Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amine-modified polyionic liquid supports enhance the efficacy of PdNPs for the catalytic hydrogenation of CO2 to formate†
Reece
Paterson
a,
Luke E.
Fahy
a,
Elisabetta
Arca
*b,
Casey
Dixon
a,
Corinne Y.
Wills
a,
Han
Yan
c,
Anthony
Griffiths
c,
Sean M.
Collins
 c,
Kejun
Wu
c,
Richard A.
Bourne
c,
Kejun
Wu
c,
Richard A.
Bourne
 c,
Thomas W.
Chamberlain
c,
Thomas W.
Chamberlain
 *c,
Julian G.
Knight
a and
Simon
Doherty
*c,
Julian G.
Knight
a and
Simon
Doherty
 *a
*a
aNewcastle University Centre for Catalysis (NUCAT), School of Chemistry, Bedson Building, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: simon.doherty@ncl.ac.uk
bSchool of Mathematics, Statistics and Physics, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK
cInstitute of Process Research & Development, School of Chemistry and School of Chemical and Process Engineering, University of Leeds, Woodhouse Lane, LS2 9JT, UK
First published on 23rd October 2023
Abstract
Palladium nanoparticles stabilised by aniline modified polymer immobilised ionic liquid is a remarkably active catalyst for the hydrogenation of CO2 to formate; the initial TOF of 500 h−1 is markedly higher than either unmodified catalyst or its benzylamine and N,N-dimethylaniline modified counterparts and is among the highest to be reported for a PdNP-based catalyst.
The global demand for a circular economy has led to the exploration of carbon dioxide (CO2) utilisation to produce value added chemicals such as fuels and feedstocks. Amongst these CO2-derived commodities, formic acid has been highlighted as a future energy carrier, either acting as a hydrogen storage medium,1 or via direct use in formic acid fuel cells.2 However, formic acid production currently depends on the hydrolysis of methyl formate, itself obtained from the carbonylation of methanol; while this process is costly and energy intensive, direct hydrogenation of CO2 to formic acid is attractive as it is single step, and can be achieved under mild conditions with the appropriate catalyst.
While Au3,4 and Ru5–7 metal nanoparticles (NP) have been reported to catalyse the hydrogenation of CO2 to formic acid/formate, studies have been dominated by catalysts based on monometallic8–12 and bimetallic13–15 Pd nanoparticles. While a range of supports have been shown to stabilise PdNPs for the hydrogenation of CO2,9–16 functionalisation of the support surface with a weakly basic amine has recently been reported to enhance catalytic activity.3 For example, an improvement in the performance of PdNPs and PdAgNPs supported on amine-grafted mesoporous carbon or SBA-15, compared with their unmodified counterparts, was attributed to several factors including; (i) the growth of small well-dispersed NPs, (ii) the formation of electron-rich Pd species resulting from charge transfer from silver, (iii) a cooperative role in facilitating the elementary steps of the catalytic cycle and (iv) increasing the CO2 adsorption capacity/adsorption rate and concentrating the CO2 around the active site.16
The incorporation of ionic liquids (ILs) is an obvious choice in the design of catalysts for CO2 hydrogenation,17a as they are not only effective at stabilizing metal nanoparticles, but they sequester CO2 and are being explored for applications in carbon capture and storage.17b In addition to their role as solvent and sorbent to solubilise CO2,6 ILs have been reported to activate CO2 and stabilise charged intermediates,8,18 as well as maintain good dispersion of the NPs and prevent aggregation,5 while basic ILs have been used as buffering media to shift the equilibrium towards free formic acid or formate.19–21 Polymer immobilized ionic liquids (PIILs) also have attractive credentials as supports for metal nanoparticles as they facilitate recovery of both the metal and the support, enhance catalyst longevity and their CO2 sorption capacity and sorption/desorption kinetics exceeds their IL counterparts.22 Moreover, the modular construction of PIILs should improve designability and enable catalysts with improved performance profiles to be developed by modifying the ionic microenvironment, varying the concentration and type of surface organic nitrogen donor, tailoring the structural integrity and porosity and controlling the metal stoichiometry for the synthesis of bimetallic NPs.23
Herein, we report the first example of amine-decorated polymer immobilised ionic liquid stabilised PdNPs as catalysts for the hydrogenation of CO2 to formate. Preliminary studies to explore the efficacy of the catalyst as a function of the composition of the support revealed that the performance depends on the type of amine and its loading and that the highest turnover frequency (TOF, measured as molformate molPd−1 h−1) of 500 h−1, obtained with PdNPs stabilised by aniline decorated PIIL, is among the highest to be reported.
The first series of amine functionalized PIIL supports 1a–d were based on a 1,2-dimethylimidazolium monomer, an imidazolium based cross-linker, and an amine modified styrene monomer, with an overall imidazolium to amine ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1). Polymers 1a–d were impregnated with tetrachloropalladate to afford precatalysts 2a–d. The solid-state 13C CP-MAS NMR spectra of 2a–d were similar to those for 1a–d with the exception of the resonances assigned to the ipso N–C atom of the aniline which appeared to shift 30 ppm to lower field, from δ 115 ppm in 1a to δ 139 ppm in 2a.
1 (Fig. 1). Polymers 1a–d were impregnated with tetrachloropalladate to afford precatalysts 2a–d. The solid-state 13C CP-MAS NMR spectra of 2a–d were similar to those for 1a–d with the exception of the resonances assigned to the ipso N–C atom of the aniline which appeared to shift 30 ppm to lower field, from δ 115 ppm in 1a to δ 139 ppm in 2a.
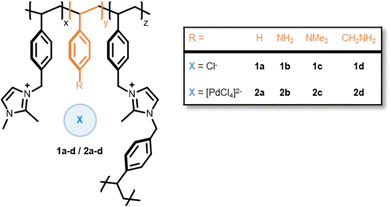 | ||
| Fig. 1 Composition of amine decorated PIIL supports 1a–d [PdCl4]2− loaded precatalysts 2a–d. Polymers contain 5 mol% cross-linker where x = 0.91, y = 1, z = 0.09. | ||
Hydrogenations were conducted in a HEL DigiCAT high pressure reactor, charged with aqueous base (20 mL, 0.65 M), 6 μmol Pd in the form of supported [PdCl4]2− precatalyst and a total pressure of 40 bar (H2: CO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
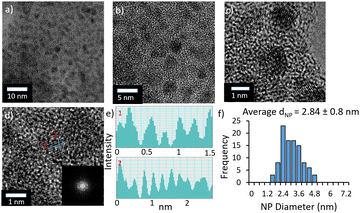
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 373 K for 2 h. Formate formation was quantified by 1H NMR spectroscopy, using dimethylsulfoxide as an internal standard.24 As the precatalysts were reduced in situ, under the conditions of catalysis, the corresponding PdNPs were isolated after use for the aqueous phase hydrogenation of CO2 and analysed by HRTEM and HAADF-STEM EDX and shown to be ultrafine and near monodisperse with average diameters between 1.87–3.7 ± 0.4–0.9 nm (Fig. 2 and ESI†). A survey of the activity as a function of the base, using unmodified precatalyst 2a, revealed that reactions conducted in the presence of K2CO3 gave a higher TOF than those with KHCO3, NaOH, NEt3 or KOH (Fig. S2a, ESI†), and as such further optimisation of the reaction parameters was performed in 0.65 M aqueous K2CO3. As there have been several other reports that the initial TOF for the hydrogenation of CO2 to formate depends on the base, future studies will investigate whether the type and amount of base affects the size and/or morphology of the NPs, and thereby the activity, or whether the base is involved in the fundamental steps of the catalysis. A study of the influence of the stirring speed and total CO2/H2 pressure on catalyst efficacy was then undertaken to minimise the effect of diffusion. The TOF increased with increasing stirring speed and reached an optimum at 750 rpm (Fig. S2c, ESI†). The TOF also increased from 56 h−1 at 10 bar of 1
1) at 373 K for 2 h. Formate formation was quantified by 1H NMR spectroscopy, using dimethylsulfoxide as an internal standard.24 As the precatalysts were reduced in situ, under the conditions of catalysis, the corresponding PdNPs were isolated after use for the aqueous phase hydrogenation of CO2 and analysed by HRTEM and HAADF-STEM EDX and shown to be ultrafine and near monodisperse with average diameters between 1.87–3.7 ± 0.4–0.9 nm (Fig. 2 and ESI†). A survey of the activity as a function of the base, using unmodified precatalyst 2a, revealed that reactions conducted in the presence of K2CO3 gave a higher TOF than those with KHCO3, NaOH, NEt3 or KOH (Fig. S2a, ESI†), and as such further optimisation of the reaction parameters was performed in 0.65 M aqueous K2CO3. As there have been several other reports that the initial TOF for the hydrogenation of CO2 to formate depends on the base, future studies will investigate whether the type and amount of base affects the size and/or morphology of the NPs, and thereby the activity, or whether the base is involved in the fundamental steps of the catalysis. A study of the influence of the stirring speed and total CO2/H2 pressure on catalyst efficacy was then undertaken to minimise the effect of diffusion. The TOF increased with increasing stirring speed and reached an optimum at 750 rpm (Fig. S2c, ESI†). The TOF also increased from 56 h−1 at 10 bar of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CO2
1 CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
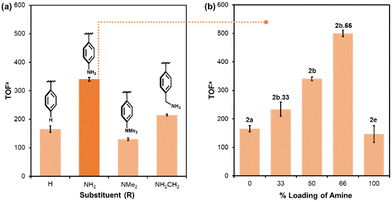
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 to an optimum of 166 h−1 at 40 bar and then decreased to 138 h−1 when the pressure was increased further to 70 bar (Fig. S2b, ESI†). In addition, the TOF decreased with decreasing reaction temperature below 100 °C and also decreased when the reaction temperature was raised to 120 °C (Fig. S2d, ESI†). Comparison of catalyst performance as a function of the amine-modified support against unmodified catalyst revealed that catalyst stabilised by PIIL modified with weakly basic aniline i.e. generated from pre-catalyst 2b, gave a dramatic enhancement in efficacy to afford a TOF of 340 h−1, compared with 166 h−1 for catalyst generated from 2a (Fig. 3a). While support modified with benzylamine (2d) also showed a positive effect on catalyst performance, with a TOF of 214 h−1, the use of N,N-dimethylaniline modified pre-catalyst 2c gave a TOF of 130 h−1, slightly lower than the 166 h−1 obtained with its unmodified counterpart 2a. Thus, while primary amines enhance catalyst performance a weakly basic amine is most effective, and a tertiary amine has a negligible impact on the efficacy. Yamashita and co-workers have also reported a similar affect as PdAgNPs supported by N,N-dimethylpropylamine modified SBA-15 was slightly less active than unmodified catalyst, for the same reaction, whereas aniline modified SBA-15 showed a large positive effect.16 The TOF's used herein to compare the efficacy of each catalyst were determined after a reaction time of 2 h, based on a study of the kinetics of hydrogenation in the early stages of the reaction using precatalyst 2a and amine-decorated precatalyst 2b.66 (vide infra) which showed that there was no observable induction period under the conditions of catalysis (Fig. S3, ESI†).
H2 to an optimum of 166 h−1 at 40 bar and then decreased to 138 h−1 when the pressure was increased further to 70 bar (Fig. S2b, ESI†). In addition, the TOF decreased with decreasing reaction temperature below 100 °C and also decreased when the reaction temperature was raised to 120 °C (Fig. S2d, ESI†). Comparison of catalyst performance as a function of the amine-modified support against unmodified catalyst revealed that catalyst stabilised by PIIL modified with weakly basic aniline i.e. generated from pre-catalyst 2b, gave a dramatic enhancement in efficacy to afford a TOF of 340 h−1, compared with 166 h−1 for catalyst generated from 2a (Fig. 3a). While support modified with benzylamine (2d) also showed a positive effect on catalyst performance, with a TOF of 214 h−1, the use of N,N-dimethylaniline modified pre-catalyst 2c gave a TOF of 130 h−1, slightly lower than the 166 h−1 obtained with its unmodified counterpart 2a. Thus, while primary amines enhance catalyst performance a weakly basic amine is most effective, and a tertiary amine has a negligible impact on the efficacy. Yamashita and co-workers have also reported a similar affect as PdAgNPs supported by N,N-dimethylpropylamine modified SBA-15 was slightly less active than unmodified catalyst, for the same reaction, whereas aniline modified SBA-15 showed a large positive effect.16 The TOF's used herein to compare the efficacy of each catalyst were determined after a reaction time of 2 h, based on a study of the kinetics of hydrogenation in the early stages of the reaction using precatalyst 2a and amine-decorated precatalyst 2b.66 (vide infra) which showed that there was no observable induction period under the conditions of catalysis (Fig. S3, ESI†).
A study of the influence of increasing the amount of aniline on the support on catalyst efficacy was undertaken by preparing precatalysts stabilised by polymers with 4-aminostyrene and imidazolium monomer ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as well as poly-4-aminostyrene (1e), which, together with 2a and 2b, form a series of precatalysts, PdCl4@xNH2PIIL, where x corresponds to the % loading of aniline (x = 0 (2a), 33 (2b.33), 50 (2b), 66 (2b.66) and 100 (2e)). Interestingly, an almost linear increase in activity was observed with increasing loading of aniline, reaching a maximum TOF of 500 h−1 with a TON of 1609 after 14 h for catalyst generated from 2b.66 (Fig. S3, ESI†), which is among the highest to be reported for a monometallic PdNP-based system. Gratifyingly, this TON is also higher than that of 839 and 874 obtained with PdAgNPs supported on phenylamine functionalised SBA-1516 or mesoporous carbon,14 respectively, and thus, future studies will target surface modified multimetallic catalysts with well-defined compositions and explore their efficacy as a function of the metals, their ratio, and the surface functionality. A TOF of 147 h−1 was obtained for catalyst supported by the homopolymer of 4-aminostyrene (1e), indicating a positive influence of the ionic liquid on catalyst efficacy as amine alone is not sufficient to achieve high activity. Interestingly, a much lower loading of Pd was obtained for 4-aminostyrene homopolymer, 1e (3.8 wt% compared to an average of 10.9 wt% for the remaining PIIL systems); this may be due to the absence of chloride anions which facilitate impregnation with the tetrachloropalladate via ion exchange.
1 as well as poly-4-aminostyrene (1e), which, together with 2a and 2b, form a series of precatalysts, PdCl4@xNH2PIIL, where x corresponds to the % loading of aniline (x = 0 (2a), 33 (2b.33), 50 (2b), 66 (2b.66) and 100 (2e)). Interestingly, an almost linear increase in activity was observed with increasing loading of aniline, reaching a maximum TOF of 500 h−1 with a TON of 1609 after 14 h for catalyst generated from 2b.66 (Fig. S3, ESI†), which is among the highest to be reported for a monometallic PdNP-based system. Gratifyingly, this TON is also higher than that of 839 and 874 obtained with PdAgNPs supported on phenylamine functionalised SBA-1516 or mesoporous carbon,14 respectively, and thus, future studies will target surface modified multimetallic catalysts with well-defined compositions and explore their efficacy as a function of the metals, their ratio, and the surface functionality. A TOF of 147 h−1 was obtained for catalyst supported by the homopolymer of 4-aminostyrene (1e), indicating a positive influence of the ionic liquid on catalyst efficacy as amine alone is not sufficient to achieve high activity. Interestingly, a much lower loading of Pd was obtained for 4-aminostyrene homopolymer, 1e (3.8 wt% compared to an average of 10.9 wt% for the remaining PIIL systems); this may be due to the absence of chloride anions which facilitate impregnation with the tetrachloropalladate via ion exchange.
Modification of the support by covalent attachment of the aniline was crucial to achieve high activity as a hydrogenation conducted with catalyst generated from 2a in the presence of the equivalent amount of aniline as that on the support in 2b.66 resulted in a slight decrease in the TOF to 116 h−1, compared with 146 h−1 obtained with 2a; moreover, the TOF decreased to 92 h−1 when the amount of aniline was increased further by 10-fold. Reassuringly, all catalysts described here were more active than commercial Pd/C (10 wt%, Sigma Aldrich), which gave an initial TOF of only 74 h−1 under the conditions identified above. In addition, pre-treatment of 6 μmol of Pd/C with a homogeneous solution of 1b.66 (12 μmol) for 16 h prior to performing a hydrogenation did not have a noticeable effect on the activity as the TOF of 51 h−1 was only marginally greater than that of 43 h−1 obtained with the same loading of Pd/C, but in the absence of 1b.66.
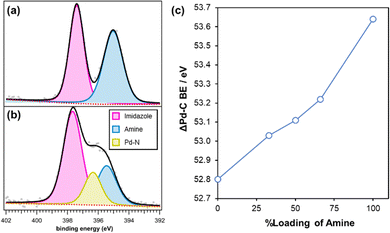
The possible coordination of amine to Pd in precatalysts 2b–d was explored by analysing the core-electron excitations of N and Pd using XPS. Charge neutralisation was used for all samples and no rescaling has been applied to quoted binding energies (BEs). Due to the limitations of using advantageous carbon as ref. 25 peak assignment was based on the characteristic BE separation between the elements. First, examination of the local N environment of polymer 1b.66 revealed two peaks at 397.4 eV and 395.0 eV characteristic of nitrogen in the imidazolium and the amine chemical environments, respectively (Fig. 4a). Following impregnation with [PdCl4]2−, the imidazolium N 1s peak did not appear to shift significantly for all samples reported in this study (397.5 ± 0.1 eV). The separation in the BE scale of this peak in comparison to the aliphatic C 1s also showed close to no deviation across samples (116.9 ± 0.1 eV). This means that the chemical environment of the N-imidazolium is unchanged, thus it can be concluded that there was no interaction between the Pd and the nitrogen atoms of the imidazolium ring. However, after loading the polymer supports with [PdCl4]2−, an additional component became apparent between 396.4–395.9 eV, which is consistent with a Pd–N interaction, as previously reported in the deconvolution of N 1s spectra (Fig. 4b).26–28 Quantitative analysis of the N 1s components was used to determine the amount of amine coordinated to surface Pd species in the various precatalysts which decreased in the order 84 at% (NH2) > 71 at% (NH2CH2) > 61 at% (NMe2).
Analysis of the Pd 3d core level for 2b.66 revealed a doublet attributed to divalent Pd coordinated to Cl with 3d5/2 and 3d3/2 peaks at 333.9 eV and 339.2 eV, respectively, as well as a shake-up satellite line at 341.1 eV.29 An additional doublet was apparent at a higher BE with 3d5/2 and 3d3/2 peaks at 335.4 eV and 340.7 eV; this doublet most likely arises from Pd coordinated to aniline as the same doublet was observed for 2b, 2b.33 and 2e. As for the N 1s spectra, the Pd BEs were compared for the various amine supported catalysts. A shift to higher BEs was observed when the amine modified pre-catalysts 2b–2e were compared to unfunctionalized 2a; by comparing the separation between the Pd 3d and aliphatic C 1s BEs the Pd BEs for the various supported precatalysts was found to shift to higher BE in the following order: PIIL < NMe2PIIL < NH2PIIL < NH2CH2PIIL (Table S3, ESI†). A shift to higher BE after coordination to an amine indicates that the Pd atoms are electron deficient due to charge transfer from the Pd 3d orbitals to the amine. Moreover, the Pd 3d BEs appeared to increase with increasing loading of amine on the polymer as shown by a comparison of the ΔBE(Pd–C) for 2a, 2b.33, 2b, 2b.66 and 2e (Fig. 4c). A Pd–N interaction was also confirmed by solid-state 15N NMR spectroscopy as the spectrum for 1e contained a signal at δ −323 ppm whereas the spectrum for precatalyst 2e contained an additional signal at δ −373 ppm (Fig. S7, ESI†); similarly, a signal at δ −319 ppm in the 15N NMR spectrum of 1b.66 shifted to δ −372 ppm in 2b.66 following impregnation with [PdCl4]2− (Fig. S8, ESI†). The high field shift of these signals indicates that the amine has become more electron-rich, consistent with the XPS analysis of 2e and 2b.66.
While coordination of a heteroatom to a metal typically results in an increase in the electron density at the metal, there have been reports in which a metal has become more electron deficient due to strong metal–support interactions. For example, PdNPs on N-doped carbon nanotubes become more electron deficient by virtue of donating electrons to pyridinic nitrogen in agreement with the results described in this communication.30 Thus, it may be possible to tune the surface electronic properties of NPs and thereby their reactivity through a judicious choice of heteroatom donor and its loading.
The Pd 3d core level of the nanoparticles recovered after in situ reduction of 2a–e, typically contained two pairs of 3d5/2 and 3d3/2 doublets; the pair with 3d5/2 BEs of 330.9–331.8 eV correspond to Pd0,31 while those with 3d5/2 BEs of 333.24–333.9 eV are due to divalent Pd, indicating that reduction to Pd0 was not complete. Unreduced Pd2+ could be indicative of Pd species strongly coordinated to Lewis basic sites that would limit their reducibility.26 Analysis of the N 1s spectra of all samples post-catalysis revealed a loss of imidazolium nitrogen. For example, the relative contribution of imidazolium nitrogen atoms to the N 1s region decreased by 45 atm% for 2b.66 post-catalysis. This is perhaps not surprising as benzyl protecting groups are typically removed using Pd-catalysed hydrogenation. For 2a, 2c and 2d the loss of imidazolium was even more significant, with no imidazolium detected at the surface of 2a post-catalysis, this was accompanied by leaching of Pd into solution which was confirmed by ICP-OES. As such, we are currently designing more robust systems to identify catalysts suitable for use in scale-up.
In conclusion, PdNPs stabilized by aniline modified polymer immobilised ionic liquid is a markedly more efficient catalyst for the hydrogenation of CO2 to formate than PdNPs stabilised by the individual components i.e. polymer immobilise ionic liquid or poly-4-aminostyrene. TOFs increased with increasing aniline content on the polyionic liquid and the optimum TOF of 500 h−1 is among the highest to be reported for a PdNP-based catalyst. The amine-modified PIIL motif is an encouraging design concept to develop new catalysts for the reversible hydrogenation of CO2 and in operando surface investigations and detailed kinetic studies are currently underway to develop a detailed understanding of the factors that influence catalyst performance.
R. P. and A. G. acknowledge the Engineering and Physical Sciences Centre for Doctoral Training (EP/S023836/1 and EP/SO22473/1, respectively for studentships. The X-ray photoelectron (XPS) data collection was performed at the EPSRC National Facility for XPS (“HarwellXPS”) under Contract No. PR16195.
Conflicts of interest
There are no conflicts to declare.Notes and references
- I. Dutta, S. Chatterjee, H. Cheng, R. K. Parsapur, Z. Liu, Z. Li, E. Ye, H. Kawanami, J. S. C. Low, Z. Lai, X. J. Loh and K.-W. Huang, Adv. Energy Mater., 2022, 12, 2103799 CrossRef CAS.
- H. Cheng, J. Zhou, H. Xie, S. Zhang, J. Zhang, S. Sun, P. Luo, M. Lin, S. Wang, Z. Pan, J. Wang, X. J. Loh and Z. Liu, Adv. Energy Mater., 2023, 13, 2203893 CrossRef CAS.
- Q. Liu, X. Yang, L. Li, S. Miao, Y. Li, Y. Li, X. Wang, Y. Huang and T. Zhang, Nat. Commun., 2017, 8, 1–8 CrossRef CAS PubMed.
- G. A. Filonenko, W. L. Vrijburg, E. J. M. Hensen and E. A. Pidko, J. Catal., 2016, 343, 97–105 CrossRef CAS.
- X. Guo, Z. Peng, A. Traitangwong, G. Wang, H. Xu, V. Meeyoo, C. Li and S. Zhang, Green Chem., 2018, 20, 4932–4945 RSC.
- P. Gautam, P. R. Upadhyay and V. Srivastava, Catal. Lett., 2019, 149, 1464–1475 CrossRef CAS.
- S. J. L. Anandaraj, L. Kang, S. DeBeer, A. Bordet and W. Leitner, Small, 2023, 19, 2206806 CrossRef PubMed.
- Y. Wu, Y. Zhao, H. Wang, B. Yu, X. Yu, H. Zhang and Z. Liu, Ind. Eng. Chem. Res., 2019, 58, 6333–6339 CrossRef CAS.
- X. Shao, X. Miao, X. Yu, W. Wang and X. Ji, RSC Adv., 2020, 10, 9414–9419 RSC.
- L. C. Lee, X. Xing and Y. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 38436–38444 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, S. Yao, X. Song, W. Huang, M. J. Hülsey and N. Yan, J. Catal., 2019, 376, 57–67 CrossRef CAS.
- K. J. Betsy, A. Lazar, A. Pavithran and C. P. Vinod, ACS Sustainable Chem. Eng., 2020, 8, 14765–14774 CrossRef CAS.
- G. Yang, Y. Kuwahara, K. Mori, C. Louis and H. Yamashita, J. Phys. Chem. C, 2021, 125, 3961–3971 CrossRef CAS.
- S. Masuda, K. Mori, Y. Futamura and H. Yamashita, ACS Catal., 2018, 8, 2277–2285 CrossRef CAS.
- H. Zhong, M. Iguchi, M. Chatterjee, T. Ishizaka, M. Kitta, Q. Xu and H. Kawanami, ACS Catal., 2018, 8, 5355–5362 CrossRef CAS.
- K. Mori, S. Masuda, H. Tanaka, K. Yoshizawa, M. Che and H. Yamashita, Chem. Commun., 2017, 53, 4677–4680 RSC.
- (a) T. Sasaki, Curr. Opin. Green Sustainable Chem., 2022, 36, 100633 CrossRef CAS; (b) S. Zeng, X. Zhang, L. Bai, X. Zhang, H. Wang, J. Wang, D. Bao, M. Li, X. Liu and S. Zhang, Chem. Rev., 2017, 117, 9625–9673 CrossRef CAS PubMed.
- G. A. Filonenko, W. L. Vrijburg, E. J. M. Hensen and E. A. Pidko, J. Catal., 2016, 343, 97–105 CrossRef CAS.
- A. Weilhard, K. Salzmann, M. Navarro, J. Dupont, M. Albrecht and V. Sans, J. Catal., 2020, 385, 1–9 CrossRef CAS.
- Z. Zhang, S. Hu, J. Song, W. Li, G. Yang and B. Han, ChemSusChem, 2009, 2, 234–238 CrossRef CAS PubMed.
- Z. Zhang, Y. Xie, W. Li, S. Hu, J. Song, T. Jiang and B. Han, Angew. Chem., Int. Ed., 2008, 47, 1127–1129 CrossRef CAS PubMed.
- (a) S. Zulfiqar, M. I. Sarwar and D. Mecerreyes, Polym. Chem., 2015, 6, 6435–6451 RSC; (b) B. Feng, Z. Zhang, J. Wang, D. Yang, Q. Li, Y. Liu, H. Gai, T. Huang and H. Song, Fuel, 2022, 325, 124853 CrossRef CAS.
- S. Doherty, J. G. Knight, H. Y. Alharbi, R. Paterson, C. Wills, C. Dixon, L. Šiller, T. W. Chamberlain, A. Griffiths, S. M. Collins, K. Wu, M. D. Simmons, R. A. Bourne, K. R. J. Lovelock and J. Seymour, ChemCatChem, 2022, 14, e202101752 CrossRef CAS.
- T. Chatterjee, E. Boutin and M. Robert, Dalton Trans., 2020, 49, 4257–4265 RSC.
- G. Greczynski and L. Hultman, Prog. Mater. Sci., 2020, 107, 100591 CrossRef CAS.
- D. A. Bulushev, M. Zacharska, E. V. Shlyakhova, A. L. Chuvilin, Y. Guo, S. Beloshapkin, A. V. Okotrub and L. G. Bulusheva, ACS Catal., 2016, 6, 681–691 CrossRef CAS.
- Z. Li, H. Yu, Y. Zhang, D. Wu, Y. Bai, S. Liu and H. Zhao, Chem. Commun., 2023, 59, 4535–4538 RSC.
- P. Chatterjee, H. Wang, J. S. Manzano, U. Kanbur, A. D. Sadow and I. I. Slowing, Catal. Sci. Technol., 2022, 12, 1922–1933 RSC.
- M. C. Militello and S. J. Simko, Surf. Sci. Spectra, 1994, 3, 402–409 CrossRef CAS.
- Z. He, B. Dong, W. Wang, G. Yang, Y. Cao, H. Wang, Y. Yang, Q. Wang, F. Peng and H. Yu, ACS Catal., 2019, 9, 2893–2901 CrossRef CAS.
- Q. Zhang, S. Zheng, J. Zhang, W. Li and Y. Fu, Catal. Lett., 2021, 151, 2537–2546 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04987f |
| This journal is © The Royal Society of Chemistry 2023 |