based on molecular design, the pore size, morphology, and function of porous materials fabricated from organic molecules can be precisely and easily controlled. Therefore, these materials have been extensively investigated for gas separation applications.1–3 Typical examples of extremely designable porous organic materials are metal–organic frameworks (MOFs), in which organic linkers and metal elements are connected by coordination bonds,2,4–6 and covalent-organic frameworks (COFs), in which organic molecules are linked by covalent bonds.7–10 However, the fabrication of robust porous structures such as MOFs and COFs usually requires harsh and/or complicated synthesis routes (e.g., microwave, voltage application, high pressure, and high temperature), and/or the use of metal elements obtained from limited resources. On the other hand, porous materials fabricated with non-covalent bonds are prepared by simple recrystallization under ambient conditions.11,12 In particular, all-organic porous materials constructed by hydrogen bonds are called hydrogen-bonded frameworks (HOFs) and are intensively investigated as one of the porous organic materials because of their high crystallinity and designability.13 Non-covalent bonds are generally considered weaker than coordination and covalent bonds. However, hydrogen bonds between strong bases and acids, such as amines and sulfonic acids, are known as “charge-assisted hydrogen bonding”, and are extremely ionic and rigid.14 The porous structures of ammonium sulfonate salts, which are constructed via charge-assisted hydrogen bonding, exhibit excellent robustness and rigidity.15–20We previously reported that ammonium sulfonate salts, composed of bulky TPMA and aromatic sulfonic acids, such as tetrahedral tetrasulfonic acid (MTBPS), form diamondoid porous organic salts (d-POSs) (Fig. 1a).15–20 In detail, four TPMA molecules and four sulfonic acid molecules form a [4 + 4] supramolecular cluster (Fig. 1a, center) by self-assembly via rigid charge-assisted hydrogen bonding. Subsequently, these clusters are self-assembled to form the diamond network, and the interpenetration of some of the diamond networks hierarchically results in the formation of d-POSs (Fig. 1a, right).15 The diamond network is composed of sulfonic acid molecules and the amino groups in TPMA; however, it does not include the triphenylmethyl group in TPMA. The bulky triphenylmethyl group suppresses the number of interpenetrations of the diamond network, and therefore d-POSs generally possess a relatively higher porosity than organic porous materials that contain complete amine structures.21 Furthermore, we previously found that the phenyl rings of TPMA were exposed on the void surface of d-POSs, and the introduction of halogen-substituents such as fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) into the para-positions of the phenyl rings of TPMA enabled d-POSs to have a variety of void structures and environments. In addition, we demonstrated that their gas adsorption properties were drastically changed depending on their void structures and environments.20
In this study, we found that not only the para-positions but also meta-positions of the phenyl rings of TPMA were exposed on the void surface. Therefore, for introducing the base component into the void surface of the d-POSs, we focused on pyrimidine, which has more base components (e.g., lone pair of nitrogen), and is a weaker base than primary amines. The diphenyl(pyrimidine-5-yl)methylamine (DPPMA) is synthesized by replacing one of the three phenyl rings of TPMA with a pyrimidine ring, where two nitrogen atoms are substituted at the meta-positions of the phenyl ring.22 A facile recrystallization method is used to combine DPPMA and MTBPS for the fabrication of a d-POS (DPPMA/MTBPS) with two nitrogen atoms, with their lone pairs exposed on the void surface. In the crystal structure of DPPMA/MTBPS, the base component of the pyrimidine rings (lone pairs of the nitrogen atoms) do not interfere with the charge-assisted hydrogen bonding between amino and sulfonic groups, and therefore DPPMA/MTBPS forms a diamond network identical to that of TPMA/MTBPS. DPPMA/MTBPS adsorbed CO2 over the primary air components (N2 and O2). Moreover, the adsorbed CO2 (49.9 mL (STP)/g at Pe/P0 = 1) was stabilized in the void via interactions with the pyrimidine ring.23,24 DPPMA/MTBPS retained CO2 down to a reasonably low pressure of Pe/P0 = 0.05, and subsequently CO2 was desorbed by decreasing the pressure to below Pe/P0 = 0.05.
Experimental section
Preparation of organic salts composed of DPPMA and MTBPS
DPPMA (5 eq) and MTBPS (1 eq) were separately dissolved in methanol, and then mixed. After solvent evaporation, the residue was washed with diethyl ether, and the organic salts were obtained as a pale-yellow powder.Preparation of the single-crystal of DPPMA/MTBPS.
The organic salt (2.0 mg) was recrystallized with benzonitrile (200 μL) in N,N-dimethylacetamide (400 μL) by gradually evaporating the solvent at 70 °C. The single crystals of DPPMA/MTBPS were obtained as colorless prisms. By repeating this process, a sufficient amount of DPPMA/MTBPS was collected for the gas adsorption experiments.Results and discussion
An organic salt with an exact TPMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTBPS molecular ratio of 4
MTBPS molecular ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was produced by simply mixing TPMA and MTBPS in a polar organic solvent. Subsequently, the facile recrystallization of the organic salt in N,N-dimethylformamide with 1,2,4-trichlorobenzene (a template molecule that facilitates the formation of porous structures) resulted in TPMA/MTBPS§ (Fig. 1b, center and S1;† for details of the preparation processes, please see the experimental section). MTBPS has a tetrahedral structure, which is consistent with the diamondoid structure, and therefore TPMA/MTBPS was selectively formed without crystal polymorphism. The single-crystal structure of TPMA/MTBPS indicated that the two diamond networks are deeply interlocked in opposite directions, forming a thermodynamically stable structure (Fig. 1b, center).
1 was produced by simply mixing TPMA and MTBPS in a polar organic solvent. Subsequently, the facile recrystallization of the organic salt in N,N-dimethylformamide with 1,2,4-trichlorobenzene (a template molecule that facilitates the formation of porous structures) resulted in TPMA/MTBPS§ (Fig. 1b, center and S1;† for details of the preparation processes, please see the experimental section). MTBPS has a tetrahedral structure, which is consistent with the diamondoid structure, and therefore TPMA/MTBPS was selectively formed without crystal polymorphism. The single-crystal structure of TPMA/MTBPS indicated that the two diamond networks are deeply interlocked in opposite directions, forming a thermodynamically stable structure (Fig. 1b, center).
Additionally, the PLATON/VOID routine calculation showed that TPMA/MTBPS has a high porosity of 40.7%. After the evacuation of a template molecule using the supercritical fluid carbon dioxide process (SCFCO2),26TPMA/MTBPS (Fig. S2a†) adsorbed CO2 over the primary air components, such as N2 and O2 (Fig. S2b†). This was ascribed to the quadrupole–quadrupole interactions27,28 between CO2 (with a strong quadrupole moment)29 and the phenyl rings exposed on the void surface, and the smaller kinetic diameter of CO2 (3.30 Å30) than those of N2 (3.64 Å30) and O2 (3.46 Å30).
This study aimed to introduce the base component into the void surface of the d-POSs. In TPMA/MTBPS, the para-positions and meta-positions of the phenyl rings of TPMA are exposed on the void surface (Fig. 1b, void surface). Thus, we hypothesized that the incorporation or substitution of base functional groups at the para-positions or meta-positions of phenyl rings would introduce the base component into the void surface. Therefore, a TPMA derivative (DPPMA) was synthesized by replacing one of the phenyl rings of TPMA with a pyrimidine ring, where two nitrogen atoms and their lone pairs were substituted at the meta-positions of the phenyl ring. Nitrogen atoms and their lone pairs on the pyrimidine ring are known showing weak basicity.23,24 Subsequently, by combining DPPMA with MTBPS, DPPMA/MTBPS similar to TPMA/MTBPS (Fig. 1b, right and S3;† for details of the synthesis processes, please see the experimental section) was successfully prepared. Notably, the single-crystal X-ray structure analysis revealed that the base component (lone pairs of the nitrogen atoms) of the pyrimidine ring do not interfere with the charge-assisted hydrogen bonding between the amino and sulfonic groups. DPPMA/MTBPS forms a diamond network identical to that of TPMA/MTBPS (Fig. 1b, right and S4†). This indicates that pyrimidine is less likely to interact with the sulfonic acid than with the amino group of TPMA because of the weaker basicity of pyrimidine than primary amines.31,32 The porosity of DPPMA/MTBPS is exhibited a slightly higher porosity of 43.8% compared to that of TPMA/MTBPS (Table S2†), because the replacement of the phenyl ring with a pyrimidine ring increases the void volume owing to the loss of the hydrogen atoms. Additionally, the porosity of DPPMA/MTBPS is the highest in the other reported d-POSs15,16,18,20 and organic porous structures with diamond networks (Table S3†).33–36 Thermogravimetric analysis (TGA) data (Fig. S5†) and the number of electrons found in the masking routine revealed that the crystals of DPPMA/MTBPS immediately following formation via crystallization included six N,N-dimethylacetamide molecules and three benzonitrile molecules per one MTBPS molecule and four DPPMA molecules, which were equivalent to 29.5% of total weight. Furthermore, as designed, the nitrogen atoms and their lone pairs on the pyrimidine rings are exposed on the void surface of DPPMA/MTBPS (Fig. 1b, right), and, per unit cell, 48 nitrogen atoms are exposed on the void surface. It is noteworthy that changing the meta-positions of the phenyl rings of TPMA successfully tuned the void environment, while maintaining the void shape of TPMA/MTBPS. Variable temperature powder X-ray diffraction (VT-PXRD) (Fig. S6†) of DPPMA/MTBPS revealed that the porous structure of DPPMA/MTBPS was maintained up to approximately 100 °C. Furthermore, DPPMA/MTBPS shows high stability for nonpolar solvents such as diethyl ether and benzene. However, the crystallinity of DPPMA/MTBPS was decreased after soaking in the polar solvents, water, and acidic/basic aqueous solutions (Fig. S7†), presumably because of the partial dissolution of DPPMA/MTBPS. Similar to TPMA/MTBPS, the template molecule in DPPMA/MTBPS was evacuated by SCFCO2 while maintaining the porous structure (Fig. 2a and S8†).
 | ||
| Fig. 2 Characterization of the diamondoid porous organic salts (DPPMA/MTBPS). (a) Powder X-ray diffraction (PXRD) patterns of DPPMA/MTBPS, simulation (black), immediately following formation via crystallization (blue), after conducting the supercritical fluid carbon dioxide (SCFCO2) process for 5 h (red). (b) Gas adsorption/desorption isotherms of DPPMA/MTBPS in the cases of nitrogen (N2, 77 K), oxygen (O2, 77 K), and carbon dioxide (CO2, 195 K). Pe denotes the pressure at gas adsorption and P0 is the condensation pressure of the adsorbate at the measurement temperature. Green line is Pe/P0 = 0.05. The amount of CO2 adsorption of DPPMA/MTBPS reaches a maximum value at Pe/P0 = 0.2 in the desorption isotherm which is common in porous structures with complicated micropores such as MOFs because of the extremely slow diffusion rate of the adsorbent in their void.25 | ||
The gas adsorption properties of DPPMA/MTBPS, after the evacuation of the template molecule, for CO2, N2, and O2 were evaluated. The PXRD patterns of TPMA/MTBPS and DPPMA/MTBPS did not change before and after the sorption experiments (Fig. S9†). Therefore, these POSs maintained their porous structures after the sorption experiments, and their gas adsorption behavior is highly reproducible even after the second time. DPPMA/MTBPS adsorbed 49.9 mL (STP)/g of CO2 at Pe/P0 = 1.0, 2.87 mL (STP)/g of N2 at Pe/P0 = 0.97, and 1.07 mL (STP)/g of O2 at Pe/P0 = 0.99 (Fig. 2b). DPPMA/MTBPS exhibited the same selective adsorption behavior for CO2 as that of TPMA/MTBPS. In addition, the CO2 adsorption isotherm revealed that DPPMA/MTBPS had a CO2-BET surface area of 192 m2 g−1 (Table S2†) and an average pore size of 10.2 Å (Fig. S10†). On the other hand, DPPMA/MTBPS did not adsorb N2 and O2, therefore the pore size distribution analysis based on the N2 and O2 adsorption isotherms were unable to be performed. The large hysteresis observed in the CO2 adsorption/desorption behavior of DPPMA/MTBPS demonstrates that CO2 is retained on the void surface by interactions with the pyrimidine rings. DPPMA/MTBPS retains the CO2 adsorbed at Pe/P0 = 1.0 when the pressure is decreased to Pe/P0 = 0.05 (Fig. 2b and S11†), whereas TPMA/MTBPS releases approximately 50% of the adsorbed CO2 (Fig. S2b†). Furthermore, the strength of interactions between pyrimidine or phenyl rings and CO2 molecules was evaluated by Fourier transform infrared (FT-IR) spectroscopy measurements under CO2 atmospheres. However, as shown in Fig. S12,† no significant differences were found between the spectra measured under vacuum and CO2 atmospheres. This means that these interactions are weaker than the general chemical interactions. Therefore, although DPPMA/MTBPS retains CO2 up to Pe/P0 = 0.05, it can readily desorb the adsorbed CO2 below Pe/P0 = 0.05. The ability to retain CO2 over a wide pressure range (0.05–1.0 atm) may play a very important role in CO2 storage and utilization processes.37
Conclusions
In this study, DPPMA, where two nitrogen atoms and their lone pairs are substituted at the meta-positions of the phenyl ring, and MTBPS were combined to form d-POSs. The pyrimidine ring did not interfere with the charge-assisted hydrogen bonding between the sulfonic and primary amino groups, and therefore DPPMA/MTBPS formed the same crystal structure as that of TPMA/MTBPS. Furthermore, as designed, the nitrogen atoms and their lone pairs on the pyrimidine rings were exposed on the void surface of DPPMA/MTBPS, and the base components were successfully introduced into the void surface while maintaining the void shape of TPMA/MTBPS. DPPMA/MTBPS adsorbed CO2 over the primary air components (N2 and O2), which was ascribed to strong quadrupole–quadrupole interactions with the phenyl rings on the void surface. DPPMA/MTBPS retained the adsorbed CO2 in the void down to a reasonably low pressure of Pe/P0 = 0.05 (originally adsorbed at Pe/P0 = 1.0), which was attributed to interactions between the pyrimidine rings and CO2. Moreover, when the pressure was decreased to below Pe/P0 = 0.05, DPPMA/MTBPS readily desorbed the adsorbed CO2. Therefore, DPPMA/MTBPS captured and retained CO2 even at a relatively low CO2 pressure of 0.08–0.13 atm, which is comparable to that of a waste incinerator,38 or 0.05 atm, which is comparable to that of a natural gas combined cycle power station.39 Therefore, the introduction of a functional component (even a base component) into the benzene rings of TPMA enables the void environment and the functionalities of POSs to be precisely designed.Author contributions
Takahiro Ami and Kouki Oka contributed equally to this work. Takahiro Ami: conceptualization, methodology, formal analysis, data curation, investigation, and writing – original draft. Kouki Oka: conceptualization, methodology, formal analysis, data curation, project administration, validation, supervision, investigation, and writing – original draft. Keiho Tsuchiya: investigation. Wataru Kosaka: investigation and writing – review & editing. Hitoshi Miyasaka: writing – review & editing. Norimitsu Tohnai: conceptualization, methodology, data curation, project administration, validation, supervision, investigation, writing – review & editing, funding acquisition, and resources.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was partially supported by Grants-in-Aids for Scientific Research (20H00381, 20H02548, 21K18925, 21H01900, and 22K14732) from MEXT, Japan. K. O. also acknowledges the support from LNest Grant from Nipponham, FUSO Innovative Technology Fund, Masuyakinen basic research foundation, and Shorai Foundation for Science and Technology.Notes and references
- Q. Qian, P. A. Asinger, M. J. Lee, G. Han, K. Mizrahi Rodriguez, S. Lin, F. M. Benedetti, A. X. Wu, W. S. Chi and Z. P. Smith, Chem. Rev., 2020, 120, 8161–8266 CrossRef CAS PubMed.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS.
- L. Zhu and Y.-B. Zhang, Molecules, 2017, 22, 1149 CrossRef PubMed.
- S. Zhou, Z. Qiu, M. Strømme and C. Xu, Energy Environ. Sci., 2021, 14, 900–905 RSC.
- J. K. Zareba, M. Nyk and M. Samoc, Cryst. Growth Des., 2016, 16, 6419–6425 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- K. Oka, B. Winther-Jensen and H. Nishide, Adv. Energy Mater., 2021, 11, 2003724 CrossRef CAS.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, 923 CrossRef CAS PubMed.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- R.-B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC.
- J. Luo, J.-W. Wang, J.-H. Zhang, S. Lai and D.-C. Zhong, CrystEngComm, 2018, 20, 5884–5898 RSC.
- I. Hisaki, C. Xin, K. Takahashi and T. Nakamura, Angew. Chem., Int. Ed., 2019, 58, 11160–11170 CrossRef CAS PubMed.
- P. Gilli, L. Pretto, V. Bertolasi and G. Gilli, Acc. Chem. Res., 2009, 42, 33–44 CrossRef CAS PubMed.
- A. Yamamoto, S. Uehara, T. Hamada, M. Miyata, I. Hisaki and N. Tohnai, Cryst. Growth Des., 2012, 12, 4600–4606 CrossRef CAS.
- A. Yamamoto, T. Hamada, I. Hisaki, M. Miyata and N. Tohnai, Angew. Chem., Int. Ed., 2013, 52, 1709–1712 CrossRef CAS PubMed.
- A. Yamamoto, T. Hasegawa, T. Hamada, T. Hirukawa, I. Hisaki, M. Miyata and N. Tohnai, Chem. – Eur. J., 2013, 19, 3006–3016 CrossRef CAS PubMed.
- A. Yamamoto, T. Hirukawa, I. Hisaki, M. Miyata and N. Tohnai, Tetrahedron Lett., 2013, 54, 1268–1273 CrossRef CAS.
- T. Miyano, N. Okada, R. Nishida, A. Yamamoto, I. Hisaki and N. Tohnai, Chem. – Eur. J., 2016, 22, 15430–15436 CrossRef CAS PubMed.
- T. Ami, K. Oka, K. Tsuchiya and N. Tohnai, Angew. Chem., 2022, 134, e20222597 Search PubMed.
- S. Yu, G. L. Xing, L. H. Chen, T. Ben and B. L. Su, Adv. Mater., 2020, 32, 2003270 CrossRef CAS PubMed.
- M. Berthelot, C. Laurence, M. Safar and F. Besseau, J. Chem. Soc., Perkin Trans. 2, 1998, 283–290 RSC.
- K. D. Vogiatzis, A. Mavrandonakis, W. Klopper and G. E. Froudakis, ChemPhysChem, 2009, 10, 374–383 CrossRef CAS PubMed.
- I. Kaljurand, T. Rodima, I. Leito, I. A. Koppel and R. Schwesinger, J. Org. Chem., 2000, 65, 6202–6208 CrossRef CAS PubMed.
- J. Ethiraj, S. Palla and H. Reinsch, Microporous Mesoporous Mater., 2020, 294, 109867 CrossRef CAS.
- J. E. Mondloch, O. Karagiaridi, O. K. Farha and J. T. Hupp, CrystEngComm, 2013, 15, 9258–9264 RSC.
- J. H. Williams, Acc. Chem. Res., 1993, 26, 593–598 CrossRef CAS.
- S. K. Valzita, Chem. Phys. Lett., 1981, 78, 421–423 CrossRef.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- B. Freeman, Y. Yampolskii and I. Pinnau, Materials science of membranes for gas and vapor separation, John Wiley & Sons Ltd., West Sussex, 2006 Search PubMed.
- T. P. Selvam, C. R. James, P. V. Dniandev and S. K. Valzita, J. Res. Pharm., 2015, 2, 1–9 Search PubMed.
- I. Juranić, Croat. Chem. Acta, 2014, 87, 343–347 CrossRef.
- H. Kim and M. P. Suh, Inorg. Chem., 2005, 44, 810–812 CrossRef CAS PubMed.
- K. M. E. Jones, A. H. Mahmoudkhani, B. D. Chandler and G. K. H. Shimizu, CrystEngComm, 2006, 8, 303–305 RSC.
- P. Metrangolo, F. Meyer, T. Pilati, D. M. Proserpio and G. Resnati, Chem. – Eur. J., 2007, 13, 5765–5772 CrossRef CAS PubMed.
- D. Beaudoin, T. Maris and J. D. Wuest, Nat. Chem., 2013, 5, 830–834 CrossRef CAS PubMed.
- R. E. Morris and P. S. Wheatley, Angew. Chem., Int. Ed., 2008, 47, 4966–4981 CrossRef CAS PubMed.
- H. Lee, S.-M. Yi, T. M. Holsen, Y.-S. Seo and E. Choi, Waste Manage., 2018, 73, 247–255 CrossRef CAS PubMed.
- J.-M. G. Amann and C. Bouallou, Energy Procedia, 2009, 1, 909–916 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2160329. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ce00086a |
| ‡ T. A. and K. O. contributed equally to this work. |
| § The crystal structure of TPMA/MTBPS has been reported in our previous work.20 Deposition Number 2160329 (DPPMA/MTBPS) contain the supplementary crystallographic data for this paper. |
| This journal is © The Royal Society of Chemistry 2023 |