Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Localised polymerisation of acrylamide using single-barrel scanning electrochemical cell microscopy†
Mahir
Mohammed
,
Bryn A.
Jones
,
Evelina
Liarou
 and
Paul
Wilson
and
Paul
Wilson
 *
*
Department of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: p.wilson.1@warwick.ac.uk
First published on 14th August 2023
Abstract
Single-barrel scanning electrochemical cell microscopy has been adapted for polymerisation of acrylamide in droplet cells formed at gold electrode surfaces. Localised electrochemical atom transfer radical polymerisation enables controlled synthesis and deposition of polyacrylamide or synthesis of polyacrylamide brushes from initiator-functionalised electrode surfaces.
The ability to create, control and interrogate interfaces with micro- to nanoscale resolution remains a challenge in the fields of microfabrication and nanotechnology. Top-down approaches are typically destructive and unsuitable for the patterning soft materials based on organic or biological macromolecules,1 required for a variety of applications e.g. (nano)medicine,2 (bio)electronics,3 and cyptography.4 Thus, the development of bottom-up, soft lithography techniques has become increasingly important. State-of-the-art techniques include micro-contact printing (μCP),5 photolithography (PL)6 and block copolymer self-assembly,7 while probe-based methods such as dip-pen nanolithography (DPN),8 polymer-pen lithography9 offer precise control over feature resolution.
Scanning electrochemical cell microscopy (SECCM)10 has been developed as a meniscus-based technique for high resolution synchronised electrochemical and topographical imaging enabled by integration of a probe positional feedback mechanism.10–12 Conventional SECCM operates on dry electrode surfaces in which electrochemical cells are confined to droplets formed between an electrolyte filled dual-barrel nanopipette and the surface of interest. This configuration of SECCM has been exploited for micro- and nanoscale electrochemical patterning of surfaces.13–16 It has also been deployed for attempted localised surface-initiated electrochemical atom transfer radical polymerisation (SI-eATRP).17 Polymerisation of N-hydroxyethylacrylamide (HEAm) was realised in droplet cells formed at gold substrates modified with a self-assembled monolayer (SAM) presenting an ATRP initiator. However, using CuII/Me6TREN at Vsurf < −0.5 V (vs. Ag+/AgCl) reduction of dissolved oxygen at the electrode resulted in formation of reactive oxygen species (ROS) in the droplets capable of initiating radical polymerisation which competed with the desired SI-eATRP process (Fig. 1, left). To improve control over the initiation process we have been investigating the use of less reducing Cu-complexes in laboratory-scale ‘plug-and-play’ simplified eATRP for translation to SECCM.18–21
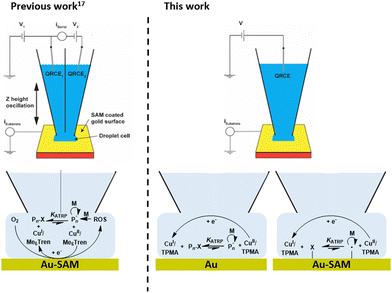 | ||
| Fig. 1 Schematic showing the operationally complex dual-barrel SECCM configuration and Cu/Me6Tren as a catalyst for localised eATRP which was found to proceed with a competing undesirable ROS-initiated mechanism (left).17 Here we employ simplified single-barrel SECCM for the first time to control eATRP both at and from the surface of a gold electrode (right). | ||
Likewise, an operationally simplified single barrel SECCM configuration has also been developed.11,22 Dual barrel SECCM involves application of a potential bias between quasi-reference counter electrodes (QRCEs) placed in the barrels of the probe. A voltage-wave generator (external or via a lock-in amplifier) is required to measure and control oscillations of the probe, sinusoidally, normal to the surface. An induced ac component of the ionic conductance (barrel) current (iac) is then used as a feedback signal to control the distance between the end of the probe and the surface. Conversely, positional feedback in single barrel SECCM is conferred by detection to surface current (isurf) only. When the nanopipette meniscus makes contact with the surface creating a two-electrode droplet electrochemical cell in which the response of isurf to a reaction at a given Vsurf can be used to monitor a reaction of interest localised to the droplet cell. This significantly simplifies the instrumentation and software required to perform experiments (e.g. no lock-in amplifier needed, Fig. S2, ESI†). The simplicity and ease of operation is a significant advantage in attempting microscale and nanoscale deposition,23 and it is still possible to perform electrochemical mapping and topographical measurements.24
Here, we report the first use of single-barrel SECCM for eATRP of acrylamide (Am) using CuII/tris(2-pyridylmethyl)amine (CuII/TPMA), overcoming some of limitations of our previous system. At appropriate Vsurf, eATRP enables controlled synthesis and deposition of polyacrylamide (PAm) at gold surfaces, whilst at initiator-functionalised electrode surfaces the controlled formation of PAm brushes is also possible (Fig. 1, right).
An aqueous solution of CuII/TPMA (6.9 mM) in a single barrel nanopipette (1 μm diameter) was delivered to a gold electrode surface in a 2 × 2 array (depicted in Fig. S3, ESI†). A threshold current (isurf = −1 × 10−11 A) was set to create an electrochemical cell confined to the dimensions of the droplet formed at the electrode surface. Voltametric analysis by cyclic voltammetry (CV) revealed the onset of a reduction event at E = −0.30 V reaching ipc = −2.6 × 10−10 A at Epc = −0.6 V (Fig. S4A, ESI†). This was assigned to the reduction of CuII/TPMA to CuI/TPMA.26,27 The absence of an oxidation peak in the anodic scan is attributed to oxidation of CuI/TPMA to CuII/TPMA by dissolved oxygen in the solution. AFM of the landing sites revealed the landing foot-print of the nanopipette but no significant feature deposition (Fig. S4B, ESI†) When acrylamide (Am) was added to the solution (10 wt%) the onset of reduction shifted to E = −0.18 V which is typical for aqueous solutions monomer in macroscopic CV.25 The magnitude of the current at Epc = −0.6 V increased to ipc = −2.6 × 10−10 A. On one hand the increase in ipc can be explained by the aqueous solution of monomer wetting the surface leading to larger droplet sizes. On the other hand, at more reducing potentials it is possible that some free radical polymerisation (FRP) of Am occurs during the CV scan which could effect the surface wettability. Indeed, AFM analysis revealed the presence of more prominent features (Fig. S4C, ESI†). Addition of 2-hydroxyethyl 2-bromoisobutyrate (HEBiB; 0.23 M) to the solution resulted in an increase in ipc from −5.7 × 10−10 A to ipc = −1.4 × 10−9 A, arising from the electrochemical reduction of CuII/TPMA to CuI/TPMA followed by fast activation of HEBiB by the in situ generated CuI/TPMA species.26,27 AFM analysis revealed the formation of prominent features under these conditions (Fig. S4D, ESI†).
Voltamettric analysis (CV) performed on aqueous solutions of Am only, using KNO3 as an electrolyte, revealed that Am was not redox active (Fig. S5, ESI†). Conversely, solutions containing Am and HEBiB in aqueous KNO3 exhibited a reduction event at E < −0.2 V, which can be attributed to direct reduction of HEBiB.28 Chronoamperometric analysis was performed on droplet cells, containing only aqueous Am and HEBiB, at gold surfaces. The potential was biased at 50 mV intervals between −0.2 V < Vsurf < −0.05 V (vs. Ag+/AgCl, QRCE) to generate i vs. t traces. Only capacitative currents were observed (Fig. S6A, ESI†). However, when CuII/TPMA was added, faradaic currents that increased with decreasing (more reducing) Vsurf were observed, indicative of the electrochemical reduction of CuII/TPMA followed by activation of HEBiB (Fig. S6B, ESI†).
Based on the results obtained from CV and i vs. t measurements, reaction solutions containing [Am]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [HEBiB]
[HEBiB]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuII/TPMA] = [20]
[CuII/TPMA] = [20]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [1]
[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [0.5]
[0.5]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
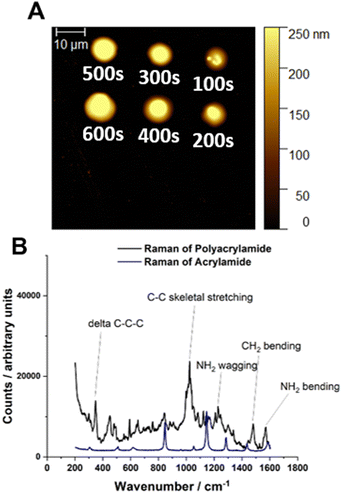
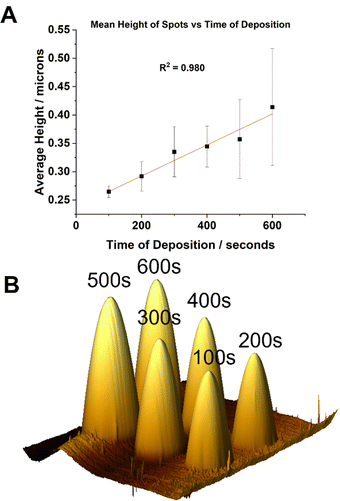
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [0.625] were prepared and loaded into single barrelled nanopipettes to be delivered to a gold electrode substrate biased to Vsurf = −0.15 V. At this surface potential the time of deposition (TOD) for the proposed eATRP of PAm within the droplet cells was varied (TOD = 100–600 s). From a qualitative point of view, analysis by AFM indicated that the dimensions of the features increased with increasing TOD (Fig. 2A). After repeated experiments the average height of the features was shown to increase with increasing TOD (Fig. S7–S9, ESI†). This is indicative of well controlled eATRP of Am occurring within the droplet cells.
[0.625] were prepared and loaded into single barrelled nanopipettes to be delivered to a gold electrode substrate biased to Vsurf = −0.15 V. At this surface potential the time of deposition (TOD) for the proposed eATRP of PAm within the droplet cells was varied (TOD = 100–600 s). From a qualitative point of view, analysis by AFM indicated that the dimensions of the features increased with increasing TOD (Fig. 2A). After repeated experiments the average height of the features was shown to increase with increasing TOD (Fig. S7–S9, ESI†). This is indicative of well controlled eATRP of Am occurring within the droplet cells.
The applied potential was then varied with TOD fixed at 200 s. Decreasing the Vsurf in −10 mV increments from −0.15 V to −0.22 V revealed little correlation between Vsurf and the amount of PAm formed within each droplet cell (Fig. S10–S12, ESI†). We were not surprised by this result as on the laboratory scale we have shown more reducing potentials yields slower reactions, lower conversions, and poor control over the polymerisation of HEAm performed at room temperature, and lower temperatures are required to achieve electrochemical control.21
The amount of polymer synthesised during SECCM-based experiments limits the methods available for sample characterisation. Conventional analysis by size-exclusion chromatography (SEC) and nuclear magnetic resonance (NMR) spectroscopy is not possible. In our previous work with used X-ray photoelectron spectroscopy (XPS) to characterise the polymer formed.17 Here, Raman spectroscopy was used to confirmed by the presence of bands assigned to C–C skeletal stretching (629 cm−1), delta C–C–C stretching (348 cm−1), NH2 wagging (1228 cm−1)/bending (1568 cm−1) and CH2 bending (1480 cm−1), which is consistent with spectra of PAm reported in literature (Fig. 2C).29 Furthermore, SEM-EDX analysis of PAm features (Fig. S13, ESI†) confirmed the deposition of organic material with strong signals for C (carbon), N (nitrogen), and O (oxygen) all present.
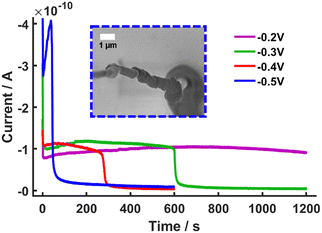
To explore the limits of the system, the reducing potential was changed from Vsurf = −0.2 V to Vsurf = −0.5 V (vs. Ag/AgCl) in 100 mV increments. This led to interesting i vs. t traces which showed an initial increase in current at the beginning of the polymerisation, follow by a sharp decrease in current to near zero values (Fig. 3A). This sharp fall in current has been observed previously when single-barrel nanopipettes, in the related scanning ion conductance microscopy (SICM), have been employed to electrochemically interrogate crystallization processes. Therein, the build-up of material in the aperture of probes resulted in ‘blocking’ and loss of the connection between the QRCE in the nanopipette and the one in the bulk solution.30,31
Here the ‘blocking’ is attributed to the formation of insulating PAm gel formed in the droplet and the barrel of the nanopipette leading to a sharp fall in current and eventual loss of the connection between the QRCE in the nanopipette and the electrode surface in this case (Fig. 3 and Fig. S14A–D and S15, ESI†). Plots of the average blocking time as a function of the Vsurf (Fig. S14E, ESI†), indicated strong correlation between Vsurf and the blocking time. The formation of the PAm gel occurs via FRP initiated by radicals formed through direct reduction of HEBiB at the electrode surface. Furthermore, the amount of CuII/TPMA deactivator, required to maintain control of the eATRP mechanism, would significantly decrease with decreasing Vsurf.32 The formation of these gel-type features is further evidence that is it possible to prepare PAm using SECCM. More significantly, it indicates that there is also a distinct mechanistic shift from a regime of controlled eATRP to uncontrolled FRP which occurs at Vsurf ≈ −0.2 V. This represents significant progress compared to our previous work in which competing initiation mechanisms could not be decoupled.17
Finally, to highlight the potential of performing localised eATRP of Am using single-channel SECCM, a self-assembled monolayer (SAM) of bis[2-(2-bromoisobutyryloxy)ethyl] disulphide was formed on the gold substrate (Fig. S1, ESI†) to carry out surface-initiated eATRP (SI-eATRP). CV was performed on a droplet cell formed on the SAM-functionalised gold electrode. Pleasingly a reduction event with an onset of E = −0.06 V, assigned to reduction of CuII/TPMA to CuI/TPMA followed by activation of the surface bound initiator, was observed (Fig. S16, ESI†).
Localised SI-eATRP of Am was performed by bringing a nanopipette, containing an aqueous solution of CuII/TPMA (6.9 mM) and Am (10 wt%), to the SAM-functionalised surface biased at Vsurf = −0.15 V. The period of the time the meniscus was held at the SAM surface was increased from 100 s to 600 s in 100 s increments. A linear correlation between reaction time and the average feature height was observed which is indicative of good control over polymer brush growth (Fig. 4A and B; Fig. S17 and S18, ESI†).33 There was very little change in the radial dimensions of the features formed (Fig. S19, ESI†). This provides additional evidence for PAm brush growth occurring perpendicular to the surface which has less of an effect on surface wettability compared to the synchronous synthesis and deposition of PAm at virgin gold electrode surfaces (vide supra). The features formed on the SAM-functionalised gold substrate remained after an aqueous rinse, providing strong evidence of SI-eATRP occurring from the initiator bound to the gold surface (Fig. S17, ESI†).
In conclusion, single-barrel SECCM has been employed to achieve localised eATRP of Am in droplet cells formed at gold electrode surfaces. The use of CuII/TPMA, provides control over the polymerisation whilst a mechanistic shift from eATRP to FRP has been observed at Vsurf ≈ −0.2 V, with eATRP favoured at Vsurf > −0.2 V. SI-eATRP of Am from a SAM-functionalised gold electrode surface demonstrated control over the PAm brush height formed as a function of reaction time. This has highlighted the potential of single-barrel SECCM for the localised synthesis and deposition of soft materials. It also provides a simple and efficient platform to screen potential polymerisation conditions for translation to dual-barrel SECCM which can be used for more complex patterning of substrates.
The authors thank the Polymer Characterization and Microscopy Research Technology Platforms. P. W. thanks the Royal Society and Tata companies (URF\R1\180274) and the EPSRC for funding (studentship, M. M.; EP/R513374/1). E. L. acknowledges the Institute of Advanced Studies and the Eutopia Science and Innovation Fellowship for funding (European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 945380).
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. B. Braunschweig, F. Huo and C. A. Mirkin, Nat. Chem., 2009, 1, 353–358 CrossRef CAS PubMed.
- J. K. Patra, et al. , J. Nanobiotechnol., 2018, 16, 71 CrossRef PubMed.
- A. Q. Zhang and C. M. Lieber, Chem. Rev., 2016, 116, 215–257 CrossRef CAS PubMed.
- Y. S. Zholdassov, D. J. Valles, S. Uddin, J. Korpanty, N. C. Gianneschi and A. B. Braunschweig, Adv. Mater., 2021, 33, e210080 CrossRef PubMed.
- B. D. Gates, Q. Xu, M. Stewart, D. Ryan, C. G. Willson and G. M. Whitesides, Chem. Rev., 2005, 105, 1171–1196 CrossRef CAS PubMed.
- B. Li, M. He, L. Ramirez, J. George and J. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 4158–4164 CrossRef CAS PubMed.
- L. Shen, C. He, J. Qiu, S.-M. Lee, A. Kalita, S. B. Cronin, M. P. Stoykovich and J. Yoon, ACS Appl. Mater. Interfaces, 2015, 7, 26043–26049 CrossRef CAS PubMed.
- M. Guardingo, P. Gonzalez-Monje, F. Novio, E. Bellido, F. Busque, G. Molnar, A. Bousseksou and D. Ruiz-Molina, ACS Nano, 2016, 10, 3206–3213 CrossRef CAS PubMed.
- F. Huo, Z. Zheng, G. Zheng, L. R. Giam, H. Zhang and C. A. Mirkin, Science, 2008, 321, 1658–1660 CrossRef CAS PubMed.
- N. Ebejer, M. Schnippering, A. W. Colburn, M. A. Edwards and P. R. Unwin, Anal. Chem., 2010, 82, 9141–9145 CrossRef CAS PubMed.
- C. L. Bentley, M. Kang and P. R. Unwin, J. Am. Chem. Soc., 2017, 139, 16813–16821 CrossRef CAS PubMed.
- C. L. Bentley, M. Kang, F. M. Maddar, F. Li, M. Walker, J. Zhang and P. R. Unwin, Chem. Sci., 2017, 8, 6583–6593 RSC.
- P. M. Kirkman, A. G. Guell, A. S. Cuharuc and P. R. Unwin, J. Am. Chem. Soc., 2013, 136, 36–39 CrossRef PubMed.
- K. McKelvey, M. A. O’Connell and P. R. Unwin, Chem. Commun., 2013, 49, 2986–2988 RSC.
- H. V. Patten, L. A. Hutton, J. R. Webb, M. E. Newton, P. R. Unwin and J. V. Macpherson, Chem. Commun., 2015, 51, 164–167 RSC.
- A. N. Patel, K. McKelvey and P. R. Unwin, J. Am. Chem. Soc., 2012, 134, 20246–20249 CrossRef CAS PubMed.
- E. E. Oseland, Z. J. Ayres, A. Basile, D. M. Haddleton, P. Wilson and P. R. Unwin, Chem. Commun., 2016, 52, 9929–9932 RSC.
- B. Zhao, M. Mohammed, B. A. Jones and P. Wilson, Chem. Commun., 2021, 57, 3897–3900 RSC.
- B. Zhao, F. Pashley-Johnson, B. A. Jones and P. Wilson, Chem. Sci., 2022, 13, 5741–5749 RSC.
- B. Zhao, J. Cheng, J. Gao, D. M. Haddleton and P. Wilson, Macromol. Chem. Phys., 2023, 7, 2300039 CrossRef.
- M. Mohammed, B. A. Jones and P. Wilson, Polym. Chem., 2022, 13, 3460–3470 RSC.
- E. Daviddi, Z. Chen, B. Beam Massani, J. Lee, C. L. Bentley, P. R. Unwin and E. L. Ratcliff, ACS Nano, 2019, 13, 13271–13284 CrossRef CAS PubMed.
- J. Hengsteler, B. Mandal, C. Nisselroy, G. P. S. Lau, T. Schlotter, T. Zambelli and D. Momotenko, Nano Lett., 2021, 21, 9093–9101 CrossRef CAS PubMed.
- V. Shkirskiy, L. C. Yule, E. Daviddi, C. L. Bentley, J. Aarons, G. West and P. R. Unwin, J. Electrochem. Soc., 2020, 167, 041507 CrossRef CAS.
- M. Fantin, A. A. Isse, K. Matyjaszewski and A. Gennaro, Macromolecules, 2017, 50, 2696–2705 CrossRef CAS.
- N. Bortolamei, A. A. Isse, A. J. D. Magenau, A. Gennaro and K. Matyjaszewski, Angew. Chem., Int. Ed., 2011, 123, 11593–11596 CrossRef.
- S. Park, P. Chmielarz, A. Gennaro and K. Matyjaszewski, Angew. Chem., Int. Ed., 2015, 54, 2388–2392 CrossRef CAS PubMed.
- M. Fantin, A. A. Isse, K. Matyjaszewski and A. Gennaro, Macromolecules, 2017, 50, 2696–2705 CrossRef CAS.
- M. K. Gupta and R. Bansil, J. Polym. Sci. Polym. Phys., 1981, 19, 353–360 CrossRef CAS.
- F. M. Maddar, D. Perry and P. R. Unwin, Crystal Growth Design, 2017, 17, 6565–6571 CrossRef CAS.
- D. Perry, A. S. Parker, A. Page and P. R. Unwin, ChemElectroChem, 2016, 3, 2212–2220 CrossRef CAS.
- A. J. D. Magenau, N. Bortolamei, E. Frick, S. Park, A. Gennaro and K. Matyjaszewski, Macromolecules, 2013, 46, 4346–4353 CrossRef CAS.
- B. Li, B. Yu, W. T. S. Huck, W. Liu and F. Zhou, J. Am. Chem. Soc., 2013, 135, 1708–1710 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc03582d |
| This journal is © The Royal Society of Chemistry 2023 |