Open Access Article
Open Access Article1,4-Dimethoxynaphthalene-2-methyl (‘DIMON’), an oxidatively labile protecting group for synthesis of polyunsaturated lipids†
Jay
Tromans
a,
Bian
Zhang
b and
Bernard T.
Golding
 *a
*a
aSchool of Natural & Environmental Sciences – Chemistry, Bedson Building, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: bernard.golding@ncl.ac.uk
bBiBerChem Research Ltd, The Biosphere, Draymans Way, Newcastle Helix, Newcastle upon Tyne, NE4 5BX, UK
First published on 23rd October 2023
Abstract
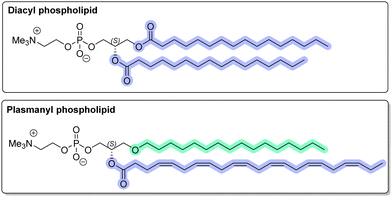
A new benzyl-type protecting group (1,4-dimethoxynaphthalene-2-methyl, ‘DIMON’) for hydroxyl functions can be selectively removed under oxidative conditions without damaging polyunsaturated fatty acyl groups. Its application is shown by the first synthesis of an ether (plasmanyl) phospholipid containing the docosa-(4Z,7Z,10Z,13Z,16Z,19Z)-hexaenoyl group.
Among the components of the human lipidome are alkyl-glycerophospholipids, which contain an alkyl group at the sn-1 position and an acyl group at the sn-2 position (so-called ether or plasmanyl phospholipids) in contrast to the familiar diacylglycerols and their corresponding phospholipids (Fig. 1).1,2 Much interest is centred on those molecules where the fatty acyl moiety is derived from the polyunsaturated arachidonic acid (20
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) or docosahexaenoic acid (22
4) or docosahexaenoic acid (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6).3 We now report the first scalable synthesis of this type of ether phospholipid, thus making these molecules readily available for biological studies and potential medical applications.
6).3 We now report the first scalable synthesis of this type of ether phospholipid, thus making these molecules readily available for biological studies and potential medical applications.
A common impediment to the use of benzyl-type protecting groups in organic synthesis, notably for glycerolipids, is that their removal under oxidative, reductive or acidic conditions is incompatible with polyunsaturated fatty acyl groups. For example, although 4-methoxybenzyl (PMB) can be used for protection and deprotection in the presence of an oleoyl group, this group could not be removed oxidatively or by any other means in the presence of acyl groups containing two or more double bonds (e.g. linoleoyl).4 Difficulty with oxidatively cleaving PMB in the presence of 1,3- and 1,4-dienes is a widely reported problem.5–8 3,4-Dimethoxybenzyl (DMB)9 can be removed oxidatively more readily than PMB, but was also unsatisfactory for molecules with diene moieties.10
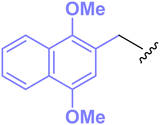
We have found a novel benzyl-type protecting group (1,4-dimethoxynaphthalene-2-methyl, ‘DIMON’), (Fig. 2) that can be removed under oxidative conditions without damaging polyunsaturated acyl groups. This group was selected after consideration of synthetic accessibility and, above all, redox potentials (E1/2, V in MeCN solvent) for methoxy-substituted aromatic systems (for a review see ref. 11): methoxybenzene (1.76),12 1,2,-dimethoxybenzene (1.45),12 1,4-dimethoxybenzene, (1.34),12 1,4-dimethoxynaphthalene (1.10),13cf. benzene (2.08),14 naphthalene (1.34).14 These data were expected to mirror the ease of removal of the corresponding benzyl-type group with a reagent [e.g. 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ)] operating via an electron transfer mechanism and therefore DIMON should be more easily removed than PMB or DMB.
The application of DIMON is illustrated by the synthesis of a variety of protected alcohols, phenols, amines and amides, and 1-alkyl-2-acyl glycerols, where the acyl groups are derived from polyunsaturated fatty acids including (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid (DHA). It is critically important that the latter acyl moiety survives removal of DIMON without double bond perturbation or acyl migration.
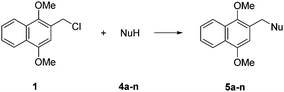
DIMON protection of heteroatoms requires 2-(chloromethyl)-1,4-dimethoxynaphthalene (1, DIMONCl), which has been prepared either by chloromethylation of 1,4-dimethoxynaphthalene15,16 or by treatment of (1,4-dimethoxynaphthalen-2-yl)methanol (2) with thionyl chloride17 or methanesulfonyl chloride18 Compound 2 is available either by reduction of methyl 1,4-dimethoxy-2-naphthoate18 or from 2-bromo-1,4-dimethoxynaphthalene via the corresponding Grignard reagent, which was reacted with dimethylformamide followed by reduction with sodium borohydride.19 We have prepared compound 2 from commercially available 2-bromo-1,4-dimethoxynaphthalene 320 by lithiation followed by treatment with formaldehyde (Scheme 1). DIMONCl 1 was obtained in quantitative yield simply from reaction of 2 in a two-phase system of diethyl ether and 12 M hydrochloric acid. Separation of the organic phase, drying and evaporation gave 2 as an air-stable, crystalline solid.
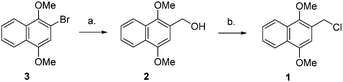 | ||
| Scheme 1 Synthesis of 2-(chloromethyl)-1,4-dimethoxynapthalene (DIMONCl 1): (a) (i) n-BuLi, −78 °C, 2 h (ii) paraformaldehyde, 2 h, rt, Et2O, Ar, 62%; (b) conc. HCl, Et2O, rt, 1 h, 100%. | ||
DIMON protection of alcohols and amides (for selected examples see Table 1) was efficiently achieved under standard conditions via the corresponding alkoxide/amide anion, which was alkylated using DIMONCl in the presence of catalytic tetrabutylammonium bromide in THF at room temperature or at reflux for the steroidal substrates. With glycidol 4e (entry 5, oxiran-2-ylmethanol), a key intermediate for phospholipid synthesis, DMF solvent was preferred to THF.
| Entry | NuH | Product | Yield (%) | Method |
|---|---|---|---|---|
| Method A: 1 equiv. NuH, 1.2 equiv. DIMONCl, 10 mol% TBAI, 1.2 equiv. NaH 60% dispersion in mineral oil, dry THF, N2, rt, 16 h.a DMF used as solvent.b Reaction at reflux. B: 1 equiv. NuH, 1.2 equiv. DIMONCl, 1.1 equiv. Cs2CO3, dry MeCN, N2, reflux, 16 h. C: 1 equiv. NuH, 1.2 equiv. DIMONCl, 1.2 equiv. NEt3, dry THF, N2, rt, 16 h. | ||||
| 1 | Eicosanol 4a | 5a | 93 | A |
| 2 | Octan-1-ol 4b | 5b | 75 | A |
| 3 | Oct-2-en-1-ol 4c | 5c | 79 | A |
| 4 | Propargyl alcohol 4d | 5d | 82 | A |
| 5 | Glycidol 4e | 5e | 85 | Aa |
| 6 | 4-Methoxybenzyl alcohol 4f | 5f | 93 | A |
| 7 | Cholesterol 4g | 5g | 91 | Ab |
| 8 | Ergosterol 4h | 5h | 89 | Aa |
| 9 | L-Menthol 4i | 5i | 71 | A |
| 10 | 4-Chlorophenol 4j | 5j | 91 | B |
| 11 | Thymol 4k | 5k | 80 | B |
| 12 | Naphth-1-ol 4l | 5l | 89 | B |
| 13 | Morpholine 4m | 5m | 68 | C |
| 14 | N-Methylbenzamide 4n | 5n | 86 | A |
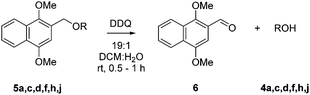
Phenols were reacted with DIMONCl using Cs2CO3 as base, whilst triethylamine sufficed for amines. DIMON was efficiently removed from protected alcohols and phenols within one hour at 20 °C by exposure to 2,3-dichloro-4,5-dicyanobenzoquinone (DDQ) in wet DCM (Table 2). After deprotection, the protecting moiety was easily separated chromatographically as 1,4-dimethoxy-2-naphthaldehyde 6 and could be recycled, whilst any residual DDQ was reduced by aqueous ascorbic acid to 2,3-dichloro-5,6-dicyano-1,4-quinol, which was extracted into an aqueous phase. Importantly, the DIMON ether was selectively oxidised over the PMB ether (Table 2, entry 4) in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by 1H NMR analysis. This agreed with the redox potentials of DIMON and PMB, and showed that DIMON can be orthogonally removed in the presence of PMB.
1 ratio by 1H NMR analysis. This agreed with the redox potentials of DIMON and PMB, and showed that DIMON can be orthogonally removed in the presence of PMB.
DIMON could also be removed under acidic catalysis (TFA in DCM or HCl in MeOH) or by catalytic hydrogenation (Pd/H2) with substrates derived from alcohols lacking a non-aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (see Scheme 2 and ESI†).
C bond (see Scheme 2 and ESI†).
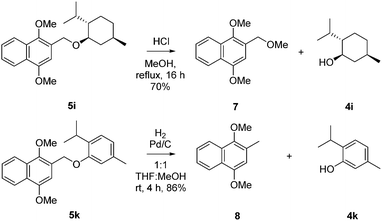 | ||
| Scheme 2 Acidic and reductive cleavage of DIMON from DIMON-menthol to give menthol and DIMON-thymol to afford thymol, respectively. | ||
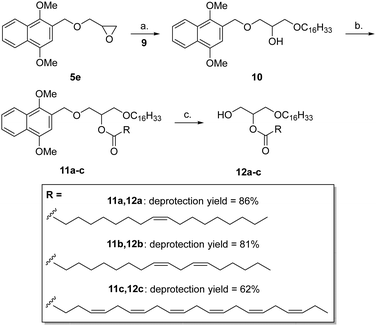
To define conditions suitable for the synthesis of alkyl phospholipids, DIMON-protected rac.-glycidol 5e was reacted with a 3-fold excess of hexadecan-1-ol 9 in the presence of either catalytic ytterbium triflate or boron trifluoride, the latter giving a better yield of compound 10 (Scheme 3). Acylation of 10 was performed with three representative fatty acids giving the corresponding esters 11a-c: from oleic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 93% yield, from linoleic acid (18
1) in 93% yield, from linoleic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in 79% and docosahexaenoic acid (22
2) in 79% and docosahexaenoic acid (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) in 91% yield.
6) in 91% yield.
All three esters were smoothly deprotected by DDQ giving the corresponding primary alcohols 12a-c in 86% (oleyl), 81% (linoleyl) and 62% yield (docosahexaenoyl) with no evidence of double bond perturbation or acyl migration.
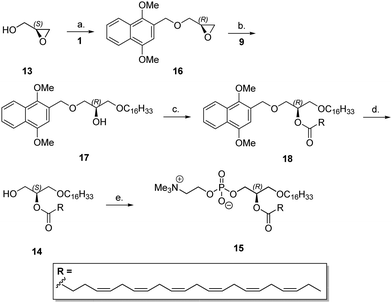
To illustrate the application of DIMON in total synthesis of polyunsaturated phospholipids, we repeated the synthetic route shown in Scheme 3 starting from (S)-glycidol 13, giving intermediate 14 in 35% overall yield (cf.Scheme 4). Compound 14 was converted into the naturally occurring plasmanyl phospholipid 15 using a known phosphorylation method, reaction with 2-chloro-1,3,2-dioxophospholane 2-oxide (COP) then trimethylamine, to afford a choline derivative.21 This is the first reported synthesis of such an ether phospholipid. The corresponding oleoyl derivative was made from 12a in a similar manner (data not shown).
Although this paper describes a specific application of DIMON, we believe that this new protecting group will find application in the synthesis of a wide range of natural products.
We thank the European Research and Development Fund (ERDF) and BiBerChem Research (Newcastle upon Tyne, UK) for financial support (to JT), the NMSF at Swansea University for HRMS and Masuma Begum for technical support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Goldfine, Prog. Lipid Res., 2010, 49, 493 CrossRef CAS PubMed.
- N. Jiménez-Rojo and H. Riezman, FEBS Lett., 2019, 593, 2378 CrossRef PubMed.
- See the collection of reviews and papers in Frontiers in Cell Developmental and Biology, e.g. J. Koch, K. Watschinger, E. R. Werner and M. A. Keller, Front. Cell Dev. Biol., 2022, 10, 864716 CrossRef PubMed.
- A. B. Maude, PhD thesis, Newcastle University, 1992.
- T. Onoda, R. Shirai and S. Iwasaki, Tetrahedron Lett., 1997, 38, 1443 CrossRef CAS.
- M. Hutchings, D. Moffat and N. S. Simpkins, Synlett, 2001, 661 CrossRef CAS.
- R. A. Pilli, I. R. Correa Jr., A. O. Maldaner and G. B. Rosso, Pure Appl. Chem., 2005, 77, 1153 CrossRef CAS.
- A. Furstner, L. C. Bouchez, J. Funel, V. Liepins, F. Poree, R. Gilmour, F. Beaufils, D. Laurich and M. Tamiya, Angew. Chem., Int. Ed., 2007, 46, 9265 CrossRef PubMed.
- K. Horita, T. Yoshioka, T. Tanaka, Y. Oikawa and A. Yonemitsu, Tetrahedron, 1986, 42, 3021 CrossRef CAS.
- K. A. Scheidt, T. D. Bannister, A. Tasaka, M. D. Wendt, B. M. Savall, G. J. Fegley and W. R. Roush, J. Am. Chem. Soc., 2002, 124, 6981 CrossRef CAS PubMed.
- N. L. Weinberg and H. R. Weinberg, Chem. Rev., 1968, 68, 449 CrossRef CAS.
- A. Zweig, W. G. Hodgson and W. H. Jura, J. Am. Chem. Soc., 1964, 86, 4124 CrossRef CAS.
- A. Zweig, A. H. Maurer and B. G. Roberts, J. Org. Chem., 1967, 32, 1322 CrossRef CAS.
- J. W. Loveland and G. R. Dimeler, Anal. Chem., 1961, 33, 1196 CrossRef CAS.
- B. R. Baker and G. H. Carlson, J. Am. Chem. Soc., 1942, 64, 2657 CrossRef CAS.
- G. A. Kraus and M. D. Hagen, J. Org. Chem., 1983, 48, 3265 CrossRef CAS.
- X. Ning, H. Qi, R. Li, Y. Li, Y. Jin, M. A. McNutt, J. Liu and Y. Yin, Eur. J. Med. Chem., 2017, 138, 343 CrossRef CAS PubMed.
- E. Vieira, A. Binggeli, V. Breu, D. Bur, W. Fischli, R. Gueller, G. Hirth, H.-P. Maerki, M. Mueller, C. Oefner, M. Scalone, H. Stadler, M. Wilhelm and W. Wostl, Bioorg. Med. Chem. Lett., 1999, 9, 1397 CrossRef CAS PubMed.
- U. Ackermann, D. Sigmund, S. D. Yeoh, A. Rigopoulos, G. O’Keefe, G. Cartwright, J. White, A. M. Scott and H. J. Tochon-Danguy, J. Labelled Compd. Radiopharm., 2011, 54, 788 CrossRef CAS.
- J.-Y. Jeong, J. Sperry, J. A. Taylor and M. A. Brimble, Eur. J. Med. Chem., 2014, 87, 220 CrossRef PubMed.
- J. M. Zeidler, W. Zimmermann and H. J. Roth, Chem. Phys. Lipids, 1990, 55, 155 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04292h |
| This journal is © The Royal Society of Chemistry 2023 |