Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intermolecular hydrogen bonding in calix[5]arene derived cavitands regulates the molecular recognition of fullerenes†
Rubén
Álvarez-Yebra‡
 ,
Alba
Sors-Vendrell‡
,
Alba
Sors-Vendrell‡
 and
Agustí
Lledó
and
Agustí
Lledó
 *
*
Institut de Química Computacional i Catàlisi (IQCC), Universitat de Girona, Maria Aurèlia Capmany 69, 17003, Girona, Spain. E-mail: agusti.lledo@udg.edu
First published on 1st September 2023
Abstract
We present a readily available calix[5]arene derived cavitand receptor that is stabilized in the closed cone conformer through intermolecular hydrogen bonding with methanol molecules. The receptor features a highly spherical aromatic surface that binds C60 and C70 fullerenes effectively, and the binding event can be regulated allosterically by the addition of methanol.
The molecular recognition of fullerenes is a topic of current interest, mostly because of their demonstrated application as photovoltaic materials.1,2 The development of new hosts is of great interest for their use in the separation of fullerenes from soot mixtures,3–7 and for their regioselective functionalization to obtain well-defined and isomerically pure derivatives.8–12 Calix[5]arene derivatives are good candidates for the molecular recognition of fullerenes because of their shape complementarity, which allows efficient π–π stacking interactions between host and guest.13–17 Despite this, calix[5]arene hosts have not found widespread use as fullerene hosts, probably because previous strategies to enhance binding and selectivity–such as embedding two calix[5]arene units in a ditopic receptor– are synthetically cumbersome.18,19 Conversely, the longitudinal extension of calixarenes with aromatic moieties is synthetically advantageous and is a viable strategy to enhance their binding ability.20,21 We have recently developed a series of hydrogen bond stabilized calix[5]arene hosts using an amide bond formation as key step for facile diversification.22–24 The hosts so far reported in our group are based on a permethylated calix[5]arene scaffold, which favours irregularly shaped conformations rather than the spherical and symmetrical bowl structures that originate from hydrogen bonding of the lower rim phenol functions in the parent calix[5]arene. With molecular recognition of fullerenes in mind, we envisaged developing a new receptor scaffold based on the parent calixarene structure with free phenolic units at the lower rim (Fig. 1). In addition, we aimed at stabilizing the folded structure by intermolecular hydrogen bonding with solvent or “helper” molecules, as opposed to structures previously reported by us that fold through intramolecular hydrogen bonding.22–24 The new approach would be more versatile synthetically speaking, allowing an easier diversification with readily available aniline precursors. Solvent assisted folding has been previously demonstrated in resorcin[4]arene derived cavitands, albeit the covalent pre-organization in this type of receptors intrinsically favours the closed vase conformation.25 In contrast, calix[5]arene derived cavitands feature a much higher degree of flexibility, which poses a challenge to stabilize cone conformers by means of intermolecular interactions.
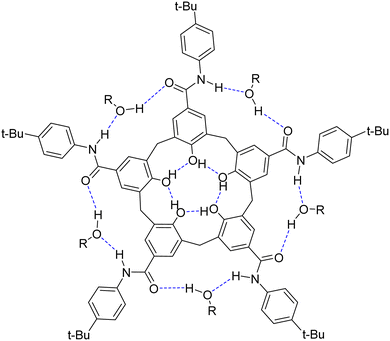 | ||
| Fig. 1 Structure of the cavitand 1 showing the stabilizing hydrogen bond network, including water or alcohol molecules. | ||
Herein, we report the first example of a calix[5]arene derived deep cavitand (1) that folds into the binding competent cone conformer with the assistance of methanol, establishing a continuous hydrogen bond seam with the amide groups of 1.
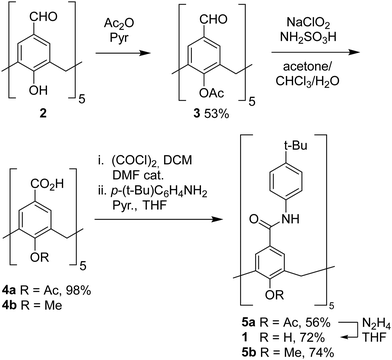
Cavitand 1 was synthesized by modifying our previously reported method with a suitable temporary protection scheme for the phenolic functions (Scheme 1). Calix[5]arene pentaaldehyde 224,26,27 was acetylated in moderate yield to obtain pentaacetate 3. The choice of protecting group is not trivial in this context, because groups larger than methyl impose significant barriers to the rotation of the aromatic panels about the methylene hinges of the calix[5]arene structure, leading to kinetically locked conformers that can hinder subsequent derivatization.28 Indeed, in the case of 3 a broad resonance is observed in the 1H NMR for the acetyl protons at 298 K. Nevertheless, this signal sharpened upon heating, making us confident that suitable conditions for subsequent derivatization could be found. Oxidation to the pentaacid derivative 4a proceeded uneventfully in excellent yield, and this precursor was then coupled with 4-(tert-butyl)aniline via the corresponding pentaacyl chloride in good yield considering the fivefold reaction. Finally, the acetyl groups of 5a were cleaved with hydrazine to obtain the targeted cavitand (1) in good yield. The O-permethylated analogue 5b was obtained in an analogous manner from pentaacid precursor 4b.
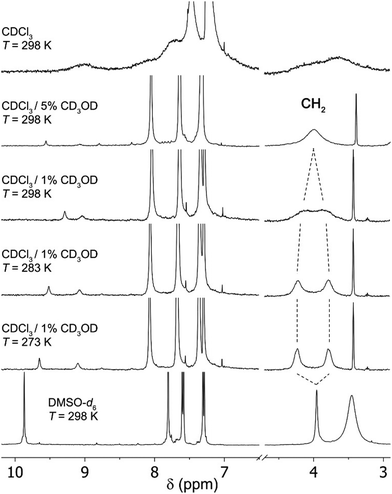
The 1H NMR spectra in CDCl3 of cavitand 1 at 298 K presents broad and poorly defined signals, indicative of mixtures of multiple slowly interconverting conformers and/or aggregation phenomena (Fig. 2). Upon addition of CD3OD into the solution (5% by volume), the resonances became sharper and well-defined, suggesting that intermolecular hydrogen bonding with methanol molecules is stabilizing monomeric cavitand species in a well-defined cone conformer. Nonetheless, this stabilizing effect is not sufficient to slow down the bowl inversion motion of the calix[5]arene core, as indicated by the appearance of the methylene bridge protons as a single resonance, rather than an AB system of diastereotopic protons as observed for related systems that are in slow exchange (in the NMR time scale). 22-24 We reasoned that while the addition of methanol would effectively provide a stabilizing effect by bridging the amide moieties along the mid-section of the cavitand, the hydrogen-bond competitive nature of methanol could disrupt the lower-rim intermolecular network of phenol groups, resulting in two opposing effects. To diminish the interference of hydrogen bonding at the lower rim, the amount of methanol was reduced to 1%, and we observed a significant broadening of the CH2 resonance, close to the coalescence point. Ultimately, upon decreasing the temperature to 283 K, the CH2 protons resolved into two separated and well-defined peaks corresponding to the expected AB spin system. The overall spectrum is consistent with a structure of averaged C5v symmetry resulting from fast rotation of the upper anilide panels about the aryl-CO bond, relative to the NMR time scale (the CH protons of the calix[5]arene core appear as a single resonance). Albeit the intensity of the NH and OH resonances of the cavitand is diminished by exchange with deuterium from CD3OD, the downfield shift observed for these resonances is in good agreement with the formation of stabilizing cooperative hydrogen bond networks. For comparison, we next assessed the behaviour in solution of 5b, an analogue of 1 lacking the ability to establish a hydrogen bond network at the lower rim. In solution of CDCl3 with 1% CH3OH, the 1H NMR spectrum of 5b displays sharp and well-defined resonances commensurate with an averaged D5h symmetry (Fig. S1, ESI†). The methylene bridge protons appear as a single sharp resonance, indicating fast cone inversion in the NMR time scale. Upon cooling, the spectrum remained unaltered even at 273 K, indicating that hydrogen bonding to methanol molecules is insufficient to stabilize 5b in folded cone conformers. Overall, these data indicates that the stabilization of 1 arise from a cooperative effect of intermolecular hydrogen bonding along the amide region and intramolecular hydrogen bonding at the phenolic lower rim. To corroborate these findings, we assessed computationally the structure of 1 bridged by 5 methanol molecules using DFT (Fig. 3). A structure minimization in implicit CHCl3 as solvent converged to a folded structure with the envisaged arrangement of methanol molecules establishing an uninterrupted cyclic hydrogen bond network with the amide groups. This arrangement preserves the array of hydrogen bonds between phenol groups at the lower rim that is characteristic of calix[5]arenes. The cavitand defines a highly spherical cavity with a total buried volume of about 1000 Å3.29
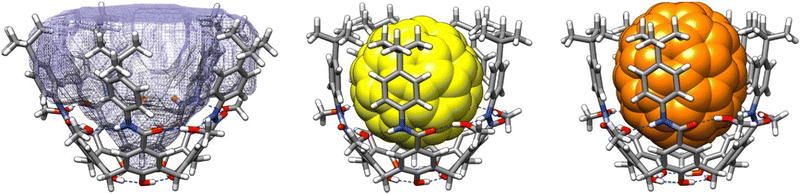 | ||
| Fig. 3 From left to right: optimized molecular models of 1·5(MeOH) (showing the available buried volume), C60⊂1·5(MeOH), and C70⊂1·5(MeOH). | ||
Having demonstrated that intermolecular hydrogen bonding to methanol is effective at stabilising the cone conformer of 1, we sought to exploit this feature in the molecular recognition of fullerenes given the existing precedents of fullerene binding by calix[5]arene derivatives. Optimization (PM7) of host–guest complex structures with C60 and C70 indicated a snug fit in both cases (Fig. 3). We next assessed qualitatively the binding of C60 and C70 by means of 1H NMR spectroscopy (Fig. S2, ESI†). Upon addition of either fullerene to a CDCl3 solution of 1, no significant changes were observed, and only broad and ill-defined resonances were observed. However, upon addition of methanol to the solution a well-resolved spectrum was obtained, suggesting that complexation occurred. The obtained spectra are commensurate with a time averaged D5h symmetrical structure resulting from fast cone–cone interconversion, and a broad peak is observed for the methylene resonances indicating a situation at the verge of coalescence. Indeed, upon cooling to 273 K the methylene signal split into the pair of diastereomeric resonances previously observed in the absence of fullerene. The OH and NH resonances became sharper and shifted downfield in agreement with a situation of higher kinetic stability of the complex. Given the fast exchange dynamics of the host–guest pair and the small shifts observed by NMR, we resorted to UV-Vis titration experiments in order to assess the corresponding binding constants (Table 1 and ESI†). The host–guest interaction of cavitand 1 and fullerenes can be visually observed by a sharp colour change upon addition of host to the fullerene solutions. For solubility reasons we used a mixture of CHCl3 and o-dichlorobenzene (o-DCB) in 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v ratio respectively.§ Upon addition of increasing amounts of 1 to C60, the colour of the solution changed from magenta to pale yellow, and the intensity of the absorption spectra increased gradually in the overall spectrum. The absorption values were extracted and fitted to a 1
10 v/v ratio respectively.§ Upon addition of increasing amounts of 1 to C60, the colour of the solution changed from magenta to pale yellow, and the intensity of the absorption spectra increased gradually in the overall spectrum. The absorption values were extracted and fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm revealing an association constant (Ka) of 6500 ± 20 M−1.30 Similarly, a solution of C70 in CHCl3/o-DCB 90
1 binding isotherm revealing an association constant (Ka) of 6500 ± 20 M−1.30 Similarly, a solution of C70 in CHCl3/o-DCB 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 experienced a change of colour from red to pale orange upon addition of increasing amounts of 1, revealing a Ka of 4020 ± 20 M−1. The formation of both complexes was also observed in the gas phase by ESI-HRMS (Fig. S3, ESI†). We next assessed the effect of methanol on the Ka, which was expected to increase by virtue of the stabilizing effect observed during NMR studies. Remarkably, titrations performed at 283 K in the presence of MeOH resulted in an increase in the Ka of both C60 and C70. With respect to titrations carried in the absence of methanol at 298 K and 283 K, a 5-fold and 10-fold increase in the Ka of C60 were obtained respectively. In the case of C70 a more moderate 1.5-fold increase in Ka was observed with respect to the reference experiment at 298 K without methanol. Somewhat surprisingly, the Ka decreased in relation to the titration experiment carried out without methanol at 283 K. These results show that intermolecular hydrogen bonding can be used to regulate allosterically the molecular recognition of fullerenes in simple calix[5]arene derived cavitands. Most remarkably, cavitand 1 provides Ka's in the 103–104 M−1 range in a highly competitive solvent (o-DCB), whereas previously reported hosts based on a single calix[5]arene macrocycle display Ka's in the 102–103 range under similar conditions.18 Constants in the range of those obtained for 1 can be replicated with receptors that feature multiple covalently tethered calix[5]arene18,19 or corannulene recognition units,31–33 but such hosts are much more challenging to synthesize and present limited diversification potential. For completeness, we also attempted fitting all our titration data to a 2
10 experienced a change of colour from red to pale orange upon addition of increasing amounts of 1, revealing a Ka of 4020 ± 20 M−1. The formation of both complexes was also observed in the gas phase by ESI-HRMS (Fig. S3, ESI†). We next assessed the effect of methanol on the Ka, which was expected to increase by virtue of the stabilizing effect observed during NMR studies. Remarkably, titrations performed at 283 K in the presence of MeOH resulted in an increase in the Ka of both C60 and C70. With respect to titrations carried in the absence of methanol at 298 K and 283 K, a 5-fold and 10-fold increase in the Ka of C60 were obtained respectively. In the case of C70 a more moderate 1.5-fold increase in Ka was observed with respect to the reference experiment at 298 K without methanol. Somewhat surprisingly, the Ka decreased in relation to the titration experiment carried out without methanol at 283 K. These results show that intermolecular hydrogen bonding can be used to regulate allosterically the molecular recognition of fullerenes in simple calix[5]arene derived cavitands. Most remarkably, cavitand 1 provides Ka's in the 103–104 M−1 range in a highly competitive solvent (o-DCB), whereas previously reported hosts based on a single calix[5]arene macrocycle display Ka's in the 102–103 range under similar conditions.18 Constants in the range of those obtained for 1 can be replicated with receptors that feature multiple covalently tethered calix[5]arene18,19 or corannulene recognition units,31–33 but such hosts are much more challenging to synthesize and present limited diversification potential. For completeness, we also attempted fitting all our titration data to a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model given existing precedents,17 resulting in a poor fit in all cases.30 Based on our volume calculations (Fig. 3), we can estimate occupancies of 26% and 29% for C60 and C70 respectively in a hypothetical closed capsule formed by two units of 1·5(MeOH), deviating significantly from Rebek's 55% rule.34
1 model given existing precedents,17 resulting in a poor fit in all cases.30 Based on our volume calculations (Fig. 3), we can estimate occupancies of 26% and 29% for C60 and C70 respectively in a hypothetical closed capsule formed by two units of 1·5(MeOH), deviating significantly from Rebek's 55% rule.34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v)
10 v/v)
In conclusion, we have synthesized a new deep cavitand receptor derived from calix[5]arene and a simple aniline that is stabilized in the cone conformation by intermolecular hydrogen bonding. The cavitand presents good complementarity with C60 and C70 fullerenes and the intermolecular hydrogen bonding manifold allows regulation of the association constants. This new host design is highly amenable to diversification, and a family of receptors could be easily obtained by varying the aniline precursor. Overall, we believe that hosts based on the structure of 1 offer great potential for the selective molecular recognition of fullerenes at a reasonable synthetic cost.
We are grateful for financial support from the Spanish Government (grants PID2020-113181GB-I00 and TED2021-130573B-I00 funded by MCIN/AEI/10.13039/501100011033 and by the European Union NextGenerationEU/PRTR) and Generalitat de Catalunya (grant 2021-SGR-623 by AGAUR). Open access funding provided thanks to the CRUE-CSIC agreement with the RSC.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Collavini and J. L. Delgado, Sustainable Energy Fuels, 2018, 2, 2480–2493 RSC.
- C.-Z. Li, H.-L. Yip and A. K. Y. Jen, J. Mater. Chem., 2012, 22, 4161–4177 RSC.
- C. García-Simón, M. Costas and X. Ribas, Chem. Soc. Rev., 2016, 45, 40–62 RSC.
- D. Canevet, E. M. Pérez and N. Martín, Angew. Chem., Int. Ed., 2011, 50, 9248–9259 CrossRef CAS PubMed.
- J. Pfeuffer-Rooschüz, S. Heim, A. Prescimone and K. Tiefenbacher, Angew. Chem., Int. Ed., 2022, 61, e202209885 CrossRef PubMed.
- E. Ubasart, C. García-Simón, M. Pujals, K. Asad, N. Chronakis, T. Parella and X. Ribas, Org. Chem. Front., 2021, 8, 4101–4105 RSC.
- G. Markiewicz, A. Jenczak, M. Kołodziejski, J. J. Holstein, J. K. M. Sanders and A. R. Stefankiewicz, Nat. Commun., 2017, 8, 15109 CrossRef PubMed.
- M. Pujals, T. Pèlachs, C. Fuertes-Espinosa, T. Parella, M. Garcia-Borràs and X. Ribas, Cell Rep. Phys. Sci., 2022, 3, 100992 CrossRef CAS.
- E. Ubasart, O. Borodin, C. Fuertes-Espinosa, Y. Xu, C. García-Simón, L. Gómez, J. Juanhuix, F. Gándara, I. Imaz, D. Maspoch, M. von Delius and X. Ribas, Nat. Chem., 2021, 13, 420–427 CrossRef CAS PubMed.
- V. Leonhardt, S. Fimmel, A.-M. Krause and F. Beuerle, Chem. Sci., 2020, 11, 8409–8415 RSC.
- C. Fuertes-Espinosa, C. García-Simón, M. Pujals, M. Garcia-Borràs, L. Gómez, T. Parella, J. Juanhuix, I. Imaz, D. Maspoch, M. Costas and X. Ribas, Chemistry, 2020, 6, 169–186 CrossRef CAS.
- Z. Lu, T. K. Ronson, A. W. Heard, S. Feldmann, N. Vanthuyne, A. Martinez and J. R. Nitschke, Nat. Chem., 2023, 15, 405–412 CrossRef CAS PubMed.
- T. Hirao and T. Haino, Chem. – Asian J., 2022, 17, e202200344 CrossRef CAS PubMed.
- A. Ikeda, Y. Suzuki, M. Yoshimura and S. Shinkai, Tetrahedron, 1998, 54, 2497–2508 CrossRef CAS.
- J. L. Atwood, L. J. Barbour, M. W. Heaven and C. L. Raston, Chem. Commun., 2003, 2270–2271 RSC.
- J. L. Atwood, L. J. Barbour and C. L. Raston, Cryst. Growth Des., 2002, 2, 3–6 CrossRef CAS.
- T. Haino, M. Yanase and Y. Fukazawa, Angew. Chem., Int. Ed. Engl., 1997, 36, 259–260 CrossRef CAS.
- T. Haino, M. Yanase, C. Fukunaga and Y. Fukazawa, Tetrahedron, 2006, 62, 2025–2035 CrossRef CAS.
- T. Haino, C. Fukunaga and Y. Fukazawa, Org. Lett., 2006, 8, 3545–3548 CrossRef CAS PubMed.
- Y.-X. Yue, Z. Zhang, Z.-H. Wang, R. Ma, M.-M. Chen, F. Ding, H.-B. Li, J.-J. Li, L. Shi, Y. Liu and D.-S. Guo, Small Struct., 2022, 3, 2200067 CrossRef CAS.
- T.-X. Zhang, J.-J. Li, H.-B. Li and D.-S. Guo, Front. Chem., 2021, 9, 710808 CrossRef CAS PubMed.
- R. Álvarez-Yebra, R. López-Coll, P. Galán-Masferrer and A. Lledó, Org. Lett., 2023, 25, 3190–3194 CrossRef PubMed.
- R. Álvarez-Yebra, R. López-Coll, N. Clos-Garrido, D. Lozano and A. Lledó, Isr. J. Chem., 2023, e202300077 CrossRef.
- D. Lozano, R. Álvarez-Yebra, R. López-Coll and A. Lledó, Chem. Sci., 2019, 10, 10351–10355 RSC.
- A. R. Far, A. Shivanyuk and J. Rebek, J. Am. Chem. Soc., 2002, 124, 2854–2855 CrossRef CAS PubMed.
- J. Garcia-Hartjes, S. Bernardi, C. A. G. M. Weijers, T. Wennekes, M. Gilbert, F. Sansone, A. Casnati and H. Zuilhof, Org. Biomol. Chem., 2013, 11, 4340–4349 RSC.
- S. Pasquale, S. Sattin, E. C. Escudero-Adán, M. Martínez-Belmonte and J. de Mendoza, Nat. Commun., 2012, 3, 785 CrossRef PubMed.
- D. R. Stewart, M. Krawiec, R. P. Kashyap, W. H. Watson and C. D. Gutsche, J. Am. Chem. Soc., 1995, 117, 586–601 CrossRef CAS.
- N. R. Voss and M. Gerstein, Nucleic Acids Res., 2010, 38, W555–W562 CrossRef CAS PubMed.
- D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC.
- A. Sacristán-Martín, D. Miguel, A. Diez-Varga, H. Barbero and C. M. Álvarez, J. Org. Chem., 2022, 87, 16691–16706 CrossRef PubMed.
- D.-C. Yang, M. Li and C.-F. Chen, Chem. Commun., 2017, 53, 9336–9339 RSC.
- A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842–3843 CrossRef CAS PubMed.
- S. Mecozzi and J. J. Rebek, Chem. – Eur. J., 1998, 4, 1016–1022 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization for compounds 3-5b. UV-Vis titration experiments. Copies of NMR (1H, 13C, 2D) and HRMS spectra of new compounds. See DOI: https://doi.org/10.1039/d3cc03780k. Spectroscopic data are available from the CORA repository, https://doi.org/10.34810/data780. Computational data are available from the ioChem-BD repository, https://doi.org/10.19061/iochem-bd-4-63 |
| ‡ These authors contributed equally. |
| § This mixture provided good solubility throughout all the titrations. Other mixtures also including toluene and CS2 were tested, but they did not completely dissolve one of components or resulted in appearance of precipitates as the titration progressed. |
| This journal is © The Royal Society of Chemistry 2023 |