Open Access Article
Open Access ArticleCarborane-based heteromolecular extended networks driven by directional C–Te⋯N chalcogen bonding interactions†
Maxime
Beau
a,
Olivier
Jeannin
a,
Marc
Fourmigué
 *a,
Emmanuel
Aubert
b,
Enrique
Espinosa
b,
Sunhee
Lee
c,
Won-Sik
Han
*a,
Emmanuel
Aubert
b,
Enrique
Espinosa
b,
Sunhee
Lee
c,
Won-Sik
Han
 c and
Ie-Rang
Jeon
c and
Ie-Rang
Jeon
 *a
*a
aUniv Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes), Campus de Beaulieu, Rennes 35000, France. E-mail: marc.fourmigue@univ-rennes.fr; ie-rang.jeon@univ-rennes.fr
bCRM2, Université de Lorraine, CNRS, Nancy, F-54500, France
cDepartment of Chemistry, Seoul Women's University, Seoul 01797, Republic of Korea
First published on 17th October 2023
Abstract
We demonstrate that o-closo-(TeMe)2carborane directs, in the presence of linear ditopic neutral Lewis bases, the formation of co-crystals with 1D extended supramolecular networks. Specifically, the network formation is systematically stabilized by short and quasi-linear C–Te⋯N chalcogen-bonding (ChB) interactions. In sum, we report efficient carborane-based tectons to rationally design high-dimensional neutral heteromolecular networks.
Icosahedral carboranes (dicarba-closo-dodecaboranes) are well-known for their rich boron content, versatile chemistry, aromaticity, and chemical and thermal stability.1–3 Owing to such intriguing properties, these molecules have been considered as an ideal building block in the field of medicinal chemistry and material science, with their uses in applications such as boron neutron capture therapy,4 biomedicine,5 molecular motors,6 gas sorption,7 and luminescent devices.8 While these materials are often conceived exploiting synthetic organic chemistry and coordination chemistry, the crystal engineering approach to build a supramolecular extended architecture has yet to be explored, regarding the proven ability of crystal engineering in materials science to finely tune the properties and even to engender new functionalities.9
Carboranes feature acidic protons on carbon atoms, acting as hydrogen bond (HB) donors as proven by structural investigations of the molecule itself or of their solvated phases.10 It is also proven that periodination of the carborane increases the acidity of the CH protons, which then act as a stronger hydrogen bond donor.11 Despite these features, their employment for crystal engineering has been largely limited to discrete assemblies. The first reported examples are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
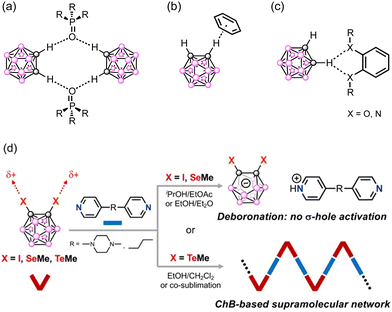
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystals of o-, m-, and p-carboranes with hexamethylphosphoramide (Scheme 1a), showing varied structures in the solid state owing to the distinctive arrangement of CH sites.12 Later, o-carboranes were also investigated for host–guest systems with aza crown ethers as a flexible host molecule13 or with cavitand molecules, such as cyclotriveratrylene14 and calixarenes.15 In the latter, the guest inclusion was stabilized mainly through the C–H⋯π hydrogen bonding (HB) interaction (Scheme 1b).16 In some cases, the CH proton of carborane is engaged in bifurcated C–H⋯O or C–H⋯N HB interactions with a chelating 1,2-dimethoxybenzene moiety or 1,10-phenanthroline (Scheme 1c).17 Of the small number of reported examples, the co-crystal of the o-carborane moiety is essentially limited to the discrete supramolecular assemblies, with only one exception of an extended network mediated by bifurcated hydrogen bonds, whose final structure is almost impossible to predict in advance.16c This fact might be attributed to the weaker directionality and predictability of the CH proton of the carborane as well as the inadequate geometrical shape of the acceptor.
1 co-crystals of o-, m-, and p-carboranes with hexamethylphosphoramide (Scheme 1a), showing varied structures in the solid state owing to the distinctive arrangement of CH sites.12 Later, o-carboranes were also investigated for host–guest systems with aza crown ethers as a flexible host molecule13 or with cavitand molecules, such as cyclotriveratrylene14 and calixarenes.15 In the latter, the guest inclusion was stabilized mainly through the C–H⋯π hydrogen bonding (HB) interaction (Scheme 1b).16 In some cases, the CH proton of carborane is engaged in bifurcated C–H⋯O or C–H⋯N HB interactions with a chelating 1,2-dimethoxybenzene moiety or 1,10-phenanthroline (Scheme 1c).17 Of the small number of reported examples, the co-crystal of the o-carborane moiety is essentially limited to the discrete supramolecular assemblies, with only one exception of an extended network mediated by bifurcated hydrogen bonds, whose final structure is almost impossible to predict in advance.16c This fact might be attributed to the weaker directionality and predictability of the CH proton of the carborane as well as the inadequate geometrical shape of the acceptor.
 | ||
| Scheme 1 Examples of hydrogen bonding interactions involving o-carborane (a)–(c) and chalcogen bonding (ChB)-based crystal engineering involving o-carborane (d, this work). | ||
In targeting carborane-based supramolecular networks, the choice of carborane-based tecton is crucial to provide more than one directional interaction with a rigid multidentate acceptor molecule. In this context, the incorporation of more robust and directional supramolecular interactions would be ideal. Recently, halogen bonding (XB)18 and chalcogen bonding (ChB)19,20 interactions have emerged as reliable and effective supramolecular interactions in the field of crystal engineering. In contrast to directional XB,21 the use of ChB in crystal engineering is challenging due to the weaker directionality related to the divalent character of a chalcogen atom. Nevertheless, design strategies of using one electron withdrawing substituent to lift the degeneracy of two σ-holes on a chalcogen atom have been proven successful to recover predictability as in XB.22
Along this line, we have recently designed o-carboranes involving an iodo- or a chalcogenomethyl substituent on each skeletal carbon vertice, o-closo-X2C2B10H10 (X = I (1), SeMe (2), and TeMe (3); Scheme 2a), and have shown their efficient XB and ChB interactions with halide anions.23 The electrostatic potential surface (ESP) calculation showed a highly activated σ-hole in the prolongation of the Ccarborane–X bond, owing to the strong electron withdrawing effect by the carborane unit. However, while the negative charge of a halide leads to strong XB or ChB interactions, their spherical shape hinders any predictability for the outcome of the co-crystal structures. By introducing a linear ditopic Lewis base, one can therefore expect two highly directional σ-holes diverging from the carborane unit to allow the formation of a controlled supramolecular 1D zig-zag chain network (Scheme 1d). Herein, we investigate the ability of o-closo-X2C2B10H10, 1–3,23 to act as XB and ChB donors in heteromolecular network assemblies through co-crystallization with such linear, neutral ditopic Lewis bases, 1,2-di(4-pyridyl)ethane and 1,4-di(4-pyridyl)piperazine (bpe and bipy-pip, respectively, Scheme 2b).
Slow evaporation of iPrOH/EtOAc solution containing an equimolar amount of 1 and bpe provided colourless prism-shaped crystals. Surprisingly, structural analyses show the deboronation of 1 to afford an anionic nido-7,8-I2C2B9H10 species, and consequently the protonation of bpe to a Hbpe+ cation, to yield [nido-7,8-I2C2B9H10]−(Hbpe)+ (Scheme 1d, Fig. 1a). Indeed, such nucleophilic attack on the electron-poor boron has been reported to occur quite commonly under strongly basic conditions (KOH/EtOH or organic amines) or in the presence of F−.24 The presence of the electron-withdrawing halogen substituents on the C–carbon vertices is known to accelerate the deboronation reaction even under mild conditions such as DMSO, MeOH, or Et2O.25
To avoid electron-withdrawing iodine substituents that facilitate the deboronation, we moved to the selenomethyl-substituted carborane, 2. Slow evaporation of EtOH/Et2O solution containing 2 and 1,4-di(4-pyridyl)piperazine (bipy-pip) provided colourless needle-shaped single crystals. Structural analyses again indicate the deboronation to obtain a salt, [nido-7,8-(SeMe)2C2B9H10]−(Hbipy-pip)+ (Scheme 1d, Fig. 1b).
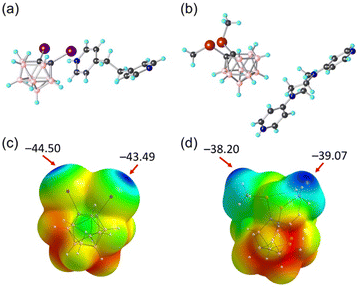
None of the obtained nido-carborane salts feature any σ-hole interaction. Instead, the anionic nido-carboranes likely engage in cage−⋯cage− and cage−⋯π interactions (Fig. S1 and S2, ESI†).26 In addition, the protonated cations, Hbpe+ and Hbipy-pip+, are self-assembled to form a 1D chain through N–H⋯N hydrogen bonding (Fig. S3, ESI†). To build a supramolecular network driven by efficient XB or ChB interaction, the key requirements are strongly activated σ-holes on the carborane-substituted halogen/chalcogen atoms and strong ditopic Lewis bases. In carborane-based systems, however, the presence of a strong Lewis base favours the deboronation reaction and the resulting anionic nido-carborane will not activate anymore the σ-hole. The loss of strongly charge-depleted region on the I and Se atoms upon deboronation is indeed confirmed by the ESP calculations, showing negative potential values of VS,max = −44.5 and −39.07 kcal mol−1 for nido-7,8-I2C2B9H10 and nido-7,8-(SeMe)2C2B9H10, respectively (Fig. 1c and d). This is in stark contrast to VS,max = +36.4 and +27.5 kcal mol−1, observed for 1 and 2, respectively. It therefore turned out to be challenging to perform XB- or ChB-based crystal engineering with the carborane substituent, which is otherwise an excellent σ-hole activating group.
Given difficulties to obtain co-crystals with 1 and 2 despite different trials, we moved our attention to the TeMe-derivative, 3. Slow evaporation of EtOH/CH2Cl2 solution containing an equimolar amount of 3 and bipy-pip provided colourless plate-shaped single crystals of 3·(bipy-pip). It crystallizes in the monoclinic system, space group P21/m, with 3 located on a mirror plane and the bipy-pip on an inversion centre, affording a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. As shown in Fig. 2, the pyridine nitrogen atom engages in a ChB with the activated σ-hole of the Te atom. The Te⋯N contact distance of 2.834(9) Å is notably shorter than the sum of van der Waals radii, leading to a very small reduction ratio (RR) = 0.79. Moreover, the C–Te⋯N angle amounts to 171.3°, highlighting the short and almost linear ChB interaction. This gives rise to the formation of the sought-after supramolecular zig–zag chain structure where carborane units are bridged by bipy-pip molecules without deboronation.
1 stoichiometry. As shown in Fig. 2, the pyridine nitrogen atom engages in a ChB with the activated σ-hole of the Te atom. The Te⋯N contact distance of 2.834(9) Å is notably shorter than the sum of van der Waals radii, leading to a very small reduction ratio (RR) = 0.79. Moreover, the C–Te⋯N angle amounts to 171.3°, highlighting the short and almost linear ChB interaction. This gives rise to the formation of the sought-after supramolecular zig–zag chain structure where carborane units are bridged by bipy-pip molecules without deboronation.
To further probe the efficiency of 3 as a tecton favouring the formation of a carborane-based network, we tried co-crystallization with the bpe linker featuring a decreased Lewis base character. Despite our efforts, a similar solution co-crystallization technique did not afford a single crystal suitable for an X-ray diffraction study. To avoid the use of the polar solvents, which could favour the deboronation reaction and could hinder the co-crystallization, we used a solvent-free co-sublimation technique,27 where 3 and bpe are placed in a two-zone furnace under static vacuum. The co-sublimation successfully afforded pale-yellow prism-shaped crystals of 3·(bpe). It crystallizes in the monoclinic system, space group P21/n, with the asymmetric unit containing one molecule of 3 and two halves of bpe located on an inversion centre, giving a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. As shown in Fig. S4 (ESI†), ChB between the ditopic carborane donor and linear acceptor again favours the formation of a 1D structure, similar to 3·(bipy-pip). Due to the less basic character of bpe, the observed Te⋯N contacts (2.855(9), 2.88(1), and 2.883(8) Å for Te1A⋯N1, Te1B⋯N1, and Te2⋯N2, respectively)‡ are slightly longer than those in 3·bipy-pip (RR = 0.79 and 0.80). Nevertheless, the ChB interaction is again highly linear (∠C–Te⋯N = 169.4–178.1°).
1 stoichiometry. As shown in Fig. S4 (ESI†), ChB between the ditopic carborane donor and linear acceptor again favours the formation of a 1D structure, similar to 3·(bipy-pip). Due to the less basic character of bpe, the observed Te⋯N contacts (2.855(9), 2.88(1), and 2.883(8) Å for Te1A⋯N1, Te1B⋯N1, and Te2⋯N2, respectively)‡ are slightly longer than those in 3·bipy-pip (RR = 0.79 and 0.80). Nevertheless, the ChB interaction is again highly linear (∠C–Te⋯N = 169.4–178.1°).
Upon co-sublimation of 3 and bpe, a few colourless prism-shaped crystals of by-product have also been observed, which turned out to be mono-substituted TeMe-carborane 4, that was co-crystalized with bpe, forming 4·bpe (Fig. 3). Given the insignificant quantity of 4·bpe found in the co-crystallization tube, 4 is likely a minor by-product in the synthesis of 3 or thermally decomposed product in the vapor medium of the co-sublimation process. 4·bpe crystallizes in the triclinic system, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with the asymmetric unit involving one molecule of 4 and two halves of bpe located on an inversion centre, giving a 1
, with the asymmetric unit involving one molecule of 4 and two halves of bpe located on an inversion centre, giving a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal. As expected, the pyridine nitrogen is engaged in directional interaction with the Te atom, affording a very short Te⋯N contact (2.819(3) Å) with RR down to 0.78. The Te⋯N contact observed in 4·bpe, shorter than that observed in 3·bpe (2.883 vs. 2.819 Å), is also coherent with the optimized structures of 3 and 4 by periodic DFT calculations without considering temperature effects (2.646 vs. 2.629 Å; Fig. S5, ESI†). Indeed, ESP calculation on 4 shows VS,max = +36.5 kcal mol−1 (Fig. S6, ESI†), which is slightly deeper than the one previously found in 3 (34.4 kcal mol−1).23 The shorter Te⋯N contacts in 4·bpe can therefore be attributed convincingly to the deeper charge depletion of Te on the mono-substituted one.
1 co-crystal. As expected, the pyridine nitrogen is engaged in directional interaction with the Te atom, affording a very short Te⋯N contact (2.819(3) Å) with RR down to 0.78. The Te⋯N contact observed in 4·bpe, shorter than that observed in 3·bpe (2.883 vs. 2.819 Å), is also coherent with the optimized structures of 3 and 4 by periodic DFT calculations without considering temperature effects (2.646 vs. 2.629 Å; Fig. S5, ESI†). Indeed, ESP calculation on 4 shows VS,max = +36.5 kcal mol−1 (Fig. S6, ESI†), which is slightly deeper than the one previously found in 3 (34.4 kcal mol−1).23 The shorter Te⋯N contacts in 4·bpe can therefore be attributed convincingly to the deeper charge depletion of Te on the mono-substituted one.
To our surprise, the remaining CH proton in 4 is also engaged in a highly directional and non-bifurcated hydrogen bond with the pyridine nitrogen, which was reported to be unsuccessful with non-substituted o-carborane and ditopic Lewis bases.17 The observed C⋯N distance of 3.160(4) Å (H⋯N 2.087 Å), with the C–H⋯N angle of 164.0°, is indeed very short for a CH⋯X hydrogen bond, which is typically weak compared to normal hydrogen bonds involving OH or NH groups.28 Indeed the VS,max value estimated on the CH proton (32.5 kcal mol−1, Fig. S6, ESI†) is as strong as the one on the Te. Such CH hydrogen bonds are now generally recognized as an important complementary interaction to ultimately direct specific structural patterns.29 In 4·bpe, the concerted manifestation of the chalcogen- and CH hydrogen-bonds indeed favours the formation of the extended chain structure, as observed in the co-crystals with ditopic ChB donor tecton 3.
In conclusion, we have demonstrated here that a supramolecular synthetic approach successfully provides carborane-based extended networks based on ChB. Harnessing carborane-based tectons in such a supramolecular synthesis appeared to be very challenging due to the deboronation side reaction in the presence of strong Lewis bases, which serve as ChB acceptors. In order to circumvent the nucleophilic attack, the choice of a carborane-based ChB donor with a less electron withdrawing –TeMe group was essential. In conjunction with that, the elimination of the solvent was found to be another key parameter to consider. Owing to the strong Te activation through carborane-substitution, the observed C–Te⋯N ChB interaction is extremely short and highly linear. These results open perspectives toward the elaboration of carborane-based supramolecular materials, which could find their place in numerous applications where carboranes play an important role.
This work has been supported by the French National Research Agency Grants (ANR-17-ERC3-0003, ANR-22-CE07-0050), a PhD grant (to M. Beau) from Région Bretagne, and a research grant from Seoul Women's University (2023-0246). We thank the CDIFX in Rennes for the use of the X-ray diffractometer and the EXPLOR mesocenter for providing access to their computing facility (project 2021CPMXX2483).
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. J. Wedge and M. F. Hawthorne, Coord. Chem. Rev., 2003, 240, 111 CrossRef CAS.
- R. N. Grimes, Carboranes, Elservier, Amsterdam, 2011 Search PubMed.
- (a) J. Aihara, J. Am. Chem. Soc., 1978, 100, 3339 CrossRef CAS; (b) Z. F. Chen and R. King, Chem. Rev., 2005, 105, 3613 CrossRef CAS PubMed.
- G. Choi, I.-R. Jeon, H. Piao and J.-H. Choy, Adv. Funct. Mater., 2018, 28, 1704470 CrossRef.
- (a) J. F. Valliant, K. J. Guenther, A. S. King, P. Morel, P. Schaffer, O. O. Sogbein and K. A. Stephenson, Coord. Chem. Rev., 2002, 232, 173–230 CrossRef CAS; (b) R. L. Julius, O. K. Farha, J. Chiang, L. J. Perry and M. F. Hawthorne, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 4808–4813 CrossRef CAS PubMed.
- S. D. Karlen, H. Reyes, R. E. Taylor, S. I. Khan, M. F. Hawthorne and M. A. Garcia-Garibay, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14973 CrossRef CAS PubMed.
- O. K. Farha, A. M. Spokoyny, K. L. Mulfort, F. Hawthorne, C. A. Mirkin and J. T. Hupp, J. Am. Chem. Soc., 2007, 129, 12680 CrossRef CAS PubMed; Y.-S. Bae, O. K. Farha, A. M. Spokoyny, C. A. Mirkin, J. T. Hupp and R. Q. Snurr, Chem. Commun., 2008, 4135 RSC.
- J. Ochi, K. Tanaka and Y. Chujo, Angew. Chem., Int. Ed., 2020, 59, 9841 CrossRef CAS PubMed.
- G. R. Desiraju, J. Am. Chem. Soc., 2013, 135, 9952 CrossRef CAS PubMed.
- (a) G. Harakas, T. Vu, C. B. Knobler and M. F. Hawthorne, J. Am. Chem. Soc., 1998, 120, 25 CrossRef; (b) H. Lee, C. B. Knobler and M. F. Hawthorne, Chem. Commun., 2000, 2485 RSC; (c) G. Barberà, C. Viñas, F. Teixidor, G. M. Rosair and A. J. Welch, J. Chem. Soc., Dalton Trans., 2002, 3647 RSC.
- A. V. Puga, F. Teixidor, R. Sillanpää, R. Kivekäs, M. Arca, G. Barberà and C. Viñas, Chem. – Eur. J., 2009, 15, 9755 CrossRef CAS PubMed.
- M. G. Davidson, T. G. Hibbert, J. A. K. Howard, A. Mackinnon and K. Wade, Chem. Commun., 1996, 2285 RSC.
- P. D. Godfrey, W. J. Grigsby, P. J. Nicolas and C. L. Raston, J. Am. Chem. Soc., 1997, 119, 9283 CrossRef CAS.
- (a) R. J. Blanch, M. Williams, G. D. Fallon, M. G. Gardiner, R. Kaddour and C. L. Raston, Angew. Chem., Int. Ed. Engl., 1997, 36, 504 CrossRef CAS; (b) M. J. Hardie, P. D. Godfrey and C. L. Raston, Chem. – Eur. J., 1999, 5, 1828 CrossRef CAS.
- (a) M. J. Hardie and C. L. Raston, Eur. J. Inorg. Chem., 1999, 195 CrossRef CAS; (b) T. E. Clark, M. Makha, C. L. Raston and A. N. Sobolev, Dalton Trans., 2006, 5449 RSC; (c) M. Makha, C. L. Raston, A. N. Sobolev, L. Barbour and P. Turner, CrystEngComm, 2006, 8, 306 RSC; (d) T. E. Clark, M. Makha, A. N. Sobolev and C. L. Raston, Dalton Trans., 2008, 4855 RSC; (e) C. L. Raston and G. W. V. Cave, Chem. – Eur. J., 2004, 10, 279 CrossRef CAS PubMed.
- (a) R. J. Blanch, M. Williams, G. D. Fallon, M. G. Gardiner, R. Kaddour and C. L. Raston, Angew. Chem., Int. Ed. Engl., 1997, 36, 504 CrossRef CAS; (b) M. J. Hardie and C. L. Raston, Eur. J. Inorg. Chem., 1999, 195 CrossRef CAS; (c) M. J. Hardie, P. D. Godfrey and C. L. Raston, Chem. – Eur. J., 1999, 5, 1828 CrossRef CAS.
- M. J. Hardie and C. L. Raston, CrystEngComm, 2001, 39, 1 Search PubMed.
- (a) G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711 CrossRef CAS; (b) G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478 CrossRef CAS PubMed.
- C. B. Aakeroy, D. L. Bryce, G. R. Desiraju, A. Frontera, A. C. Legon, F. Nicotra, K. Rissanen, S. Scheiner, G. Terraneo, P. Metrangolo and G. Resnati, Pure Appl. Chem., 2019, 91, 1889 CrossRef CAS.
- (a) A. F. Cozzolino, I. Vargas-Baca, S. Mansour and A. H. Mahmoudkhani, J. Am. Chem. Soc., 2005, 127, 3184 CrossRef CAS PubMed; (b) P. C. Ho, P. Szydlowski, J. Sinclair, P. J. W. Elder, J. Kubel, C. Gendy, L. M. Lee, H. Jenkins, J. F. Britten, D. R. Morim and I. Vargas-Baca, Nat. Commun., 2016, 7, 11299 CrossRef CAS PubMed; (c) G. E. Garett, G. L. Gibson, R. N. Straus, D. S. Seferos and M. S. Taylor, J. Am. Chem. Soc., 2015, 137, 4126 CrossRef PubMed; (d) A. Kremer, A. Fermi, N. Biot, J. Wouters and D. Bonifazi, Chem. – Eur. J., 2016, 22, 5665 CrossRef CAS PubMed.
- (a) P. Metrangolo, G. Resnati, T. Pilati and S. Biella, Struct. Bonding, 2008, 126, 105 CrossRef CAS; (b) M. Fourmigué, Curr. Opin. Solid State Mater. Sci., 2009, 13, 36 CrossRef.
- (a) H.-T. Huynh, O. Jeannin and M. Fourmigué, Chem. Commun., 2017, 53, 8467 RSC; (b) A. Dhaka, O. Jeannin, I.-R. Jeon, E. Aubert, E. Espinosa and M. Fourmigué, Angew. Chem., Int. Ed., 2020, 132, 23789 CrossRef.
- M. Beau, S. Lee, S. Kim, W.-S. Han, O. Jeannin, M. Fourmigué, E. Aubert, E. Espinosa and I.-R. Jeon, Angew. Chem., Int. Ed., 2021, 60, 366 CrossRef CAS PubMed.
- (a) R. A. Wiesboeck and M. F. Hawthorne, J. Am. Chem. Soc., 1964, 86, 1642 CrossRef CAS; (b) V. I. Bregadze, Chem. Rev., 1992, 92, 209 CrossRef CAS.
- (a) Y. Xie, Y. Wang, J. Miao, C. Hu, Z. Zhang, Y. Cui and Y. Li, Eur. J. Inorg. Chem., 2017, 4559 Search PubMed; (b) C. L. Powell, M. Schulze, S. J. Black, A. S. Thomson and M. D. Threadgill, Tetrahedron Lett., 2007, 1251 CrossRef CAS.
- (a) D. Tu, J. Li, F. Sun, H. Yan, J. Poater and M. Solà, JACS Au, 2021, 1, 2047 CrossRef CAS PubMed; (b) D. Tu, H. Yan, J. Poater and M. Solà, Angew. Chem., Int. Ed., 2020, 59, 9018 CrossRef CAS PubMed.
- P. M. J. Szell, S. A. Gabriel, E. Caron-Poulin, O. Jeannin, M. Fourmigué and D. L. Bryce, Cryst. Growth Des., 2018, 18, 6227 CrossRef CAS.
- T. Steiner, New J. Chem., 1998, 1099 RSC.
- G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond, Oxford University Press, Oxford, UK, 1991 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic data, additional structure figures and calculation results, and crystallographic information files (CIFs). CCDC 2284097–2284101. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc04338j |
‡ The –Te1Me group is disordered over two positions with 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 occupancy. 48 occupancy. |
| This journal is © The Royal Society of Chemistry 2023 |