Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards the quantification of the chemical mechanism of light-driven water splitting on GaN photoelectrodes
Artem
Shushanian
 a,
Daisuke
Iida
a,
Daisuke
Iida
 b,
Yu
Han
b,
Yu
Han
 a and
Kazuhiro
Ohkawa
a and
Kazuhiro
Ohkawa
 *b
*b
aChemistry Program, Physical Science and Engineering Division King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: kazuhiro.ohkawa@kaust.edu.sa
bElectrical and Computer Engineering Program, Computer, Electrical and Mathematical Sciences and Engineering Division King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia
First published on 2nd August 2023
Abstract
We present results from a study addressing the unbiased water-splitting process and its side reactions on GaN-based photoelectrodes decorated with NiOx, FeOx, and CoOx nanoparticles. Observations involving physicochemical analyses of liquid and vapour phases after the experiments were performed in 1 M NaOH under ambient conditions. A water-splitting process with GaN-based photoelectrodes results in the generation of hydrogen gas and hydrogen peroxide. Quantification of the water-splitting chemical mechanism gave numerical values indicating an increase in the device performance and restriction of the GaN electrocorrosion with surface modifications of GaN structures. The hydrogen generation efficiencies are ηH2(bare GaN) = 1.23%, ηH2(NiOx/GaN) = 4.31%, ηH2(FeOx/GaN) = 2.69%, and ηH2(CoOx/GaN) = 2.31%. The photoelectrode etching reaction moieties Qetch/Q are 11.5%. 0.21%, 0.26% and 0.20% for bare GaN, NiOx/GaN, FeOx/GaN, and CoOx/GaN, respectively.
Storage of solar energy in chemical bonds is a suitable route towards diversification of mass production of essential substances in industry. Application of III-nitride materials offers the possibility of light-driven splitting of the water to hydrogen gas and products of water oxidation.1 In addition to the intrinsic thermal and chemical stability of III-nitrides,2–6 they show a tendency to enhance their performances and durability when modified with nanoparticles (NPs) based on transition metal oxides7–10 and noble metals,11–13 and their combinations.14–16
The lifetime of III-nitride-based photoanodes varies with different NP materials and deposition techniques, so we need to study the reaction products to quantify the decrease in the electrode etching. Also, we expect to clarify the amounts of anodic water oxidation products as the oxygen gas is the only product that has previously been reported.7,9,11,17,18 Due to the significant impact of transition metal oxide NPs on the properties of the water-splitting process on III-nitrides, we suggest measuring their dissolution rates.
In this work, we present an approach to quantifying the water-splitting process and its corresponding side reactions on GaN-based photoelectrodes (Table 1). We observed that ultraviolet (UV)-light-driven reactions on bare GaN, NiOx/GaN, FeOx/GaN, and CoOx/GaN structures form the basis for further studies on the processes driven by visible light on III-nitride alloys and can help select the optimal surface modification of III-nitride-based devices for water splitting.
| Photoanode | NPs | Electrolyte | Product analysis | YearRef. | |
|---|---|---|---|---|---|
| Gas | Liquid | ||||
| a Only the generation of H2 is mentioned. | |||||
| GaN | None | 1 M KOH | ±a | − | 20051 |
| Patterned GaN | None | 1–5 M NaOH | ± | − | 200719 |
| GaN powder | None, RuO2, Rh2yCryOx | 2.4 M MeOH, 0.01 M AgNO3 | + | − | 200720 |
| GaN nanowires | Pt | 1 M H2SO4 | + | − | 201711 |
| GaN, β-Ga2O3/GaN | Ni3(PO4)2 | 0.5 M NaOH | + | − | 202217 |
| GaN | Au | 1 M NaOH | − | − | 202212 |
| GaN | None, FeOx, CoOx, NiOx | 1 M NaOH | + | + | This work |
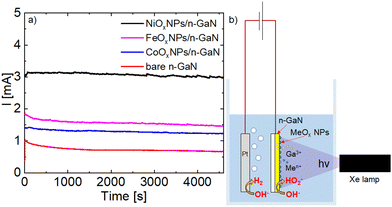
Fig. 1a represents the photocurrent generated as a result of zero-bias UV irradiation of GaN-based structures in 1 M NaOH solution with the Pt counterelectrode in a configuration shown in Fig. 1b. NiOx decorated structures provided the highest reaction current (∼3.0 mA), and samples with FeOx and CoOx NPs showed lower currents of 1.5 and 1.3 mA, respectively. The bare GaN photoelectrode provided the lowest reaction current of ∼0.7 mA. We calculated the total charges of reactions Q (C) via integration of current evolutions I (A) with the exposition time t (s), as shown in eqn (1):
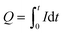 | (1) |
In our previous studies on n-GaN etching, we found hydrogen peroxide (H2O2) to be a major product of water oxidation, so we expected the photoelectrode to generate peroxides as a main product of the water splitting reaction.21,22 Theoretical investigations of the water-splitting process on the GaN surface propose the dimerization of hydroxyl species during oxygen generation.23,24 Also, the studies on water splitting with carbon nitride photoelectrodes report the generation of hydrogen peroxide.25–28 To quantify the amounts of peroxide formed, we used the iodine reverse titration in acidic media. Hydrogen gas and the hydrogen peroxide generation efficiencies (ηH2 and ηH2O2) were calculated according to the following equation (eqn (2) and Table 2):
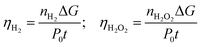 | (2) |
| Photoelectrode | Q (C) | n H2 (mol) | n H2O2 (mol) | η H2 (%) | η H2O2 (%) |
|---|---|---|---|---|---|
| Bare GaN | 3.3 | 1.6 × 10−5 | 1.4 × 10−5 | 1.23 | 1.08 |
| NiOxNPs/GaN | 14.1 | 5.6 × 10−5 | 5.5 × 10−5 | 4.31 | 4.23 |
| FeOxNPs/GaN | 7.2 | 3.5 × 10−5 | 3.3 × 10−5 | 2.69 | 2.54 |
| CoOxNPs/GaN | 5.9 | 3.0 × 10−5 | 2.9 × 10−5 | 2.31 | 2.23 |
We quantitatively characterized the water-splitting reaction by identifying hydrogen gas at the cathode and peroxides in the electrolyte. In this situation, we assumed that a two-electron water oxidation occurred that resulted in the products we observed. Hydroxide anions were reduced to hydroxide anions on a working electrode side (eqn (3)):
| 3OH− → HO2− + H2O + 2e− | (3) |
| 4OH− → O2 + 2H2O + 4e− | (4) |
| 2H2O + 2e− → H2 + 2OH− | (5) |
Qetch = 6F(nGa − nGa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BG) BG) | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), nGa (mol) is the concentration of Ga in the electrolyte after the experiment, and nGa
485 C mol−1), nGa (mol) is the concentration of Ga in the electrolyte after the experiment, and nGa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BG (mol) is a background concentration of Ga, measured after the storage of the similar samples in 1 M NaOH for 4600 s without UV-light irradiation. We assume that threading dislocations propagating through the whole thickness of the GaN structures provide channels for access of the n-GaN layer to the electrolyte.29 As the reaction current remained relatively constant within the exposition time, we consider that the etching of GaN takes the same moiety of the total charge of the water-splitting process. Modification of the GaN structures with CoOx resulted in the lowest moiety of the charge spent on the electrooxidation of the GaN photoelectrode.
BG (mol) is a background concentration of Ga, measured after the storage of the similar samples in 1 M NaOH for 4600 s without UV-light irradiation. We assume that threading dislocations propagating through the whole thickness of the GaN structures provide channels for access of the n-GaN layer to the electrolyte.29 As the reaction current remained relatively constant within the exposition time, we consider that the etching of GaN takes the same moiety of the total charge of the water-splitting process. Modification of the GaN structures with CoOx resulted in the lowest moiety of the charge spent on the electrooxidation of the GaN photoelectrode.
Table 3 shows the results of the inductively coupled plasma-mass spectrometry (ICP-MS) measurements of the electrolytes after keeping the samples that were exposed or not exposed to UV light. When the light was off, GaN hardly dissolved in 1 M NaOH; however, the decoration of its surface with oxide NPs limited this process. Coating the GaN structures with oxide NP produced an increase in the water splitting reaction rate and slowed the corrosion of GaN as shown before.30 In this study, we observed the highest reaction rate for the sample modified with NiOx NPs, while the concentrations of Ni after both experiments with and without UV light exposure were higher than the concentrations of other metals in the electrolyte after the corresponding experiments on FeOx/GaN and CoOx/GaN. The highest inhibition of the GaN electrooxidation reaction was observed for the CoOx/GaN system where the concentrations of Co are the lowest after the experiments with and without UV light. In agreement with the calculated values of Qetch/Q, the introduction of metal oxide NPs potentially increases the durability of the GaN photoelectrodes by 54.8 times with the NiOx NPs, by 44.2 times with the FeOx NPs, and by 57.5 times with the CoOx NPs.
| Photoelectrode | n Ga (mol) | n Me (mol) |
n
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BG (mol) BG (mol) |
n
Me
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BG (mol) BG (mol) |
Q etch (C) | Q etch/Q (%) |
|---|---|---|---|---|---|---|
| a Corresponding metal (Ni, Fe, or Co) concentration after the water-splitting experiment driven by UV light on the MeOxNPs/GaN photoelectrode. b Corresponding metal (Ni, Fe, or Co) background concentrations, measured after storing the MeOxNPs/GaN photoelectrode in the electrolyte with no exposure to UV light. c Limit of detection. | ||||||
| Bare GaN | 7.3 × 10−7 | <LODc | 7.1 × 10−8 | <LOD | 0.38 | 11.5 |
| NiOxNPs/GaN | 5.8 × 10−8 | 1.1 × 10−6 | 7.0 × 10−9 | 6.9 × 10−8 | 0.030 | 0.21 |
| FeOxNPs/GaN | 3.7 × 10−8 | 6.1 × 10−7 | 4.0 × 10−9 | 4.7 × 10−8 | 0.019 | 0.26 |
| CoOxNPs/GaN | 2.3 × 10−8 | 2.3 × 10−7 | 2.2 × 10−9 | 9.0 × 10−9 | 0.012 | 0.20 |
Observations on the concentrations of the metals after the experiment show the involvement of the oxide NPs in the process. Thus, we observed several processes on MeOx/GaN that run under UV irradiation: (1) water splitting to hydrogen gas and peroxides, (2) electrooxidation of GaN described, and (3) interactions of metal oxides with the reaction layers. We quantitatively proved that inhibition of the GaN etching reaction via modification of the GaN structures with metal oxide NPs occurs.
In summary, we presented the water-splitting process on bare GaN, NiOx/GaN, FeOx/GaN, and CoOx/GaN structures. Introduction of the NiOx NPs shows the highest increase in reaction rate while the CoOx NPs provide the highest durability of the GaN-based devices. The major product of the water splitting on GaN structures is a result of the two-electron decomposition of water to hydrogen peroxide and hydrogen gas. Our approach may help optimize modifications of the III-nitride-based photoelectrodes in the future. A detailed understanding of the mechanisms of water oxidation reactions and the side processes would clarify the possibility of effective solar energy storage in commercially valuable chemical products.
The working electrode was a thin film structure (top to bottom) with unintentionally doped (uid)-GaN (100 nm, n ≈ mid. 1016 cm−3)/Si-doped n-GaN (3 μm, n = 3 × 1018 cm−3)/uid-GaN (2 μm, n ≈ mid. 1016 cm−3), grown using the metalorganic vapour-phase epitaxy method on patterned c-plane sapphire. We decorated the top layer with spin-coated and annealed MeOx NPs described elsewhere for Me = Co8 and Me = Ni, Fe.9 We ran the experiments on a light-driven water-splitting apparatus for 4600 s in a two-electrode configuration in which a Pt wire was used as a counter electrode with no applied electrical bias. A Xe arc lamp (300 W) filtered with a UV spectroscopic mirror irradiated the surface of the working electrodes with the power density of 100 mW cm−2. We used a 1 M NaOH solution prepared by dissolving NaOH (Fisher Scientific, ≥97.5% [w/w%]) in deionized water (MilliQ) as an electrolyte in our experiments.
Light-driven water splitting on GaN resulted in the generation of products in both liquid and vapour phases. We collected vapour phase products near the electrodes and analyzed them using a gas chromatograph (Shimadzu GC-8A). To measure the concentrations of metals in the electrolyte after the experiment, we used ICP-MS (Thermo iCAP). Relative standard deviations for the calibrations were 7.7% for 56Fe and 3.4% for 57Fe in kinetic energy distribution (KED) mode, 3.1% (KED) and 2.8% (standard mode) for 59Co, 0.8% (KED) and 2.5% (standard) for 60Ni, 0.4% (KED) and 10.6 (standard) for 62Ni, 0.6% (KED) and 1.3% (standard) for 69Ga, and 1.5% (KED) and 0.8% (standard) for 71Ga. The background concentrations of these metals were calculated after the storage of the similar samples in the electrolyte with no UV irradiation for 4600 s. We used 103Rh as an internal standard for ICP-MS measurements. We found and quantified the peroxide amounts by carefully diluting our aliquots with sulfuric acid (Fisher Scientific, 95.0 to 98.0% [w/w%]) and application of iodine reverse titration with potassium iodide (Fluka, Z99% [w/w%]) and sodium thiosulfate pentahydrate (Fisher Scientific, 99.5 to 101.0% [w/w%]) solutions.
We normalized the reaction currents, generated gas volumes, peroxide, and metal concentrations to 1 cm2 of irradiated GaN photoelectrodes for the correct comparison and analysis.
A. S.: investigation, methodology, writing – original draft; D. I.: validation, writing – review & editing; Y. H.: supervision, writing – review & editing; K. O.: conceptualization, project administration, writing – review & editing.
This work was financially supported by King Abdullah University of Science and Technology (KAUST) (BAS/1/1676-01-01). We would like to show our gratitude to the staff of the KAUST Analytical Core Lab, in particular Andrei Zybinskii, for their help with the ICP-MS calibration and measurements.
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Fujii, T. Karasawa and K. Ohkawa, Jpn. J. Appl. Phys., 2005, 44, L543 CrossRef CAS.
- F. Medjdoub, J.-F. Carlin, M. Gonschorek, E. Feltin, M. A. Py, D. Ducatteau, C. Gaquiere, N. Grandjean and E. Kohn, 2006 International Electron Devices Meeting, IEEE, 2006, pp. 1–4.
- B. Imer, F. Wu, M. D. Craven, J. S. Speck and S. P. DenBaars, Jpn. J. Appl. Phys., 2006, 45, 8644 CrossRef CAS.
- D. Maier, M. Alomari, N. Grandjean, J.-F. Carlin, M.-A. Diforte-Poisson, C. Dua, A. Chuvilin, D. Troadec, C. Gaquière, U. Kaiser, S. L. Delage and E. Kohn, IEEE Trans. Device Mater. Reliab., 2010, 10, 427–436 CAS.
- S. Chen and L.-W. Wang, Chem. Mater., 2012, 24, 3659–3666 CrossRef CAS.
- A. R. Acharya, Himalayan Phys., 2014, 5, 22–26 CrossRef.
- T. Hayashi, M. Deura and K. Ohkawa, Jpn. J. Appl. Phys., 2012, 51, 112601 CrossRef.
- M. Velazquez-Rizo, D. Iida and K. Ohkawa, Jpn. J. Appl. Phys., 2019, 58, SCCC23 CrossRef CAS.
- M. Velazquez-Rizo, D. Iida and K. Ohkawa, Sci. Rep., 2020, 10, 12586 CrossRef PubMed.
- C. W. Lee, F.-W. Lin, P.-H. Liao, M.-L. Lee and J.-K. Sheu, J. Phys. Chem. C, 2021, 125, 16776–16783 CrossRef CAS.
- P. Varadhan, H.-C. Fu, D. Priante, J. R. D. Retamal, C. Zhao, M. Ebaid, T. K. Ng, I. Ajia, S. Mitra, I. S. Roqan, B. S. Ooi and J.-H. He, Nano Lett., 2017, 17, 1520–1528 CrossRef CAS PubMed.
- A. Abdullah, M. A. Johar, A. Waseem, I. V. Bagal, M. A. Hassan, J. K. Lee and S.-W. Ryu, Mater. Sci. Eng., B, 2022, 275, 115514 CrossRef CAS.
- Z. Li, R. Li, H. Jing, J. Xiao, H. Xie, F. Hong, N. Ta, X. Zhang, J. Zhu and C. Li, Nat. Catal., 2023, 6, 80–88 CrossRef CAS.
- K. Maeda, N. Sakamoto, T. Ikeda, H. Ohtsuka, A. Xiong, D. Lu, M. Kanehara, T. Teranishi and K. Domen, Chem. – Eur. J., 2010, 16, 7750–7759 CrossRef CAS PubMed.
- M. G. Kibria, H. P. Nguyen, K. Cui, S. Zhao, D. Liu, H. Guo, M. L. Trudeau, S. Paradis, A.-R. Hakima and Z. Mi, ACS Nano, 2013, 7, 7886–7893 CrossRef CAS PubMed.
- H. N. Nong, L. Gan, E. Willinger, D. Teschner and P. Strasser, Chem. Sci., 2014, 5, 2955–2963 RSC.
- M. Arunachalam, K. R. Subhash, K. S. Ahn, C. S. Kim, J. S. Ha, S. W. Ryu and S. H. Kang, ACS Appl. Energy Mater., 2022, 5, 2169–2183 CrossRef CAS.
- T. Sekimoto, H. Hashiba, S. Shinagawa, Y. Uetake, M. Deguchi, S. Yotsuhashi and K. Ohkawa, Jpn. J. Appl. Phys., 2016, 55, 88004 CrossRef.
- I. Waki, D. Cohen, R. Lal, U. Mishra, S. P. DenBaars and S. Nakamura, Appl. Phys. Lett., 2007, 91, 93519 CrossRef.
- K. Maeda, K. Teramura, N. Saito, Y. Inoue and K. Domen, Bull. Chem. Soc. Jpn., 2007, 80, 1004–1010 CrossRef CAS.
- A. Shushanian, D. Iida, Z. Zhuang, Y. Han and K. Ohkawa, RSC Adv., 2022, 12, 4648–4655 RSC.
- A. Shushanian, D. Iida, Y. Han and K. Ohkawa, New J. Chem., 2022, 46, 23013–23018 RSC.
- J. Wang, L. S. Pedroza, A. Poissier and M. Fernández-Serra, J. Phys. Chem. C, 2012, 116, 14382–14389 CrossRef CAS.
- A. V. Akimov, J. T. Muckerman and O. V. Prezhdo, J. Am. Chem. Soc., 2013, 135, 8682–8691 CrossRef CAS PubMed.
- Y. Kofuji, Y. Isobe, Y. Shiraishi, H. Sakamoto, S. Tanaka, S. Ichikawa and T. Hirai, J. Am. Chem. Soc., 2016, 138, 10019–10025 CrossRef CAS PubMed.
- Y. Kofuji, S. Ohkita, Y. Shiraishi, H. Sakamoto, S. Ichikawa, S. Tanaka and T. Hirai, ACS Sustainable Chem. Eng., 2017, 5, 6478–6485 CrossRef CAS.
- B. Yan, Z. Chen and Y. Xu, Chem. - Asian J., 2020, 15, 2329–2340 CrossRef CAS PubMed.
- J. Chen, N. Kang, J. Fan, C. Lu and K. Lv, Mater. Today Chem., 2022, 26, 101028 CrossRef CAS.
- F. C. P. Massabuau, P. H. Griffin, H. P. Springbett, Y. Liu, R. V. Kumar, T. Zhu and R. A. Oliver, APL Mater., 2020, 8, 8–13 Search PubMed.
- W. Ohara, D. Uchida, T. Hayashi, M. Deura and K. Ohkawa, Mater. Res. Soc. Symp. Proc., 2012, 1446, 1–5 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |