Open Access Article
Open Access ArticleSpecific N-terminal attachment of TMTHSI linkers to native peptides and proteins for strain-promoted azide alkyne cycloaddition†
Matt
Timmers
 *ab,
Wouter
Peeters
c,
Niels J.
Hauwert
*ab,
Wouter
Peeters
c,
Niels J.
Hauwert
 d,
Cristianne J. F.
Rijcken
b,
Tina
Vermonden
d,
Cristianne J. F.
Rijcken
b,
Tina
Vermonden
 a,
Ingrid
Dijkgraaf
a,
Ingrid
Dijkgraaf
 *c and
Rob M. J.
Liskamp
*c and
Rob M. J.
Liskamp
 *bce
*bce
aDepartment of Pharmaceutics, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht 3584 CG, The Netherlands
bCristal Therapeutics, Maastricht 6229 EV, The Netherlands
cDepartment of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht 6229 ER, The Netherlands
dSymeres, Kerkenbos 1013, Nijmegen 6546 BB, The Netherlands
eSchool of Chemistry, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: robert.liskamp@glasgow.ac.uk
First published on 30th August 2023
Abstract
The site specific attachment of the reactive TMTHSI-click handle to the N-terminus of peptides and proteins is described. The resulting molecular constructs can be used in strain-promoted azide alkyne cycloaddition (SPAAC) for reaction with azide containing proteins e.g., antibodies, peptides, nanoparticles, fluorescent dyes, chelators for radioactive isotopes and SPR-chips etc.
Selective modification of the N-terminus of peptides or proteins remains a challenge in view of the usually abundant presence of functional and hence reactive side chains of amino acids. However, in many cases site specific N-terminal modification is required to circumvent interference with active or binding domains. These domains play crucial roles in the biological activity of for example antibodies, cell penetrating peptides, signalling or immunologically active peptides and as a consequence should not be modified. Recently, MacDonald et al.1 have developed a highly suitable reagent for specific N-terminal modification of native peptides and proteins. They showed a specific reaction of the 2-pyridinecarboxaldehyde (2PCA) moiety with the N-terminus of peptides and proteins. The aim of this research was to combine this versatile 2PCA reagent with a highly reactive click moiety capable of a fast strain promoted azide alkyne cycloaddition (SPAAC). After N-terminal specific introduction of this novel molecular combination the click moiety can then undergo a mild and efficient reaction with relatively easy accessible azide containing compounds, not requiring the presence of a copper catalyst‡. We realised that it might be challenging to develop a molecular combination of a reactive click moiety and 2PCA followed by its specific N-terminal introduction. However, the relatively easy accessibility of a diversity of azides present in antibodies and other proteins, peptides, nucleic acids, nano particles, fluorescent dyes, chelators for radioactive isotopes, SPR surfaces etc. made this the preferred approach instead of synthesis of an azide containing 2PCA reagent, which then has to react with much more difficult accessible click moiety containing antibodies etc.
We wished to combine the 2PCA reagent with our recently published tetramethylthiocycloheptyne sulfoximine (TMTHSI)-click handle.2 This click reagent is the fastest reacting SPAAC reagent to date. TMTHSI is small, and not sterically crowded to reduce any observer effect, that is a biological response induced by the steric bulk of the reagent on the formed construct.2,3 This molecular combination of TMTHSI and 2PCA should then specifically react with the N-terminus of a peptide or protein after which a SPAAC reaction is possible. Thus, we describe the synthesis of TMTHSI-2PCA linker constructs, show the specific N-terminal introduction onto peptides and a protein as well as purification of the obtained molecular constructs. Finally, SPAAC of selected TMTHSI molecular constructs is illustrated especially by the successful preparation of a protein construct from an azide containing protein component and the N-terminal TMTHSI moiety containing Evasin-3 protein.
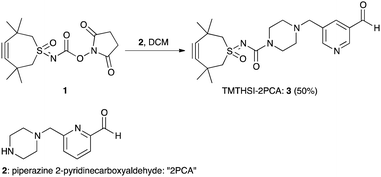
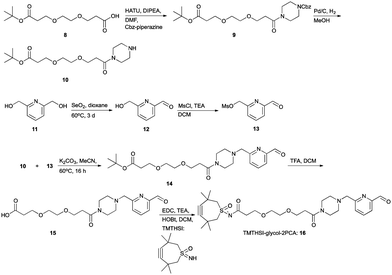
The first click construct (3) was conveniently obtained in 50% yield by combining piperazine-2PCA (2) and TMTHSI in a reaction with our earlier described TMTHSI-hydroxy succinimide derivative 1 (Scheme 1 and ESI†).
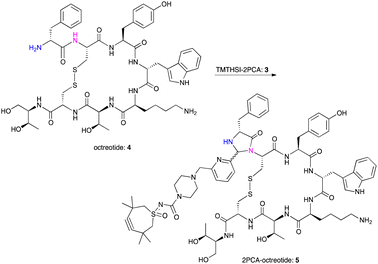
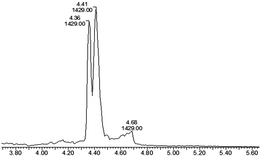
Octreotide 4, a clinically used peptide for the treatment of acromegaly, was used for selective coupling to this new TMTHSI-2PCA reagent 3. Since octreotide is a cyclized peptide, containing several functionalities including a lysine side chain NH2 and a disulfide bridge, it is a relevant model to evaluate the N-terminal specificity of reagent 3. After reaction with 15 eq. of TMTHSI-2PCA 3, a >90% conversion was achieved based on the decrease of octreotide peak shown by HPLC (Scheme 2, details in ESI,† Section S2). Octreotide was selectively functionalized on the N-terminus as was proven by LC-MS (Fig. 1). Not only was the expected mass of the modified octreotide observed, but also proof of the occurrence of cyclization, resulting from the N-terminal specific Edman-degradation-like cyclization reaction4 was apparent from the presence of two diastereomers, having the same mass and which could be separated by LC-MS.
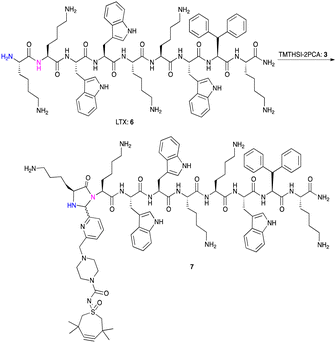
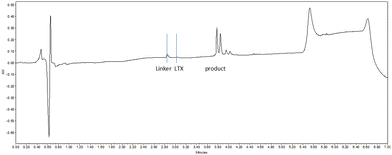
Next, we exposed the oncolytic LTX-315 peptide5,66 containing several (five) lysine residues to TMTHSI-2PCA 3 (Scheme 3). Here too, a LTX peptide adduct 7 was formed having only one TMTHSI-linker moiety (Fig. 2). As expected, no (stable) Schiff base adducts were formed with the side chains of the lysine residues. As with octreotide, the N-terminal modification of LTX-315 was also apparent from the formation of a diastereomeric mixture (Fig. 2).
Thus, the TMTHSI-2PCA 3 reagent is a suitable reagent for N-terminus site specific introduction of a TMTHSI-containing linker to peptides.
For larger peptides and proteins, a TMTHSI derivative (16) with a larger distance between the aldehyde reacting with the N-terminus and the TMTHSI-moiety was envisioned (Scheme 4), to avoid any steric encumbrance in both the N-terminal specific reaction and the subsequent SPAAC. A suitable distance between the SPAAC moiety and the site of attachment of the reagent in a sizable peptide or protein will be beneficial for the final bioconjugate. The larger distance formed by an ethylene glycol spacer may also be favourable for water solubility of the construct. The TMTHSI-glycol-2PCA derivative 16 was obtained in a straightforward 7-step synthesis in an overall yield of 25% starting from ethylene glycol derivative 8 and pyridine bismethylene alcohol 11 (Scheme 4, details in ESI,† Section S3).
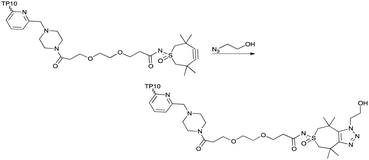
This TMTHSI-glycol-2PCA derivative 16 was used in the N-terminal modification of the peptide angiopep2 17 (Scheme 5). Angiopep2 is a conjugate peptide to facilitate intracellular delivery and blood–brain barrier transport of oligonucleotides.7–10 The crude reaction mixture 18 required extensive purification to remove the excess of unreacted TMTHSI-glycol-2PCA 16. Fortunately, excess of TMTHSI-glycol-2PCA can be removed by incubation with hydroxylamine-containing beads (Scheme 5). These beads capture the aldehyde of TMTHSI-glycol-2PCA by formation of an oxime, which is illustrated in the work-up of the reaction of peptide TP10 (AGYLLGKINLKALAALAKKIL, membrane-transporting peptide11–13) 17 with hydroxyl amine beads (Scheme 5, bottom and ESI,† Section S4).
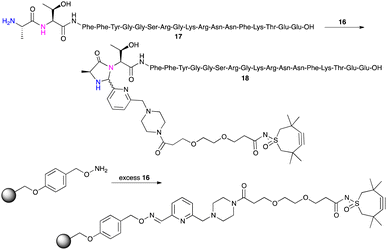 | ||
| Scheme 5 Reaction of 17 with TMTHSI-glycol-2PCA (top) and capture of excess of TMTHSI-glycol-2PCA 16 using hydroxyl amine beads (bottom). | ||
The TMTHSI-moiety integrity and reactivity was monitored by addition of azido-ethanol to the worked-up product of the N-terminal modified TP10 peptide (Scheme 6). This resulted in a complete shift of the diastereomeric peptides to the left, a more polar region of the HPLC-trace indicating a full reactivity of the TMTHSI-modified peptide (ESI,† Section S5).
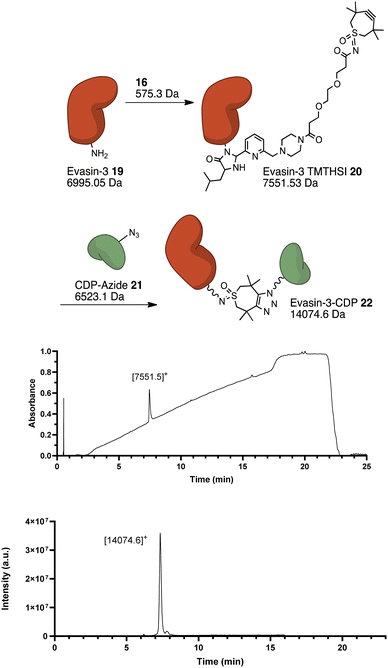
Finally, TMTHSI-glycol-2PCA was evaluated for its ability to specifically react with the N-terminus of a protein. Here, Evasin-3 19 was selected, This is a protein which shows a high affinity to CXC-type chemokines, in particular CXCL1 and CXCL8, inflammatory chemokines associated with the development of numerous diseases14 such as cancer and atherosclerosis. N-terminal modification of 19 with 16 will enable attachment of the bioconjugate to a bacterial chimeric designer protein (CDP)15 to boost the immune recognition (Fig. 3).
Thus, 12 equivalents of 16 were reacted with Evasin-3 19 for 26 hours. While no full conversion of Evasin-3 19 was observed (ESI,† Section S6), separation on HPLC was possible and therefore we could purify Evasin-3-TMTHSI 20 by preparative HPLC in a yield of 40%. Considering that only 12 equivalents of 16 were used and preparative HPLC purification was performed, a yield of 40% of pure product was quite satisfactory.
Next, conjugation with azide-functionalised CDP 21 was carried out (Fig. 3). Two equivalents of Evasin-3-TMTHSI 20 were added dropwise to 1 equivalent of CDP-azide 21. After reacting for 3.5 hours, the reaction mixture was purified using preparative HPLC. This resulted in a pure Evasin-CDP conjugate 22, as was confirmed by MS. The yield of this conjugate after purification was 60%, which is certainly acceptable considering loss of product during preparative HPLC.
We have synthesized clickable, N-terminal site-specific constructs TMTHSI-2PCA and TMTHSI-glycol-2PCA. The synthesis of both derivatives proceeded without problems, which certainly is not obvious using very reactive click compounds (e.g., BARAC).16 These reagents have proven to be N-terminal specific, and the reaction via cyclization was demonstrated by observation of the formation of diastereomers on HPLC and confirmed by mass spectrometry. Pleasantly, also in the N-terminal reaction no side products have been observed despite the possible formation of an intermediate diene, in principle capable of reacting with the TMTHSI-moiety.4 Furthermore, we found that it was possible to use a much smaller excess of 2PCA-reagent than was earlier reported1 and that if necessary, excess can be efficiently removed by capture using functionalized (hydroxyl amine containing) beads. This purification strategy together with HPLC purification has been used to determine yields which is an improvement compared to determination of conversion yields solely based on MS.1
These clickable N-terminal specific constructs can also be used in larger proteins, as was demonstrated by the clicking of Evasin-3 with CDP-azide. These combined findings demonstrate the high potential of these TMTHSI-2PCA reagents for in-solution N-terminal specific introduction of a click handle in peptides and proteins. As such this approach affords pure N-terminally click-reactive peptides and proteins leaving their internal functional domains unaffected. It is expected that this specific bioconjugation strategy will soon enable a wealth of therapeutic and diagnostic applications facilitated by the convenient SPAAC-based possible introduction of a variety of azide containing (bio)molecular constructs originating from antibodies, nucleic acids, nanoparticles, surfaces etc.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. I. MacDonald, H. K. Munch, T. Moore and M. B. Francis, One-step site-specific modification of native proteins with 2-pyridinecarboxyaldehydes, Nat. Chem. Biol., 2015, 11(5), 326–331 CrossRef CAS PubMed.
- J. Weterings, C. J. F. Rijcken, H. Veldhuis, T. Meulemans, D. Hadavi, M. Timmers, M. Honing, H. Ippel and R. M. J. Liskamp, TMTHSI, a superior 7-membered ring alkyne containing reagent for strain-promoted azide–alkyne cycloaddition reactions, Chem. Sci., 2020, 11(33), 9011–9016 RSC.
- S. B. Engelsma, L. I. Willems, C. E. Van Paaschen, S. I. Van Kasteren, G. A. Van Der Marel, H. S. Overkleeft and D. V. Filippov, Acylazetine as a dienophile in bioorthogonal inverse electron-demand Diels–Alder ligation, Org. Lett., 2014, 16(10), 2744–2747 CrossRef CAS PubMed.
- F. P. J. T. Rutjes, How to pick a single amine?, Nat. Chem. Biol., 2015, 11(5), 306–307 CrossRef CAS PubMed.
- B. Sveinbjørnsson, K. A. Camilio, B. E. Haug and Ø. Rekdal, LTX-315: a first-in-class oncolytic peptide that reprograms the tumor microenvironment, Future Med. Chem., 2017, 9(12), 1339–1344 CrossRef PubMed.
- H. Zhou, S. Forveille, A. Sauvat, T. Yamazaki, L. Senovilla, Y. Ma, P. Liu, H. Yang, L. Bezu and K. Müller, et al., The oncolytic peptide LTX-315 triggers immunogenic cell death, Cell Death Dis., 2016, 7(3), e2134 CrossRef CAS PubMed.
- H. Gao, S. Zhang, S. Cao, Z. Yang, Z. Pang and X. Jiang, Angiopep-2 and activatable cell-penetrating peptide dual-functionalized nanoparticles for systemic glioma-targeting delivery, Mol. Pharmaceutics, 2014, 11(8), 2755–2763 CrossRef CAS PubMed.
- S. Habib and M. Singh, Angiopep-2-Modified Nanoparticles for Brain-Directed Delivery of Therapeutics: A Review, Polymers, 2022, 14(4), 712 CrossRef CAS PubMed.
- Y. Lei, S. Chen, X. Zeng, Y. Meng, C. Chang and G. Zheng, Angiopep-2 and cyclic RGD dual-targeting ligand modified micelles across the blood–brain barrier for improved anti-tumor activity, J. Appl. Polym. Sci., 2022, 139(24), 52358 CrossRef CAS.
- X. X. Shi, W. M. Miao, D. W. Pang, J. S. Wu, Q. S. Tong, J. X. Li, J. Q. Luo, W. Y. Li, J. Z. Du and J. Wang, Angiopep-2 conjugated nanoparticles loaded with doxorubicin for the treatment of primary central nervous system lymphoma, Biomater. Sci., 2020, 8(5), 1290–1297 RSC.
- M. Z. Islam, H. Ariyama, J. M. Alam and M. Yamazaki, Entry of cell-penetrating peptide transportan 10 into a single vesicle by translocating across lipid membrane and its induced pores, Biochemistry, 2014, 53(2), 386–396 CrossRef CAS PubMed.
- U. Soomets, M. Lindgren, X. Gallet, M. Hällbrink, A. Elmquist, L. Balaspiri, M. Zorko, M. Pooga, R. Brasseur and Ü. Langel, Deletion analogues of transportan, Biochem. Biophys. Acta, 2000, 1467(1), 165–176 CAS.
- C. Zhang, W. Ren, Q. Liu, Z. Tan, J. Li and C. Tong, Transportan-derived cell-penetrating peptide delivers siRNA to inhibit replication of influenza virus in vivo, Drug Des., Dev. Ther., 2019, 13, 1059–1068 CrossRef CAS PubMed.
- S. S. Denisov, J. H. Ippel, A. C. A. Heinzmann, R. R. Koenen, A. Ortega-Gomez, O. Soehnlein, T. M. Hackeng and I. Dijkgraaf, Tick saliva protein Evasin-3 modulates chemotaxis by disrupting CXCL8 interactions with glycosaminoglycans and CXCR2, J. Biol. Chem., 2019, 294(33), 12370–12379 CrossRef CAS PubMed.
- A. Blanas, H. Karsjens, A. de Ligt, E. J. M. Huijbers, K. van Loon, S. S. Denisov, C. Durukan, D. J. M. Engbersen, J. Groen and S. Hennig, et al., Vaccination with a bacterial peptide conjugated to SARS-CoV-2 receptor-binding domain accelerates immunity and protects against COVID-19, Science, 2022, 25(8), 104719 CAS.
- M. F. Debets, J. C. M. van Hest and F. P. J. T. Rutjes, Org. Biomol. Chem., 2013, 11, 6439–6455 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc03397j |
| ‡ Copper catalysts are usually considered toxic and therefore have to be removed carefully, before any use of the resulting molecular constructs in vitro or in vivo. In some cases this is even hardly possible, e.g., when a metal chelator has to be introduced for a radio-active isotope which can also bind copper-ions. |
| This journal is © The Royal Society of Chemistry 2023 |