Open Access Article
Open Access ArticleFerrimagnetic and spin glass behaviour in SrMn2+3Ti4+14M3+4O38 (M = Ti and Fe) synthetic crichtonites†
José Luis
Rosas-Huerta
*a,
Ruiqi
Chen
ab,
Clemens
Ritter
c,
Oleg
Siidra
 b,
Marie
Colmont
b,
Marie
Colmont
 a and
Angel M.
Arévalo-López
a and
Angel M.
Arévalo-López
 *a
*a
aUnité de Catalyse et Chimie du Solide (UCCS) – UMR CNRS 8181, Université de Lille – Centrale Lille, Université Artois, ENSCL, Lille, F-59000, France. E-mail: joseluis.rosashuerta@univ-lille.fr; angel.arevalo-lopez@univ-lille.fr
bDepartment of Crystallography, Institute of Earth Sciences, St. Petersburg State University, University emb. 7/9, 199034, St. Petersburg, Russia
cInstitut Laue-Langevin, BP 156, Grenoble Cedex 38042, France
First published on 12th October 2023
Abstract
Novel SrMn3Ti14M4O38 (M = Ti and Fe) compounds with a crichtonite-type structure are reported herein. M = Ti shows a ferrimagnetic behavior at TN = 15 K, while M = Fe creates a ferromagnetic cluster-glass at Tf = 8 K via positional disorder. This family offers a promising magnetic playground.
Minerals have been the primary inspirational source for materials discoveries, and the ne plus ultra example is the perovskite family,1 showing one of the widest ranges of properties and structural variations, e.g. ferroelectricity, photovoltaic, superconducting or magnetic properties.1–3 Following these lessons and in the search for magnetic materials, this article focuses on the scarcely studied crichtonite-type family of compounds.
The crichtonite family can be represented with an AB3C18O38 simplified formula. This mineral group includes for instance loveringite, crichtonite, senaite, davidite, landauite, lindsleyite, or matiasite with A = Ca, Sr, Pb, U, Na, Ba and K, respectively.4–7
Natural crichtonites have been studied mostly in terms of their crystal structure, complex isomorphic substitutions and their hardness.5,7–10 Synthetic crichtonites have been observed as predominant or secondary phases in high pressure experiments,11–13 often coexisting with AM12O19 magnetoplumbites for the K(Ti13Cr4FeZrMg2)O38, Ba(Ti12Cr4Fe2ZrMg2)O38 and (Ba0.5K0.5)(Ti13Cr3.5FeZrMg2.5)O38 compositions prepared at 11–12 GPa and 1500–1600 °C.14 Solid-state, sol–gel or hydrothermal methods have also been applied successfully in the synthesis of BaMn3Ti18O38, Ca2Zn4Ti16O38, Ca(Ca,Mn,Ti)21O38, BiMg3V18O38 and SrFe3V18O38.6,15–18 The latter show either Ti3+/Ti4+ or V3+/V4+ mixed-valence oxidation states in the C site, and together with B = Mn2+ or Fe3+ open the possibility for the occurrence of complex magnetic behavior.
In this work we report two new compounds, SrMn3Ti18O38 (SMTO) and SrMn3Ti14Fe4O38 (SMTFO), which belong to the crichtonite family. Their crystal structures were characterized with X-ray (XRD) and neutron powder diffraction (NPD) along with their magnetic properties at low temperature. They illustrate the potential of this family group as a novel playground for magnetic materials with cations in different environments and oxidation states.
SMTO and SMTFO were synthesized by solid-state reaction under vacuum. Their respective precursors SrTi16.5O34 and SrTi14O29 were obtained from stoichiometric amounts of SrCO3 and TiO2 heated in air at 900 °C for 12 h. Afterwards, they were stoichiometrically mixed with MnO, Ti metal and TiO2 for SMTO and with MnO2, Fe and Fe2O3 for SMTFO. The powders were compressed into 80 mg pellets and placed into quartz tubes and sealed under vacuum. The synthesis temperature was increased to 950 °C in 3 h and held for 12 h; it was then raised to 1100 °C in 1.5 hours with a dwell time of 48 h. Finally, the samples were cooled to room temperature by turning off the furnace.
Initial powder XRD characterization shows that both compounds crystallize with the crichtonite-type structure (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), see Fig. S1 (ESI†). The SMTO phase shows a small amount of TiO2 precursor [7.2(4) wt%], while SMTFO is phase pure. High-resolution NPD was carried out at the Institut Laue-Langevin (ILL) in the D20 beamline with λ = 1.36 Å (118° take-off angle) for an accurate crystal structure determination since the different scattering factor between Ti (−3.43 fm), Mn (−3.73 fm) and Fe (9.45 fm) allows the identification of a possible cation ordering. NPD analyses were made through the Fullprof suite.19
), see Fig. S1 (ESI†). The SMTO phase shows a small amount of TiO2 precursor [7.2(4) wt%], while SMTFO is phase pure. High-resolution NPD was carried out at the Institut Laue-Langevin (ILL) in the D20 beamline with λ = 1.36 Å (118° take-off angle) for an accurate crystal structure determination since the different scattering factor between Ti (−3.43 fm), Mn (−3.73 fm) and Fe (9.45 fm) allows the identification of a possible cation ordering. NPD analyses were made through the Fullprof suite.19
Rietveld refinements of the NPD data are summarized in Table 1 and are shown in Fig. 1d and e. Both compounds crystallize in the desired crichtonite-type structure, with a small amount of unreacted TiO2 precursor (7.22 wt%) for SMTO as observed from XRD. The crichtonite structure has general formula XIIA1VIB1IVB22VIC16VIC26VIC36O38, where the roman numerals indicate the coordination with oxygen atoms. The large A1 position is occupied in this case by Sr2+, whereas Mn2+ is located at the B1 and B2 positions in octahedral and tetrahedral coordination, respectively. Ti3+(Fe3+) cations are located in the C1–3 octahedral positions (see Fig. 1a). The structure comprises two different T and O layers in a (TOT*)3 rhombohedral sequence with T* related to T by an inversion centre. The latter consists of C1–C2 octahedra sharing edges (Ti1 and Ti2 in Table 1) which form hexagonal rings linked together by B2 tetrahedra (Mn2) and A1 (Sr), as shown in Fig. 1b. Layer O involves twelve C3 (Ti3 and Fe3) octahedra describing a larger hexagonal unit with a B1 (Mn1) octahedron at the center, see Fig. 1c. The linkage between T and O layers is only through vertices while between T and T* is through Ti1–Ti2 edges. The cell parameters for SMTO (a = 10.4282(1) Å, c = 20.8410(3) Å, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) are larger in comparison with SMFTO (a = 10.4200(1) Å, c = 20.8147(4) Å) due to the different ionic radii and this suggests that Fe3+ (0.645 Å) substitutes Ti3+ (0.67 Å) and not Ti4+ (0.605 Å). Neutron diffraction refinements confirmed the preferential occupation of Fe into the Ti3 position after several trials considering Fe in the other available positions. This is also supported by bond valence sum (BVS) calculations as Ti1 and Ti2 positions confirm a 4+ oxidation state but a smaller ∼3.5 + /3.1+ for Ti3/Fe3. Thus, the nominal formula results in Sr2+Mn32+Ti144+Ti43+O38 and Sr2+Mn32+Ti144+Fe43+O38 for SMTO and SMFTO respectively, with a Ti3(Fe3) 4+
) are larger in comparison with SMFTO (a = 10.4200(1) Å, c = 20.8147(4) Å) due to the different ionic radii and this suggests that Fe3+ (0.645 Å) substitutes Ti3+ (0.67 Å) and not Ti4+ (0.605 Å). Neutron diffraction refinements confirmed the preferential occupation of Fe into the Ti3 position after several trials considering Fe in the other available positions. This is also supported by bond valence sum (BVS) calculations as Ti1 and Ti2 positions confirm a 4+ oxidation state but a smaller ∼3.5 + /3.1+ for Ti3/Fe3. Thus, the nominal formula results in Sr2+Mn32+Ti144+Ti43+O38 and Sr2+Mn32+Ti144+Fe43+O38 for SMTO and SMFTO respectively, with a Ti3(Fe3) 4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3+ mixed valence position in a 1
3+ mixed valence position in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio.
2 ratio.
| SMTO | SMTFO | |
|---|---|---|
| a Refined site occupancy factors for Ti3/Fe3 were 0.548(3)/0.451(3)%.Biso (Å2) were constrained by elements with Sr = 0.9(1)|1.3(2), Mn = 0.7(1)|0.7(1), Ti,Fe = 0.45(4)|1.13(7) and O = 0.59(2)|0.59(2) for SMTO|SMTFO. | ||
| a (Å) | 10.4282(1) | 10.4200(1) |
| c (Å) | 20.8410(3) | 20.8147(4) |
| c/a | 1.9985(1) | 1.9976(1) |
| Volume (Å3) | 1962.78(4) | 1957.17(5) |
| Crichtonite (wt%) | 92.8 (0.7) | 100.0 |
| TiO2 (wt%) | 7.2(0.4) | — |
| R wp (%) | 7.61 | 11.10 |
| R Bragg (%) | 2.43 | 5.36 |
| R exp (%) | 3.56 | 4.77 |
| χ 2 (%) | 4.57 | 5.38 |
| SMTO | |||||
|---|---|---|---|---|---|
| Wyckoff | x | y | z | BVS | |
| Sr1 | 3a | 0 | 0 | 0 | 1.83(6) |
| Mn1 | 6c | 0 | 0 | 1/2 | 1.87(8) |
| Mn2 | 3b | 2/3 | 1/3 | 0.6454(4) | 2.07(1) |
| Ti1 | 18f | 0.2394(7) | 0.3142(7) | 0.3985(2) | 4.08(4) |
| Ti2 | 18f | 0.3278(6) | 0.0841(7) | 0.3928(2) | 3.93(3) |
| Ti3 | 18f | 0.5222(5) | 0.3754(6) | 0.4970(3) | 3.52(3) |
| O1 | 18f | 0.1922(4) | 0.1324(4) | 0.4358(2) | 1.88(2) |
| O2 | 18f | 2/3 | 1/3 | 0.5450(3) | 2.12(2) |
| O3 | 18f | 0.7211(4) | 0.5409(3) | 0.6711(2) | 2.21(2) |
| O4 | 18f | 0.4009(4) | 0.4388(4) | 0.4494(1) | 2.12(2) |
| O5 | 18f | 0.3611(4) | 0.2612(4) | 0.5589(1) | 1.99(2) |
| O6 | 18f | 0.3647(4) | 0.2649(4) | 0.3411(1) | 1.84(2) |
| O7 | 6c | 0.4973(4) | 0.2039(5) | 0.4418(2) | 2.10(2) |
| SMTFO | |||||
|---|---|---|---|---|---|
| Wyckoff | x | y | z | BVS | |
| Sr1 | 3a | 0 | 0 | 0 | 1.788(8) |
| Mn1 | 6c | 0 | 0 | 1/2 | 1.98(1) |
| Mn2 | 3b | 2/3 | 1/3 | 0.6456(5) | 2.29(3) |
| Ti1 | 18f | 0.2446(1) | 0.3163(1) | 0.4014(3) | 4.10(6) |
| Ti2 | 18f | 0.3335(9) | 0.0867(1) | 0.3908(4) | 3.90(5) |
| Ti3/Fe3a | 18f | 0.5250(1) | 0.3740(2) | 0.4989(7) | 3.56/3.06(6) |
| O1 | 18f | 0.1923(5) | 0.1318(6) | 0.4375(2) | 1.78(4) |
| O2 | 18f | 2/3 | 1/3 | 0.5453(4) | 2.26(3) |
| O3 | 18f | 0.7202(7) | 0.5364(5) | 0.6709(3) | 2.04(3) |
| O4 | 18f | 0.4052(6) | 0.4378(7) | 0.4503(2) | 2.13(4) |
| O5 | 18f | 0.3570(6) | 0.2538(6) | 0.5594(2) | 1.95(4) |
| O6 | 18f | 0.3671(6) | 0.2704(6) | 0.3414(2) | 1.99(8) |
| O7 | 6c | 0.4939(6) | 0.2045(7) | 0.4426(2) | 2.09(3) |
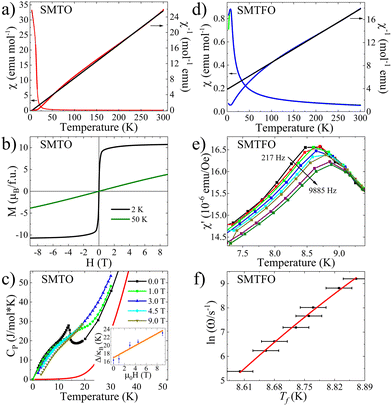
Magnetic properties were measured in a Dynacool-PPMS from Quantum Design. Temperature dependence of the magnetic susceptibility (χ) of SMTO shows a magnetic transition at TN = 15 K without any FC-ZFC divergence under 1 kOe applied field, see Fig. 2a. The fit of the reciprocal susceptibility to the Curie–Weiss law above 210 K suggests predominant ferromagnetic (FM) interactions with θC = 5.0(1) K. The calculated effective magnetic moment of 9.6(1)μB/f.u. is slightly reduced from the expected value of 10.82μB for 3 Mn2+ (S = 5/2) and 4 Ti3+ (S = 1/2); this could be due to the small amount of unreacted TiO2 and thus an error in the considered mass, but it could also be due to an orbital contribution from the t2g degeneracy of the 3d1 electrons in Ti3+ or to a reduced fitted range. Field dependent magnetization measurements at 2 and 50 K are shown in Fig. 2b. No hysteresis was detected, in accordance with the χ(T) behavior. At low temperature, a sharp increase of the magnetization can be observed and it can access 9μB with only 0.4 T and reaches 10.7μB at 9 T. NPD in the absence of a magnetic field confirms the ferrimagnetic (FiM) behavior of SMTO as described later.
Heat capacity measurements for SMTO show a sharp transition at TC = 15 K in agreement with the FiM transition, see Fig. 2c. After the phonon contribution was subtracted (see Fig. S2 for details, ESI†), the magnetic entropy released of 47.65 J mol−1 K−1 accounts for around 70% of the theoretical value S = R(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1) + 4
ln(2S + 1) + 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1)) = 67.74 J mol−1 K−1 for three Mn2+ (S = 5/2) and four Ti3+ (S = 1/2). A second feature appears at lower temperature, this flattens, broadens and shifts to higher temperatures as a magnetic field is applied. This was attributed to a Schottky anomaly and its contribution was calculated through the equation: CSch = NR(Δ/kBT)2eΔ/kBT/(1 + eΔ/kBT)2, where R is the ideal gas constant (R = 8.314 J mol−1 K−1) and Δ is the Schottky gap. Δ/kB showed a value of 16.38 K at 2 K. This value reflects a linear relation with the applied field, see inset of Fig. 2c, well-known for a magnetic Schottky term caused by orphan or magnetic impurities.20
ln(2S + 1)) = 67.74 J mol−1 K−1 for three Mn2+ (S = 5/2) and four Ti3+ (S = 1/2). A second feature appears at lower temperature, this flattens, broadens and shifts to higher temperatures as a magnetic field is applied. This was attributed to a Schottky anomaly and its contribution was calculated through the equation: CSch = NR(Δ/kBT)2eΔ/kBT/(1 + eΔ/kBT)2, where R is the ideal gas constant (R = 8.314 J mol−1 K−1) and Δ is the Schottky gap. Δ/kB showed a value of 16.38 K at 2 K. This value reflects a linear relation with the applied field, see inset of Fig. 2c, well-known for a magnetic Schottky term caused by orphan or magnetic impurities.20
For the SMTFO, the magnetic susceptibility presents a transition at 8.0(5) K with a FC-ZFC divergence, pointing towards a canted AFM or spin-glass behavior, see Fig. 2d. Curie–Weiss fit to the inverse susceptibility above 210 K results in θWC = −82.6(4) K supporting strong AFM interactions. The calculated effective moment of 13.1(1)μB per f.u. is reduced from the expected moment of 15.65μB for 3 Mn2+ (S = 5/2) and 4 Fe3+ (S = 5/2).
It also shows a large frustration index of f = |θ|/T = 10.25. NPD data collected at 2 K showed only short-range order correlations, see Fig. S3 (ESI†). AC-magnetic susceptibility was subsequently measured and revealed a spin-glass behavior, see Fig. 2e. The freezing temperature Tf varies according to the Vogel–Fulcher equation ω = ω0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ea/kB(Tf − T0)), where ω is the frequency, 1/ω0 is the intrinsic relaxation time (t0), Ea is the activation energy, kB is the Boltzmann constant and T0 is the Vogel–Fulcher temperature (Fig. 2f). The fittings were made considering the two extreme t0 values for spin-glass compounds 10−7s ≤ t0 ≤ 10−13 s, obtaining Ea/kB = 0.5 and 1 with T0 = 8.22 and 8.02 K, respectively. The very low activation energies in SMTFO reflect that Tf changes only 0.25 K when the frequency changes four orders of magnitude. For spin-glasses, the relative shift per frequency decade, δTf = ΔTf/[TfΔ
exp(Ea/kB(Tf − T0)), where ω is the frequency, 1/ω0 is the intrinsic relaxation time (t0), Ea is the activation energy, kB is the Boltzmann constant and T0 is the Vogel–Fulcher temperature (Fig. 2f). The fittings were made considering the two extreme t0 values for spin-glass compounds 10−7s ≤ t0 ≤ 10−13 s, obtaining Ea/kB = 0.5 and 1 with T0 = 8.22 and 8.02 K, respectively. The very low activation energies in SMTFO reflect that Tf changes only 0.25 K when the frequency changes four orders of magnitude. For spin-glasses, the relative shift per frequency decade, δTf = ΔTf/[TfΔ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (f)], is often calculated. In SMTFO we estimate δTf = 0.007, which is an order of magnitude smaller than for canonical spin-glass systems and thus suggests the formation of an FM cluster-glass state.21,22
(f)], is often calculated. In SMTFO we estimate δTf = 0.007, which is an order of magnitude smaller than for canonical spin-glass systems and thus suggests the formation of an FM cluster-glass state.21,22
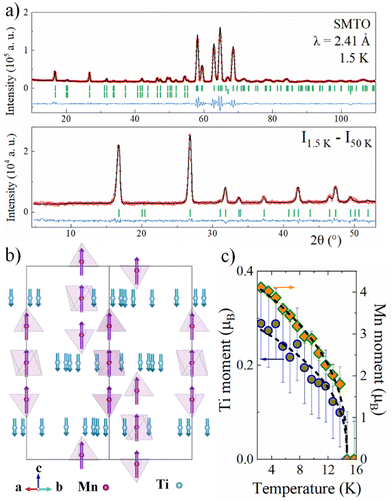
NPD of SMTO collected in D20 with λ = 2.41 Å at low temperature revealed the appearance of magnetic peaks below 15 K, see Fig. S4 (ESI†). These extra reflections coincide with the crystal structure and can be indexed with a k0 = [0 0 0] propagation vector (Γ point of the first Brillouin zone). ISODISTORT was used to determine the possible magnetic structures and space groups.23,24 The final Rietveld refinement was obtained with Fullprof with the mΓ1+ irreducible representation with the rhombohedral magnetic space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (#148.17). Fig. 3a shows the full pattern with both crystal and magnetic structures at 1.5 K along with the difference pattern between 1.5 and 50 K data to evidence the magnetic intensity gained. The magnetic structure shown in Fig. 3b consists of a ferrimagnetic arrangement between Mn and Ti spins along the z axis. The refined moments at 1.5 K converged to 4.11(2)μB and 0.30(2)μB for Mn2+ and Ti3+ respectively (Rmag = 3.02%, χ2 = 1.48). Attempts to refine only Mn moments resulted in fits of lower quality (Rmag = 4.93%, χ2 = 2.09), thus proving the contribution of the titanium to the model. The slightly reduced moments from the expected 2SMn = 5μB and 2STi = 1μB possibly reflect the disorder of the 3+/4+ mixed valency state in the Ti3 position. However, the ideal FiM disposition of 15μB from 3 Mn2+ with −4μB from 4 Ti3+ explains the values of 10.7μB at 9 T observed in the M vs. H curve at 2 K. The temperature evolutions of the Mn and Ti moments are shown in Fig. 3c, they follow a critical law μ(T) = μ1[1 − (T/TN)]β with μ1 = 0.29(3)μB and 4.37(5)μB for Ti and Mn, TN = 14.68(1) K and β = 0.39(1). The critical exponent is close to the theoretical value of β = 0.367 for a 3D Heisenberg magnet, which is appropriate for the structure described before. Observation of unsaturated moments is common in Mn2+ oxides as observed for instance in Mn2FeReO6 (3.5μB) or MnTiO3 (3.9μB).25,26
(#148.17). Fig. 3a shows the full pattern with both crystal and magnetic structures at 1.5 K along with the difference pattern between 1.5 and 50 K data to evidence the magnetic intensity gained. The magnetic structure shown in Fig. 3b consists of a ferrimagnetic arrangement between Mn and Ti spins along the z axis. The refined moments at 1.5 K converged to 4.11(2)μB and 0.30(2)μB for Mn2+ and Ti3+ respectively (Rmag = 3.02%, χ2 = 1.48). Attempts to refine only Mn moments resulted in fits of lower quality (Rmag = 4.93%, χ2 = 2.09), thus proving the contribution of the titanium to the model. The slightly reduced moments from the expected 2SMn = 5μB and 2STi = 1μB possibly reflect the disorder of the 3+/4+ mixed valency state in the Ti3 position. However, the ideal FiM disposition of 15μB from 3 Mn2+ with −4μB from 4 Ti3+ explains the values of 10.7μB at 9 T observed in the M vs. H curve at 2 K. The temperature evolutions of the Mn and Ti moments are shown in Fig. 3c, they follow a critical law μ(T) = μ1[1 − (T/TN)]β with μ1 = 0.29(3)μB and 4.37(5)μB for Ti and Mn, TN = 14.68(1) K and β = 0.39(1). The critical exponent is close to the theoretical value of β = 0.367 for a 3D Heisenberg magnet, which is appropriate for the structure described before. Observation of unsaturated moments is common in Mn2+ oxides as observed for instance in Mn2FeReO6 (3.5μB) or MnTiO3 (3.9μB).25,26
Summarizing, the change from a ferrimagnetic spin arrangement in SMTO (TN = 15 K) to a cluster-glass in SMTFO (Tf = 8 K) originates from cation disorder in the Ti3 position due to Fe3+ substitution. However, this opens the possibility for the crichtonite family to be considered as magnetic materials. The AB3C18O38 formula even further simplified to AM21O38 is in close relation to the industrially important M- and W-hexaferrites, i.e. AM12O19 and AM18O27.27 The mineral family shows a great chemical variety and further directed cationic substitutions may show the full potential as a magnetic playground.
We thank the ILL for beamtime provided at D20 (DOI:https://doi.org/10.5291/ILL-DATA.5-31-2921). A. M. A. L. thanks the ANR-AMANTS project (19-CE08-0002-01). J. L. R. H. thanks the SECTEI/098/2022-Mexico for the post-doctoral fellowship. R. C. thanks Centrale Lille for a PhD scholarship. The Chevreul Institute (FR 2638), Region Hauts-de-France, and FEDER are acknowledged for funding the X-ray diffractometers and the PPMS magnetometer.
Conflicts of interest
There are no conflicts to declare.References
- R. J. D. Tilley, Perovskites: Structure-Property relationships, Wiley, 2016, pp. 327 Search PubMed.
- R. H. Mitchell, Perovskites: Modern and Ancient, Almax Press, 2002, pp. 318 Search PubMed.
- N. S. Arul and V. D. Nithya, Revolution of Perovskite, Springer, 2020, pp. 320 Search PubMed.
- B. M. Gatehouse, I. E. Grey, I. H. Campbell and P. Kelly, Am. Mineral., 1978, 63(1–2), 28 Search PubMed.
- P. R. Kelly, I. H. Campbell, I. E. Grey and B. M. Gatehouse, Can. Mineral., 1979, 17, 635 Search PubMed.
- R. C. Peterson and I. E. Grey Csiro, Can. Mineral., 1995, 33(5), 1083 Search PubMed.
- R. K. Rastsvetaeva, S. M. Aksenov, N. V. Chukanov and L. A. D. Menezes, Dokl. Chem., 2014, 455, 53 CrossRef.
- S. J. Mills, L. Bindi, M. Cadoni, A. R. Kampf, M. E. Ciriotti and G. Ferraris, Eur. J. Mineral., 2012, 24(6), 1061 CrossRef.
- L. A. D. Menezes Filho, N. V. Chukanov, R. K. Rastsvetaeva, S. M. Aksenov, I. V. Pekov, M. L. S. C. Chaves, R. P. Richards, D. Atencio, P. R. G. Brandão, R. Scholz, K. Krambrock, R. L. Moreira, F. S. Guimarães, A. W. Romano, A. C. Persiano, L. C. A. de Oliveira and J. D. Ardisson, Mineral. Mag., 2015, 79(2), 269 CrossRef.
- P.-A. Wülser, N. Meisser, J. Brugger, K. Schenk, S. Ansermet, M. Bonin and F. Bussy, Eur. J. Mineral., 2006, 17(6), 933 CrossRef.
- V. Butvina, A. Spivak, T. Setkova and O. Safonov, Minerals, 2023, 13(2), 192 CrossRef.
- W. L. Gong, R. C. Ewing, L. M. Wang and H. S. Xie, MRS Online Proc. Libr., 1994, 353, 807 CrossRef.
- T. H. Green and N. J. Pearson, Mineral. Mag., 1987, 51(359), 145–149 CrossRef.
- J. Konzett, H. Yang and D. J. Frost, J. Petrol., 2005, 46(4), 749 CrossRef.
- F. Zhao, Z. Yue, J. Pei, Z. Gui and L. Li, J. Solid State Chem., 2006, 179(6), 1720 CrossRef.
- R. C. Peterson, I. E. Grey, L. M. D. Cranswick and C. Li, Can. Mineral., 1998, 36, 763 Search PubMed.
- S. Uma and A. W. Sleight, J. Solid State Chem., 2002, 164(1), 138 CrossRef.
- T. M. Smith Pellizzeri, C. D. McMillen, Y. Wen, G. Chumanov and J. W. Kolis, Eur. J. Inorg. Chem., 2019, 4838 Search PubMed.
- J. Rodríguez-Carvajal, Physica B, 1993, 192(1–2), 55 CrossRef.
- T. Zhu, B. Zhu, O. Mentré, S. Lee, D. Chen, Y. Jin, W. Zhu, Á. M. Arévalo-López, C. Minaud, K. Y. Choi and M. Lü, Chem. Mater., 2023, 35, 3951 CrossRef.
- N. Marcano, P. A. Algarabel, L. F. Barquín, J. P. Araujo, A. M. Pereira, J. H. Belo, C. Magén, L. Morellón and M. R. Ibarra, Phys. Rev. B: Condens. Matter Mater. Phys., 2019, 99, 054419 CrossRef.
- T. Klimczuk, H. W. Zandbergen, Q. Huang, T. M. McQueen, F. Ronning, B. Kusz, J. D. Thompson and R. J. Cava, J. Phys.: Condens. Matter, 2009, 21, 105801 CrossRef PubMed.
- H. T. Stokes, D. M. Hatch and B. J. Campbell, ISODISTORT, ISOTROPY Software Suite, https://iso.byu.edu Search PubMed.
- B. J. Campbell, H. T. Stokes, D. E. Tanner and D. M. Hatch, J. Appl. Crystallogr., 2006, 39, 607 CrossRef.
- A. M. Arevalo-Lopez, G. McNally and J. P. Attfield, Angew. Chem., Int. Ed., 2015, 54, 12074 CrossRef PubMed.
- A. M. Arevalo-Lopez and J. P. Attfield, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 104416 CrossRef.
- T. Kimura, Annu. Rev. Condens. Matter Phys., 2012, 3(93), 110 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04336c |
| This journal is © The Royal Society of Chemistry 2023 |