Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fully tuneable ethylene–propylene elastomers using a supported permethylindenyl-phenoxy (PHENI*) catalyst†
Clement G.
Collins Rice
 ,
Louis J.
Morris
,
Louis J.
Morris
 ,
Jean-Charles
Buffet
,
Jean-Charles
Buffet
 ,
Zoë R.
Turner
,
Zoë R.
Turner
 and
Dermot
O’Hare
and
Dermot
O’Hare
 *
*
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: dermot.ohare@chem.ox.ac.uk
First published on 21st September 2023
Abstract
Using a highly active supported permethylindenyl-phenoxy (PHENI*) titanium catalyst, high molecular weight ethylene–propylene (EPM) and ethylene–propylene–diene (EPDM) elastomers are prepared using slurry-phase catalysis. Final copolymer composition was found to reflect the monomer feed ratio in a linear fashion, to access a continuum of material properties with a single catalyst. Post-polymerisation crosslinking of EPDM was also demonstrated in a model sulfur vulcanisation system.
Conventional rubbers are highly crosslinked polymers which are challenging to process and have poor recyclability.1 Thermoplastic elastomers (TPEs) are an emerging class of materials that bridge the gap between classical thermoset rubbers and thermoplastics, having the elastic properties of the former and the processing flexibility and recyclability of the latter.2,3 This requires a biphasic structure with crystalline and amorphous regions. Block copolymers have proved promising with distinct hard and soft segments facilitating TPE behaviour.4–6 Hyperbranched polyethylene has also been investigated as a direct route to the production of polyolefin elastomers.3,7
Copolymers of ethylene and propylene (EPM) are an important class of TPE, with extensive research relating the physical properties to the molecular weight, composition, and chain structure.8–10 In particular, the crystalline microstructure of EPM has been shown to strongly influence yield stress and rupture energy density.9 EPM displays homogeneous deformation, like most rubbers, but also yield-like behaviour more typical of thermoplastics. It has been shown that EPM composition controls not only crystallinity but also compatibility with polymer matricies,11 polyolefin blends,12,13 and recycled polyolefins.14
Vanadium-based catalysts dominate the industrial production of EPM,15 though group four complexes have also been studied,16 with the Keltan ACE™ titanium κ1-amidinate complexes being used to produce EPM commercially.17 In general, the overwhelming preference for ethylene coordination-insertion relative to propylene (rE ≫ rP) leads to long sequences of consecutive ethylene units and this limits the compositional tunability that could potentially be possible to achieve.18–20 The amidinate complexes produce EPM with 39–75 wt% ethylene from a feed containing 33% C2 monomer.17,19 A silica-supported zirconocene reported by Caballero et al. is a limited example of a catalyst able to produce EPM across the whole composition range by controlling the gas phase E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio. Even in this case, a clear preference for ethylene is observed with 13% incorporated from a 3
P ratio. Even in this case, a clear preference for ethylene is observed with 13% incorporated from a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97 E
97 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P feed.10
P feed.10
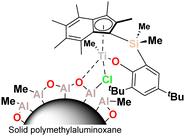
Following our recent report of group four permethylindenyl-phenoxy (PHENI*) catalysts, and their outstanding performance for ethylene and propylene homopolymerisation,21,22 we now describe the application of the PHENI* complex Me2SB(tBu2ArO,I*)TiCl2 ({(η5-C9Me6)Me2Si(2,4-tBu2-C6H2O)}TiCl2), heterogenised on solid polymethylaluminoxane (sMAO),231 (Fig. 1), for the production of EPM elastomers. sMAO is well-established as a dual function activating support for metallocene and post-metallocene catalysts.24,25 The catalyst system displays an unusual monomer-agnostic behaviour resulting in access to fully tuneable compositions of high molecular weight EPM.
The sMAO-supported PHENI* complex sMAO-Me2SB(tBu2ArO,I*)TiCl2 ([AlsMAO]0/[Ti]0 = 200; 1) was prepared,21,22 and utilised in slurry-phase olefin copolymerisations. Polymerisations were carried out within a temperature of polymerisation (Tp) range of 30 ≤ Tp ≤ 90 °C under 2 bar total pressure with 10 mg of 1 (∼712 nmol Ti) and either MAO or TIBA as cocatalyst ([Al]0/[Ti]0 = 1000) in 50 mL hexanes as a diluent.
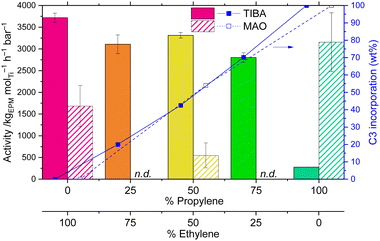
In the homopolymerisation of ethylene, 1/TIBA showed the greatest catalytic activity, while for homopolypropylene the highest activities were achieved with 1/MAO (Fig. 2). Therefore, E/P copolymerisations were performed using a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 monomer feed mixture and either MAO or TIBA. In this case, 1/TIBA displayed significantly higher copolymerisation activity than 1/MAO: 3313 and 548 kgEPM molTi−1 h−1 bar−1 respectively. It has previously been noted that TIBA can transalkylate with MAO, leading to iso-butyl substituted aluminoxanes that are almost inactive towards propylene polymerisation.26 When using 1/TIBA, E/P copolymerisation activity was comparable to that of ethylene homopolymerisation (3719 kgPE molTi−1 h−1 bar−1), though in a highly dissimilar diffusion regime: while polyethylene was formed as well dispersed solid particles,21 EPM is hexane-soluble at all compositions studied, and forms as a gel. Copolymerisations were subsequently run using E
50 monomer feed mixture and either MAO or TIBA. In this case, 1/TIBA displayed significantly higher copolymerisation activity than 1/MAO: 3313 and 548 kgEPM molTi−1 h−1 bar−1 respectively. It has previously been noted that TIBA can transalkylate with MAO, leading to iso-butyl substituted aluminoxanes that are almost inactive towards propylene polymerisation.26 When using 1/TIBA, E/P copolymerisation activity was comparable to that of ethylene homopolymerisation (3719 kgPE molTi−1 h−1 bar−1), though in a highly dissimilar diffusion regime: while polyethylene was formed as well dispersed solid particles,21 EPM is hexane-soluble at all compositions studied, and forms as a gel. Copolymerisations were subsequently run using E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios of 25
P ratios of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 and 75
75 and 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 with 1/TIBA as the catalyst system (Fig. 2). In these cases, activity is approximately independent of monomer feed composition, being within 1 standard deviation of each other.
25 with 1/TIBA as the catalyst system (Fig. 2). In these cases, activity is approximately independent of monomer feed composition, being within 1 standard deviation of each other.
Copolymer composition demonstrated precise tuneability, with the monomer ratio in the EPM corresponding almost exactly to that in the feed gas mixture. By GPC–IR, the EPM copolymers were found to contain 20, 42, and 71 wt% propylene (Fig. 2) from E/P feeds of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50
25, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and 25
50, and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 respectively; this was confirmed by quantitative solution-phase 13C{1H} NMR spectroscopy, which gave values of 16, 50, and 75 mol% propylene. Remarkably, the polymer composition was not found to significantly depend on the temperature of polymerisation (30 ≤ Tp ≤ 90 °C), being in the range 40–47 wt% propylene incorporation when 50
75 respectively; this was confirmed by quantitative solution-phase 13C{1H} NMR spectroscopy, which gave values of 16, 50, and 75 mol% propylene. Remarkably, the polymer composition was not found to significantly depend on the temperature of polymerisation (30 ≤ Tp ≤ 90 °C), being in the range 40–47 wt% propylene incorporation when 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 E
50 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P feed gas was utilised (Fig. S3 and S4, ESI†). Such direct tuneability of the comonomer composition as an almost 1-to-1 function of the feed gas is an unusual feature of the PHENI* catalyst system.
P feed gas was utilised (Fig. S3 and S4, ESI†). Such direct tuneability of the comonomer composition as an almost 1-to-1 function of the feed gas is an unusual feature of the PHENI* catalyst system.
By contrast, the [O–NS] titanium complex used by Yao et al. produced EPM with up to 29.5 mol% propylene incorporation at 60 °C, even when the feed was 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 E
67 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P. Whilst the incorporation was approximately linear over the composition regimes studies in that case, it was far from a 1–to–1 relationship.20 The commercial Keltan ACE™ complexes have demonstrated activities as high as 133
P. Whilst the incorporation was approximately linear over the composition regimes studies in that case, it was far from a 1–to–1 relationship.20 The commercial Keltan ACE™ complexes have demonstrated activities as high as 133![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 kgEPM molTi−1 h−1 bar−1 and molecular weights of 1.3–1.7 MDa. In general, though, these κ1-amidinate complexes preferentially incorporate ethylene, with just one example of an EPM, having 61 wt% P, approaching the composition of the feed (33
750 kgEPM molTi−1 h−1 bar−1 and molecular weights of 1.3–1.7 MDa. In general, though, these κ1-amidinate complexes preferentially incorporate ethylene, with just one example of an EPM, having 61 wt% P, approaching the composition of the feed (33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 E
67 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P).17 Vanadium compounds dominate the commercial production of ethylene–propylene copolymers. The vanadium(III) NHC complexes reported by Zhang et al. were able to incorporate up to 39 mol% propylene under a 75
P).17 Vanadium compounds dominate the commercial production of ethylene–propylene copolymers. The vanadium(III) NHC complexes reported by Zhang et al. were able to incorporate up to 39 mol% propylene under a 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 E
25 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P monomer supply.27 Caballero et al. used the catalyst {(η5-2-Me-C9H5)2SiMe2}ZrCl2 to synthesise a complete range of EPM compositions, though the system preferentially polymerised ethylene, with a 3
P monomer supply.27 Caballero et al. used the catalyst {(η5-2-Me-C9H5)2SiMe2}ZrCl2 to synthesise a complete range of EPM compositions, though the system preferentially polymerised ethylene, with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97 E
97 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P feed resulting in a polymer with 13 mol% ethylene.10 The titanium half-sandwich complexes reported by Zhang et al., which use X-type anionic nitrogen-based donors, demonstrated activities of up to 2390 kgEPM molTi−1 h−1.19 In this case, an E
P feed resulting in a polymer with 13 mol% ethylene.10 The titanium half-sandwich complexes reported by Zhang et al., which use X-type anionic nitrogen-based donors, demonstrated activities of up to 2390 kgEPM molTi−1 h−1.19 In this case, an E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 33
P ratio of 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 resulted in 38 mol% propylene incorporation, and a viscosity-average molecular weight, Mη, of 340 kDa. The activity of PHENI* compares favourably to this as well as offering full compositional tunability.
67 resulted in 38 mol% propylene incorporation, and a viscosity-average molecular weight, Mη, of 340 kDa. The activity of PHENI* compares favourably to this as well as offering full compositional tunability.
The resulting EPM produced by 1/TIBA was found to be amorphous by DSC, consistent with the formation of a random copolymer. Polymer molecular weight and distribution were estimated from GPC as well as short-chain branching (SCB) density and composition from an inline IR detector. Weight-average molecular weight (Mw) of EPM (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 50
P 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) was found to be greater when the scavenger was MAO rather than TIBA, 290 and 150 kDa respectively, due to a high molecular weight shoulder in the chromatogram. This is reflected in a greater dispersity (Đ) of 3.1 when using MAO compared to 2.3 with TIBA, close to the ideal value for single-site polymerisation. Both Mw and Đ were largely independent of feed composition, with Mw in the range 150–160 kDa from 25–75% propylene.
50) was found to be greater when the scavenger was MAO rather than TIBA, 290 and 150 kDa respectively, due to a high molecular weight shoulder in the chromatogram. This is reflected in a greater dispersity (Đ) of 3.1 when using MAO compared to 2.3 with TIBA, close to the ideal value for single-site polymerisation. Both Mw and Đ were largely independent of feed composition, with Mw in the range 150–160 kDa from 25–75% propylene.
Engineering stress–strain curves were obtained for vacuum compression moulded samples of EPM (Fig. 3). Compared to HDPE of comparable Mw, the EPM synthesised with 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 E
25 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P shows a much greater strain at break (εB = 974% cf. 170%) afforded by the elastomeric properties of aPP segments. Strain hardening is observed after a yield-like point at εy = 60% associated with the crystalline behaviour of the polyethylene segments. By contrast, at higher propylene incorporations the EPM displays poor mechanical performance with decreased strength and εB. In these cases the amorphous nature of the aPP segments is responsible for the degraded behaviour, while the Mw is insufficient to achieve the desirable elastic properties of UHMWaPP.22
P shows a much greater strain at break (εB = 974% cf. 170%) afforded by the elastomeric properties of aPP segments. Strain hardening is observed after a yield-like point at εy = 60% associated with the crystalline behaviour of the polyethylene segments. By contrast, at higher propylene incorporations the EPM displays poor mechanical performance with decreased strength and εB. In these cases the amorphous nature of the aPP segments is responsible for the degraded behaviour, while the Mw is insufficient to achieve the desirable elastic properties of UHMWaPP.22
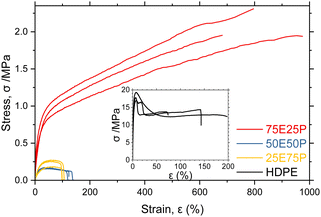 | ||
| Fig. 3 Engineering stress–strain curves of EPM as a function of composition. Inset: Engineering stress–strain curve of a commercial HDPE (Mw = 225 kDa, Đ = 25). | ||
Following the successful synthesis of EPM polymers at high activity and with tuneable composition, 1/TIBA was used in the terpolymerisation of ethylene, propylene, and 5-ethylidene-2-norbornene (ENB). As well as being easier to handle than butadiene, ENB is commonly used as a diene in multicomponent polymerisations. Only the internal norbornyl olefin participates in polymerisation, with the ethylidene providing pendant unsaturation to the polymer backbone for post-synthesis vulcanisation such as using UV light, sulfur (Scheme 1), or organic peroxides.28–31 Excellent physical and mechanical properties, including resistance to ozone, heat, chlorine, oxygen, and weathering, make EPDM the most suitable rubbers for outdoor applications, tubing, insulating materials, and automotive applications.32
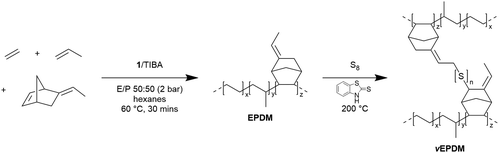 | ||
| Scheme 1 E/P/ENB terpolymerisation, and subsequent sulfur vulcanisation to form vEPDM, the structure of which is adapted from the mechanism provided by Verbruggen et al.33 | ||
Under a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 E
50 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
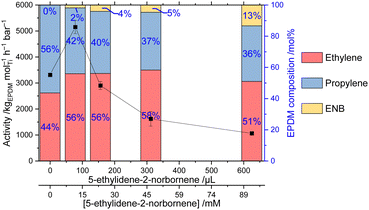
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P feed (2 bar), after initially increasing by 56% to 5155 kgEPDM mol−1 h−1 bar−1 at 78 μL ENB (12 mM) relative to homopolymerisation, terpolymerisation activity decreased as [ENB] increased (Fig. 4), reaching 1065 kgEPDM mol−1 h−1 bar−1 at 625 μL (93 mM). Mw increased slightly with increasing ENB loading, reaching 204 kDa at 625 μL, 36% higher than the E
P feed (2 bar), after initially increasing by 56% to 5155 kgEPDM mol−1 h−1 bar−1 at 78 μL ENB (12 mM) relative to homopolymerisation, terpolymerisation activity decreased as [ENB] increased (Fig. 4), reaching 1065 kgEPDM mol−1 h−1 bar−1 at 625 μL (93 mM). Mw increased slightly with increasing ENB loading, reaching 204 kDa at 625 μL, 36% higher than the E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P copolymerisation in the absence of ENB, resulting from poor chain transfer to ENB.34
P copolymerisation in the absence of ENB, resulting from poor chain transfer to ENB.34
The composition of the EPDM polymers was determined by solution-phase 1H NMR spectroscopy, according to the assignments and integral analysis of Leone et al.35 The proportion of ENB in the terpolymer increases with increasing [ENB] from 1.8 mol% at 78 μL (12 mM) to 13.4 mol% at 625 μL (93 mM) (Fig. 4). The complex (EBI)ZrCl2 studied by Ali et al. produced EPDM with composition 79.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.2
18.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.30 mol% (E
2.30 mol% (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ENB) from an 80
ENB) from an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 E
20 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P feed with 60 mM ENB at an activity of 3.55 × 106 kgEPDM molZr−1 h−1.34 Malmberg et al. used the same catalyst and achieved ENB incorporations of up to 16 mol% at a 600 mM concentration.36 The vanadium(V) complexes studied by Leone et al. produced polymer with composition 80.3
P feed with 60 mM ENB at an activity of 3.55 × 106 kgEPDM molZr−1 h−1.34 Malmberg et al. used the same catalyst and achieved ENB incorporations of up to 16 mol% at a 600 mM concentration.36 The vanadium(V) complexes studied by Leone et al. produced polymer with composition 80.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5
6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.2 mol% from an 18
13.2 mol% from an 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 feed at an activity of 3006 kgEPDM molV−1 h−1.35 Compared to these complexes, the PHENI* catalyst enchains ENB more efficiently, with higher incorporation at smaller concentrations, and produces EPDM with a composition that more closely matches the monomer feed.
9 feed at an activity of 3006 kgEPDM molV−1 h−1.35 Compared to these complexes, the PHENI* catalyst enchains ENB more efficiently, with higher incorporation at smaller concentrations, and produces EPDM with a composition that more closely matches the monomer feed.
A system comprising sulfur and the common accelerant 2-mercaptobenzothiazole (C6H4(NH)SC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, MBT) was used to vulcanise the as produced EPDM.33,37–42 Relatively high loadings of S8 (10 phr; parts per hundred rubber) and MBT (5 phr) were used to ensure, in the absence of optimisations, sufficiently high conversion of ENB residues. Vulcanisations were performed in 50 μL DSC pans, and the reactions were followed by dynamic DSC protocols established by Restrepo-Zapata et al.41 The thermograms show endothermic features corresponding to the melting points of sulphur (113 °C) and MBT (177–181 °C), and an exotherm between 180–230 °C, which is indicative of curing occurring by sulfur crosslinking (Fig. S6, ESI†). Furthermore, the vulcanisation was followed by dynamic rheology (Fig. S7, ESI†). During the initial temperature ramp the onset of crosslinking was observed at 175 °C corresponding to a maximum in tan
S, MBT) was used to vulcanise the as produced EPDM.33,37–42 Relatively high loadings of S8 (10 phr; parts per hundred rubber) and MBT (5 phr) were used to ensure, in the absence of optimisations, sufficiently high conversion of ENB residues. Vulcanisations were performed in 50 μL DSC pans, and the reactions were followed by dynamic DSC protocols established by Restrepo-Zapata et al.41 The thermograms show endothermic features corresponding to the melting points of sulphur (113 °C) and MBT (177–181 °C), and an exotherm between 180–230 °C, which is indicative of curing occurring by sulfur crosslinking (Fig. S6, ESI†). Furthermore, the vulcanisation was followed by dynamic rheology (Fig. S7, ESI†). During the initial temperature ramp the onset of crosslinking was observed at 175 °C corresponding to a maximum in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (δ), with the storage modulus, G′, increasing rapidly as the reaction progresses from a starting value of 25 kPa to a final value of 1.1 MPa after 140 minutes of curing at 200 °C. The storage modulus is associated with the elastic response of a viscoelastic material and so the observed increase corresponds to the presence of crosslinks which are able to store elastic potential energy.
(δ), with the storage modulus, G′, increasing rapidly as the reaction progresses from a starting value of 25 kPa to a final value of 1.1 MPa after 140 minutes of curing at 200 °C. The storage modulus is associated with the elastic response of a viscoelastic material and so the observed increase corresponds to the presence of crosslinks which are able to store elastic potential energy.
In the infrared spectrum of the resulting vulcanised material (vEPDM) additional transitions are observed compared to EPDM arising from C–H and C–C vibrations corresponding to the crosslinks,43 and C–S vibrations are seen in the region 775–600 cm−1 (Fig. S8, ESI†).44 The solid-state 13C CPMAS NMR spectrum of vEPDM shows a broad resonance around 110–155 ppm, not present in the solution-phase spectrum of EPDM, and which corresponds to C–S resonances at the crosslink junctions α to the pendant olefin. Winters et al. showed that broad resonances in ssNMR spectra at δ 117 and 151 ppm arise from EPDM vulcanisation.38
In conclusion, the PHENI* catalyst has demonstrated excellent catalytic performance towards ethylene–propylene copolymerisations. The synthesis of EPM and EPDM is reported with high activities, high molecular weights, and fully tuneable composition. In all cases, composition can be controlled and tuned by the ratio of monomers in the feed, highlighting the desirable and unusually monomer-agnostic behaviour of this catalyst. The versatility of this chemistry has been demonstrated by the terpolymerisation with a diene and the latent unsaturation undergoing post-polymerisation crosslinking.
C. G. C. R., L. J. M., J.-C. B., and Z. R. T. would like to thank SCG Chemicals PLC for financial support. C. G. C. R. additionally acknowledges funding from the Engineering and Physical Sciences Research Council IAA (EP/X525777/1). The authors thank Ms Liv Thobru, Ms Sara Rund Herum, and Ms Rita Jenssen (Norner AS, Norway) for GPC analysis.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- A. A. Yehia, Polym. Plast. Technol. Eng., 2004, 43, 1735–1754 CrossRef CAS.
- A. S. Mohite, Y. D. Rajpurkar and A. P. More, Polym. Bull., 2022, 79, 1309–1343 CrossRef CAS.
- K. Lian, Y. Zhu, W. Li, S. Dai and C. Chen, Macromolecules, 2017, 50, 6074–6080 CrossRef CAS.
- K. S. O’Connor, A. Watts, T. Vaidya, A. M. LaPointe, M. A. Hillmyer and G. W. Coates, Macromolecules, 2016, 49, 6743–6751 CrossRef.
- C. M. Koo, M. A. Hillmyer and F. S. Bates, Macromolecules, 2006, 39, 667–677 CrossRef CAS.
- Y. Zhao, Y. Ma, Y. Xiong, T. Qin, Y. Zhu, H. Deng, J. Qin, X. Shi and G. Zhang, Polymer, 2022, 254, 125075 CrossRef CAS.
- Q. Mahmood and W.-H. Sun, R. Soc. Open Sci., 2018, 5, 180367 CrossRef PubMed.
- W. J. Wang and S. Zhu, Macromolecules, 2000, 33, 1157–1162 CrossRef CAS.
- K. J. Wright and A. J. Lesser, Macromolecules, 2001, 34, 3626–3633 CrossRef CAS.
- M. J. Caballero, I. Suarez, B. Coto, R. Van Grieken and B. Monrabal, Macromol. Symp., 2007, 257, 122–130 CrossRef CAS.
- P. Doshev, G. Lohse, S. Henning, M. Krumova, A. Heuvelsland, G. Michler and H.-J. Radusch, J. Appl. Polym. Sci., 2006, 101, 2825–2837 CrossRef CAS.
- L. D'Orazio, R. Greco, C. Mancarella, E. Martuscelli, G. Ragosta and C. Silvesrte, Polym. Eng. Sci., 1982, 22, 536–544 CrossRef.
- S. Jose, S. Thomas, P. K. Biju and J. Karger-Kocsis, J. Polym. Res., 2013, 20, 303 CrossRef.
- G. Radonjič and N. Gubeljak, Macromol. Mater. Eng., 2002, 287, 122–132 CrossRef.
- A. M. F. Phillips, H. Suo, M. D. F. C. Guedes da Silva, A. J. L. Pombeiro and W. H. Sun, Coord. Chem. Rev., 2020, 416, 213332 CrossRef CAS.
- R. Kravchenko and R. M. Waymouth, Macromolecules, 1998, 31, 1–6 CrossRef CAS.
- G. van Doremaele, M. van Duin, M. Valla and A. Berthoud, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2877–2891 CrossRef CAS.
- C. Cozewith and G. V. Strate, Macromolecules, 1971, 4, 482–489 CrossRef CAS.
- Z. Q. Zhang, J. T. Qu, S. Zhang, Q. P. Miao and Y. X. Wu, Polym. Chem., 2018, 9, 48–59 RSC.
- Z. Yao, D. F. Ma, Z. X. Xiao, W. L. Yang, Y. X. Tu and K. Cao, RSC Adv., 2017, 7, 10175–10182 RSC.
- C. G. Collins Rice, J.-C. Buffet, Z. R. Turner and D. O'Hare, Chem. Commun., 2021, 57, 8600–8603 RSC.
- C. G. Collins Rice, J.-C. Buffet, Z. R. Turner and D. O'Hare, Polym. Chem., 2022, 13, 5597–5603 RSC.
- T. J. Williams, J. V. Lamb, J.-C. Buffet, T. Khamnaen and D. O’Hare, RSC Adv., 2021, 11, 5644–5650 RSC.
- A. F. R. Kilpatrick, J.-C. Buffet, P. Nørby, N. H. Rees, N. P. Funnell, S. Sripothongnak and D. O’Hare, Chem. Mater., 2016, 28, 7444–7450 CrossRef CAS.
- E. Kaji and E. Yoshioka, US8404880B2, 2013.
- H. Kurokawa and T. Sugano, Macromol. Symp., 1995, 97, 143–149 CrossRef CAS.
- S. Zhang, W. C. Zhang, D. D. Shang and Y. X. Wu, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 553–561 CrossRef CAS.
- W. Wang and B. Qu, Polym. Degrad. Stab., 2003, 81, 531–537 CrossRef CAS.
- G. Milani and F. Milani, J. Appl. Polym. Sci., 2012, 124, 311–324 CrossRef CAS.
- E. A. Snijders, A. Boersma, B. Van Baarle and P. Gijsman, Polym. Degrad. Stab., 2005, 89, 484–491 CrossRef CAS.
- M. Van Duin, R. Orza, R. Peters and V. Chechik, Macromol. Symp., 2010, 291, 66–74 CrossRef.
- I. Surya, M. Muniyadi and H. Ismail, Polym. Compos., 2021, 42, 1698–1711 CrossRef CAS.
- M. A. L. Verbruggen, L. van der Does, J. W. M. Noordermeer and M. van Duin, J. Appl. Polym. Sci., 2008, 109, 976–986 CrossRef CAS.
- A. Ali, M. K. Tufail, M. I. Jamil, W. Yaseen, N. Iqbal, M. Hussain, A. Ali, T. Aziz, Z. Fan and L. Guo, Molecules, 2021, 26, 2037 CrossRef CAS PubMed.
- G. Leone, G. Zanchin, R. Di Girolamo, F. De Stefano, C. Lorber, C. De Rosa, G. Ricci and F. Bertini, Macromolecules, 2020, 53, 5881–5894 CrossRef CAS.
- A. Malmberg and B. Löfgren, J. Appl. Polym. Sci., 1997, 66, 35–44 CrossRef CAS.
- S. Cesca, J. Polym. Sci., Part D: Macromol. Rev., 1975, 10, 1–230 Search PubMed.
- R. Winters, W. Heinen, M. A. L. Verbruggen, J. Lugtenburg, M. van Duin and H. J. M. de Groot, Macromolecules, 2002, 35, 1958–1966 CrossRef CAS.
- M. Mortazavi, H. Arabi, S. Ahmadjo, M. Nekoomanesh and G. H. Zohuri, J. Appl. Polym. Sci., 2011, 122, 1838–1846 CrossRef CAS.
- N. Sanches, S. Cassu, M. Diniz and R. Lazzarini Dutra, Polimeros, 2014, 24, 269–275 CrossRef.
- N. C. Restrepo-Zapata, T. A. Osswald and J. P. Hernández-Ortiz, Polym. Eng. Sci., 2015, 55, 2073–2088 CrossRef CAS.
- D. Z. Pirityi and K. Pölöskei, Polymers, 2021, 13, 1116 CrossRef CAS PubMed.
- F. J. Linnig and J. E. Stewart, Rubber Chem. Technol., 1958, 31, 719–736 CrossRef.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies, John Wiley & Sons Ltd, 3rd edn, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, polymerisation data, polymer characterisation data. Copolymerisation of propylene and 1-hexene is discussed in the ESI. See DOI: https://doi.org/10.1039/d3cc03791f |
| This journal is © The Royal Society of Chemistry 2023 |