Open Access Article
Open Access ArticleControlling molecular shuttling in a rotaxane with weak ring recognition sites†
Nina
Bukhtiiarova
ab,
Alberto
Credi
 *ab and
Stefano
Corra
*ab and
Stefano
Corra
 *ab
*ab
aCLAN-Center for Light Activated Nanostructures, Istituto per la Sintesi Organica e Fotoreattività, CNR area della ricerca Bologna, via Gobetti, 101, 40129, Bologna, Italy
bDipartimento di Chimica Industriale “Toso-Montanari”, Alma Mater Studiorum – Università di Bologna, viale del Risorgimento, 4, Bologna 40136, Italy. E-mail: stefano.corra@unibo.it
First published on 9th October 2023
Abstract
We describe a rotaxane molecular shuttle encompassing triazolium and tertiary ammonium units as weak recognition sites for the ring. Such a design, which differs from that of typical controllable rotaxanes, allows the precise tuning of the ring distribution among the two sites – i.e., the coconformational equilibrium – by changing the solvent polarity or the nature of the counteranions. Shuttling of the ring between the two stations can also be toggled by acid–base stimuli. Such an approach is paradigmatic to obtain rotaxanes that can sense environmental changes and transduce them into a coconformational response and opens avenues for novel applications in sensing and stimuli-responsive materials.
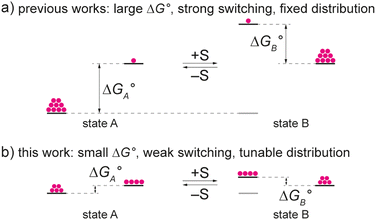
Controllable molecular shuttles are a class of bistable rotaxanes whose axle component is endowed with two different recognition sites (stations) for the ring component. These rotaxanes can exist in two isomers, called coconformers, which display different stabilities and are in equilibrium. Upon application of a suitable chemical, electrical or optical stimulus, the relative affinity of the macrocycle for the two stations can be modified, causing the former to move from one station to the other.1,2 More precisely, the transformation triggered by the stimulus determines a new coconformational equilibrium, in which the distribution of the ring among the stations is different from the original one. The reversibility of the switching process, usually by application of an opposite stimulus, enables the reset to the original distribution.
Because of these unique characteristics, arising by the presence of the mechanical bond, molecular shuttles and related species have become attractive for utilization in fields such as functional materials, sensing, and information storage, as well as molecular machines and motors.1,3 To ensure a clear-cut coconformational switching, it is important that, in either state, the macrocycle interacts with the two stations with a markedly different strength, and that the pattern is inverted upon changing the state. In this manner, it can be ensured that the ring resides exclusively on station A in the original state, while it moves completely to station B in the switched state.1,4
A popular and successful design for chemically controllable reversible molecular shuttles is based on a secondary ammonium station encircled by a crown ether which, upon deprotonation of the ammonium center, shuttles to a pH-insensitive weaker station.5 Among others, 4,4′-bipyridinium or triazolium units are good candidates for the role of the weaker station in combination with sec-ammonium.6 As the strength of interaction between a crown ether such as dibenzo-24-crown-8 (DB24C8) and sec-ammonium centers is remarkably high,7 in rotaxanes displaying this motif the dominant coconformer is the one in which the macrocycle sits onto the sec-ammonium station, regardless of the nature of the secondary station.6
The deprotonation of the ammonium center with a base switches off the recognition of the ring, thereby reversing the unbalanced distribution. Overall, it can be said that complete ring shuttling from one station to the other is achieved. This result requires (i) a large free energy difference between the two coconformations in either state, and (ii) the ability of the stimulus to change significantly such difference (Fig. 1a).
In 2008, Takata and coworkers showed that the sec-ammonium station of a rotaxane can be converted into a tert-ammonium moiety that exhibits a much lower affinity for DB24C8.8 This approach could be utilized to make rotaxanes with coconformational equilibria characterized by much lower ΔG° and a markedly different switching behaviour. We envisioned that the combination of low affinity tert-ammonium and triazolium stations should result in a bistable rotaxane where subtle variations in the energy of the ground state suffice to bias the coconformational equilibrium (Fig. 1b). To date, however, only one rotaxane comprising a tert-ammonium and a urea station that responds to chemical stimuli (Na+ ions or protonation/deprotonation) has been described.9
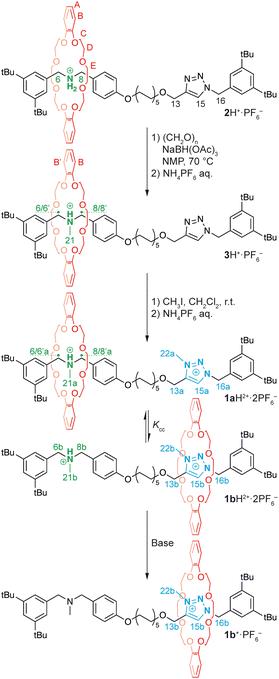
Herein, we report on the preparation and operation of a bistable molecular shuttle endowed with a tert-ammonium and a triazolium station (1aH2+, Scheme 1) in which the coconformational equilibrium is highly sensitive to the polarity of the medium. We also demonstrate that the relative distribution of the rings between the stations can be adjusted to different values by modifying the ion-pairing interactions between the positively charged stations and their counteranions. The position of the ring between the two stations can also be switched by classical acid/base treatment.
The synthesis of target rotaxane 1aH2+ is depicted in Scheme 1. The sec-ammonium [2]rotaxane 2H+·PF6− was synthesized by stoppering the pseudorotaxane complex formed between the DB24C8 and the axle equipped with a sec-ammonium recognition motif by copper-catalyzed azide–alkyne cycloaddition (CuAAC) (see the ESI†). In order to avoid simultaneous interaction with the two weak stations—tert-ammonium and triazolium—in the target rotaxane, a C10 spacer alkyl chain was introduced. The 1H NMR spectral features of 2H+·PF6− in CD2Cl2 are consistent with a [2]rotaxane structure where the DB24C8 encircles the dibenzyl ammonium moiety. The tert-ammonium station was installed by methylation of the sec-ammonium using a variation of the Eschweiler-Clarke reaction.8 Reaction of 2H+·PF6− with paraformaldehyde and triacetoxyborohydride (20 equivalents each) in N-methyl-2-pyrrolidone at 70 °C, followed by anion exchange, allowed the isolation of 3H+·PF6− in good yield. Methylation of the ammonium moiety was confirmed by the appearance, in the 1H NMR spectrum, of a doublet at 2.93 ppm corresponding to the methyl group (H21) bound to the nitrogen. Due to the loss of the symmetry plane containing the axle, combined with the slow nitrogen inversion on the NMR timescale,10 the methylene protons become diastereotopic. Specifically, H6 and H6’, as well as H8 and H8’, resonate at different frequencies and display scalar coupling. Additionally, due to the constrains of the mechanical bond, HB splits into two sets of resonances (HB and HB′), syn and anti with respect to the NMe group (see the ESI†). The signals associated to the triazole portion of the molecule remain mostly unchanged, indicating that, even upon methylation, the majority of rings encircle the ammonium station. ROESY data also confirm this conclusion. It is worth noting that this rotaxane equipped with a tert-ammonium and a neutral triazole station can also function as a pH-operated molecular switch (see the ESI†).
The target rotaxane 1H2+·2PF6− was obtained by regioselective methylation of the triazole moiety of rotaxane 3H+·PF6− with iodomethane, followed by anion exchange.6,11 The 1H NMR spectrum of 1H2+·2PF6− in CD2Cl2 shows that each signal exists as two distinct sets of resonances in a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio. In particular, the triazolium singlet (H15) displays two distinct resonances at 9.37 and 8.23 ppm associated with the encircled and free triazolium stations, respectively (Fig. 2a). The same ratio is observed for the two resonances associated with the methyl group of the tert-ammonium moiety (H21, Fig. 2a). The two sets of resonances can thus be attributed to the two distinct coconformers of rotaxane 1H2+, in which the ring encircles either the tertiary ammonium (1aH2+·2PF6−) or the triazolium (1bH2+·2PF6−) station (Scheme 1).
5 ratio. In particular, the triazolium singlet (H15) displays two distinct resonances at 9.37 and 8.23 ppm associated with the encircled and free triazolium stations, respectively (Fig. 2a). The same ratio is observed for the two resonances associated with the methyl group of the tert-ammonium moiety (H21, Fig. 2a). The two sets of resonances can thus be attributed to the two distinct coconformers of rotaxane 1H2+, in which the ring encircles either the tertiary ammonium (1aH2+·2PF6−) or the triazolium (1bH2+·2PF6−) station (Scheme 1).
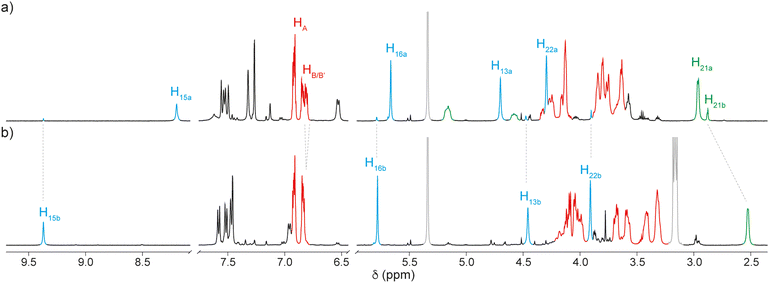 | ||
Fig. 2 Partial 1H NMR spectra (500 MHz, CD2Cl2, 298 K) of a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture of rotaxane 1aH2+·2PF6− and 1bH2+·2PF6− (a) and the same sample after addition of 1 equivalent of tBu4NCl displaying mainly 1bH2+·2Cl− (b). Signals are color-coded and numbered according to Scheme 1. The residual solvent and the tetrabutylammonium signals are color-coded in grey. 5 mixture of rotaxane 1aH2+·2PF6− and 1bH2+·2PF6− (a) and the same sample after addition of 1 equivalent of tBu4NCl displaying mainly 1bH2+·2Cl− (b). Signals are color-coded and numbered according to Scheme 1. The residual solvent and the tetrabutylammonium signals are color-coded in grey. | ||
The presence of both coconformers points to a similar affinity of the DB24C8 for the two stations. In the low polarity CD2Cl2 solvent with the non-coordinating PF6− counterion, 95% of the rings encircle the ammonium station; this figure corresponds to a coconformational equilibrium constant (Kcc = [1bH2+]/[1aH2+]) of about 0.05 and a free energy difference between the two coconformers of less than 2 kcal mol−1. On the basis of this small ΔG°, we hypothesized that even minor perturbations of the medium (e.g. the addition of a different anion or a change in solvent polarity) could affect significantly the population of the two coconformers. We initially investigated the influence of solvent polarity on the coconformational equilibrium. 1H NMR spectra of rotaxane 1aH2+·2PF6− were recorded in acetone-d6, a solvent remarkably more polar than CD2Cl2. Spectra at 298 K revealed significant line broadening indicative of a fast dynamics of exchange between the two stations on the NMR timescale, most likely due to the lower association of the ring to the cationic stations in highly polar solvents. The chemical shift of protons H15 indicates that in such a polar environment the majority of the rings encircle the triazolium station (see the ESI†). To accurately determine the relative population of the two coconformers, the temperature was lowered to 223 K to obtain a slow exchange process on the NMR timescale. In these conditions, the [1bH2+·2PF6−]/[1aH2+·2PF6−] ratio is 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, corresponding to a Kcc of about 2.3 (ΔG° = –0.5 kcal mol−1, see the ESI†).
30, corresponding to a Kcc of about 2.3 (ΔG° = –0.5 kcal mol−1, see the ESI†).
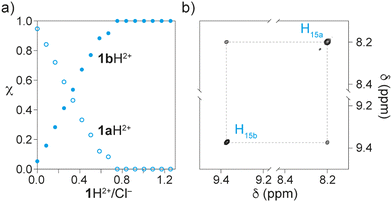
In a second series of experiments, chloride anions, which are known to pair with the ammonium site,7,12 were added to bias the equilibrium towards the triazolium station in CD2Cl2. We measured the relative population of the two stations at increasing concentration of tetrabutylammonium chloride. To our delight, all distributions between the two stations, ranging from 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to less than 1
5 to less than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 ammonium/triazolium (Fig. 2b), could be obtained, as shown by the relative integration of the signals for the free and complexed triazolium station at 8.23 and 9.37 ppm, respectively (Fig. 3a). Moreover, a complete shift of the equilibrium towards 1bH2+ was reached upon the addition of about one equivalent of Cl−, suggesting that ring shuttling takes place as a consequence of the competing ion-pairing equilibrium (Fig. 3a).12 Finally, the NH+ proton becomes strongly deshielded, shifting from 7.6 ppm to 12.7 in the presence of chloride anions, while the formation of a tight ion pair between the ammonium and the chloride is indicated by the upfield shift of H21b. Interestingly, the absence of hydrogen bonding between the DB24C8 ring and the ammonium NH+ restores the fast nitrogen inversion on the NMR timescale. The diastereotopic CH2 signals become isochronous and protons HB of the ring are magnetically equivalent. These observations confirm that the ring has moved from the ammonium to the triazolium station.13 The identity of 1bH2+·2Cl− was unambiguously proven by deprotonating the ammonium station: the deprotonated rotaxane 1b+·PF6− is afforded, in which the rings are located around the triazolium station (see the ESI†). The presence of a single coconformation is confirmed by the presence of sharp resonances for all protons. Comparison of the 1H NMR spectra of 1b+·PF6− and 1bH2+·2Cl− showed remarkably similar spectral features. In particular, the signal at 9.37 ppm is associated with the complexed triazolium station, while deprotonation of the ammonium was indicated by the disappearance of the doublet at 2.9 ppm and the appearance of a sharp singlet at 2.2 ppm, consistent with the methyl group of a tertiary amine. This experiment also confirms the possibility to operate the system as a classical acid/base molecular switch.
99 ammonium/triazolium (Fig. 2b), could be obtained, as shown by the relative integration of the signals for the free and complexed triazolium station at 8.23 and 9.37 ppm, respectively (Fig. 3a). Moreover, a complete shift of the equilibrium towards 1bH2+ was reached upon the addition of about one equivalent of Cl−, suggesting that ring shuttling takes place as a consequence of the competing ion-pairing equilibrium (Fig. 3a).12 Finally, the NH+ proton becomes strongly deshielded, shifting from 7.6 ppm to 12.7 in the presence of chloride anions, while the formation of a tight ion pair between the ammonium and the chloride is indicated by the upfield shift of H21b. Interestingly, the absence of hydrogen bonding between the DB24C8 ring and the ammonium NH+ restores the fast nitrogen inversion on the NMR timescale. The diastereotopic CH2 signals become isochronous and protons HB of the ring are magnetically equivalent. These observations confirm that the ring has moved from the ammonium to the triazolium station.13 The identity of 1bH2+·2Cl− was unambiguously proven by deprotonating the ammonium station: the deprotonated rotaxane 1b+·PF6− is afforded, in which the rings are located around the triazolium station (see the ESI†). The presence of a single coconformation is confirmed by the presence of sharp resonances for all protons. Comparison of the 1H NMR spectra of 1b+·PF6− and 1bH2+·2Cl− showed remarkably similar spectral features. In particular, the signal at 9.37 ppm is associated with the complexed triazolium station, while deprotonation of the ammonium was indicated by the disappearance of the doublet at 2.9 ppm and the appearance of a sharp singlet at 2.2 ppm, consistent with the methyl group of a tertiary amine. This experiment also confirms the possibility to operate the system as a classical acid/base molecular switch.
From a kinetic point of view, the analysis of the 1H NMR spectra in CD2Cl2 revealed significant line broadening regardless of the relative population of the stations. This observation is indicative of an exchange equilibrium between the two coconformers that is intermediate-to-slow on the NMR timescale as confirmed also by exchange NMR (EXSY) experiments (see the ESI†). The in-phase cross-peaks between protons H15a and H15b are indicative of chemical exchange between 1aH2+ and 1bH2+ (Fig. 3b), which can occur by ring shuttling.
This combined set of data indicates that shuttling between the two chemically distinct stations occurs without the need to formally deactivate one of them and that fine tuning of the coconformational equilibrium constant can be achieved.
In conclusion, we reported the synthesis and operation of a rotaxane molecular switch equipped with two weak stations for a DB24C8 macrocycle. Due to the tiny energy difference between the two coconformers, the corresponding equilibrium is highly influenced by the medium (for example, changes in the solvent polarity or the presence of ions). Specifically, the nature and concentration of anions, capable of pairing with the positively charged tert-ammonium station, can be exploited to fine tune the distribution of the rings between the two stations. These findings open interesting possibilities to make mechanically interlocked molecules that can sense anions and probe environmental changes in solution and, in perspective, in materials. Bistable rotaxanes with chemically distinct stations but with no strong bias in the ring distribution could be useful also from the viewpoint of molecular machinery.
Financial support from the EU (H2020 ITN grant ‘‘Art- MoMa’’ no. 860434) is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.References
- (a) C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, Hoboken, 2016 CrossRef; (b) V. Balzani, A. Credi and M. Venturi, Molecular Devices and Machines - Concepts and Perspectives for the Nanoworld, Wiley-VCH, Weinheim, 2008 CrossRef.
- (a) S. Silvi, M. Venturi and A. Credi, J. Mater. Chem., 2009, 19, 2279–2294 RSC; (b) A. C. Fahrenbach, C. J. Bruns, H. Li, A. Trabolsi, A. Coskun and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 482–493 CrossRef CAS PubMed; (c) M. N. Chatterjee, E. R. Kay and D. A. Leigh, J. Am. Chem. Soc., 2006, 128, 4058–4073 CrossRef CAS PubMed.
- (a) P. Wu, B. Dharmadhikari, P. Patra and X. Xiong, Nanoscale Adv., 2022, 4, 3418–3461 RSC; (b) J. Berná, D. Leigh, M. Lubomska, S. M. Mendoza, E. M. Perez, P. Rudolf, G. Teobaldi and F. Zerbetto, Nat. Mater., 2005, 4, 704–710 CrossRef PubMed; (c) M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed; (d) K. M. Bak, K. Porfyrakis, J. J. Davis and P. D. Beer, Mater. Chem. Front., 2020, 4, 1052–1073 RSC; (e) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS PubMed; (f) E. Moulin, L. Faour, C. C. Carmona-Vargas and N. Giuseppone, Adv. Mater., 2020, 32, 1906036 CrossRef CAS PubMed; (g) M. Baroncini, S. Silvi and A. Credi, Chem. Rev., 2020, 120, 200–268 CrossRef CAS PubMed.
- (a) G. Ragazzon, A. Credi and B. Colasson, Chem. – Eur. J., 2017, 23, 2149–2156 CrossRef CAS PubMed; (b) G. Ragazzon, C. Schäfer, P. Franchi, S. Silvi, B. Colasson, M. Lucarini and A. Credi, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9385 CrossRef CAS PubMed.
- (a) P. R. Ashton, P. J. Campbell, P. T. Glink, D. Philp, N. Spencer, J. F. Stoddart, E. J. T. Chrystal, S. Menzer, D. J. Williams and P. A. Tasker, Angew. Chem., Int. Ed. Engl., 1995, 34, 1865–1869 CrossRef CAS; (b) D. Thibeault and J.-F. Morin, Molecules, 2010, 15, 3709–3730 CrossRef CAS PubMed.
- (a) F. Coutrot, ChemistryOpen, 2015, 4, 556–576 CrossRef CAS PubMed; (b) H. V. Schroder and C. A. Schalley, Chem. Sci., 2019, 10, 9626–9639 RSC; (c) P. R. Ashton, R. Ballardini, V. Balzani, I. Baxter, A. Credi, M. C. T. Fyfe, M. Teresa Gandolfi, M. Gómez-López, M.-V. Martínez-Díaz, A. Piersanti, N. Spencer, J. F. Stoddart, M. Venturi, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 1998, 120, 11932–11942 CrossRef CAS; (d) E. Busseron and F. Coutrot, J. Org. Chem., 2013, 78, 4099–4106 CrossRef CAS PubMed.
- The binding constants between crown ether and dibenzylammonium in CH2Cl2 are 104–106 M−1. The actual value is highly dependent on the molecular structure and on the presence of ion-pairing equilibria and can vary largely. For thorough studies on these aspects see: (a) J. W. Jones and H. W. Gibson, J. Am. Chem. Soc., 2003, 125, 7001–7004 CrossRef CAS PubMed; (b) H. W. Gibson, J. W. Jones, L. N. Zakharov, A. L. Rheingold and C. Slebodnick, Chem. – Eur. J., 2011, 17, 3192–3206 CrossRef CAS PubMed; (c) P. R. Ashton, R. A. Bartsch, S. J. Cantrill, R. E. Hanes, S. K. Hickingbottom, J. N. Lowe, J. A. Preece, J. F. Stoddart, V. S. Talanov and Z.-H. Wang, Tetrahedron Lett., 1999, 40, 3661–3664 CrossRef CAS; (d) J. Groppi, L. Casimiro, M. Canton, S. Corra, M. Jafari-Nasab, G. Tabacchi, L. Cavallo, M. Baroncini, S. Silvi, E. Fois and A. Credi, Angew. Chem., Int. Ed., 2020, 59, 14825–14834 CrossRef CAS PubMed.
- (a) K. Nakazono, S. Kuwata and T. Takata, Tetrahedron Lett., 2008, 49, 2397–2401 CrossRef CAS; (b) S. Suzuki, K. Nakazono and T. Takata, Org. Lett., 2010, 12, 712–715 CrossRef CAS PubMed.
- T.-W. Lu, C.-F. Chang, C.-C. Lai and S.-H. Chiu, Org. Lett., 2013, 15, 5742 CrossRef CAS PubMed.
- (a) J. M. Lehn, Dynamic Stereochemistry. Fortschritte der Chemischen Forschung, Springer, Berlin, Heidelberg, 1970, vol 15/3, pp. 311–377 Search PubMed; (b) M. J. S. Dewar and W. B. Jennings, J. Am. Chem. Soc., 1971, 93, 401–403 CrossRef CAS.
- F. Coutrot and E. Busseron, A New Glycorotaxane Molecular Machine Based on an Anilinium and a Triazolium Station, Chem. – Eur. J., 2008, 14, 4784–4787 CrossRef CAS PubMed.
- (a) M. Montalti and L. Prodi, Chem. Commun., 1998, 1461–1462 RSC; (b) M. Clemente-León, C. Pasquini, V. Hebbe-Viton, J. Lacour, A. Dalla Cort and A. Credi, Eur. J. Org. Chem., 2006, 105–112 CrossRef.
- Similar results were obtained upon addition of an excess of p-toluensulfonate anion.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04483a |
| This journal is © The Royal Society of Chemistry 2023 |