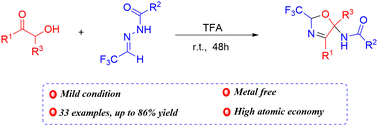
Junjiao Wang, Yongwei Shang, Xiujuan Zhao, Zhenli Cui, Yang Li, Ke-Hu Wang, Danfeng Huang, Yulai Hu, Na Wang and Lei Feng
Chem. Commun., 2023,59, 14293-14296
DOI:
10.1039/D3CC03854H,
Communication