Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aryl–aryl cross-coupling reactions without reagents or catalysts: photocyclization of ortho-iodoaryl ethers and related compounds via triplet aryl cation intermediates†
Wei
Sun
,
Luke
Wilding-Steele
,
Richard C. D.
Brown
 and
David C.
Harrowven
and
David C.
Harrowven
 *
*
Department of Chemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK. E-mail: dch2@soton.ac.uk
First published on 14th August 2023
Abstract
Cyclisations of benzyl ortho-iodoaryl ethers to benzo[c]chromenes can be effected without reagents or catalysts by irradiation with UVC under flow. Reactions proceed via triplet aryl cation generation, 5-exo and 3-exo-cyclisations, and rearomatisation. They have wide scope, are easy to effect and extend to a myriad of related ring systems.
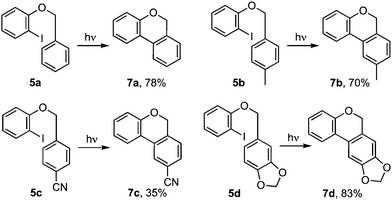
Cyclisations of benzyl ortho-iodoaryl ethers to benzo[c]chromenes, e.g.5 → 4 or 7 (Scheme 1) are typically achieved by radical cyclisation,1 or palladium catalysed cross-coupling reactions.2 The products given by the two approaches can differ when cyclisation involves addition to a substituted arene. Thus, while the Pd catalysed cyclisation occurs ortho- to the tethering chain, giving benzoisochromene 7,2 cyclisation via aryl radical intermediate 1 principally gives benzoisochromene 4 through a neophyl rearrangement via ipso-cyclisation to 2 and fragmentation to 3.1,3 Herein we show how it is possible to induced cyclisation without reagents or catalysts through irradiation with UVC.4 Notably, reactions give ortho-addition products 7 rather than those derived by a radical-induced neophyl rearrangement 4, implicating the intermediacy of triplet aryl cations 3[9] and 3[10],5 as supported by TD-DFT calculations.6
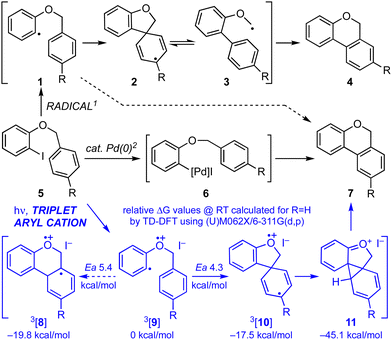 | ||
| Scheme 1 Regiochemical outcomes for the common cyclisation reactions of benzyl ortho-iodoaryl ethers [R = H for calculated relative ΔG values via triplet aryl cation 9]. | ||
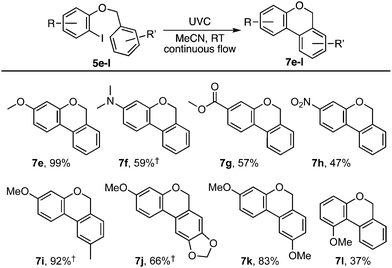
Our investigation began with benzyl ortho-iodophenyl ether 5a which, on irradiation with UVC under continuous flow in acetonitrile at ambient temperature, gave benzoisochromene 7a in 78% yield (Scheme 2). To determine if the reaction proceeded via radical intermediate 1, the method was extended to substrates 5b–d with a para-substituent on the benzyl ether residue. In each case cyclisation gave regioisomer 7 rather than 4 indicating that they did not proceed via aryl radical intermediates 1b–d (see ESI† for further discussion).1 We next examined the reaction by TD-DFT,6 and found that the 5-exo-trig cyclisation mode also appeared to be favoured by the corresponding triplet aryl cation 3[9], with 6-exo/endo-trig cyclisation to 3[8] having a higher activation energy. However, relaxation of the thus formed intermediate 3[10] led to spontaneous cyclisation to tetracycle 11, setting up a facile double aromatisation with loss of HI to give the observed product 7. The yields attained in these early experiments indicated that benzyl residues carrying an electron withdrawing substituent (5c) were poorer substrates than those carrying electron donating substituents (5b,d). To probe the impact of substituents further, a series of benzyl o-iodoaryl ethers were prepared with substituents on the iodinated arene, 5e–h, and with substituents on both arenes, 5i–l (Table 1). These experiments showed that yields were enhanced by electron donating substituents on the iodinated arene (7e,f,i–k) and were supressed by electron withdrawing substituents (7g,h). Notably, the reaction was also given in modest yield by benzyl 2-iodo-3-methoxyphenyl ether 5l. In this case we believe a complex product mixture was given due to the sensitivity of the starting material and product 7l towards the HI formed as a by-product of the reaction.7
Indeed, while the method was being developed, several starting materials and products proved susceptible to benzyl ether cleavage by HI.7 For example, photolysis of p-nitrobenzyl o-iodophenyl ether led to a complex product mixture from which p-nitrobenzyl iodide was isolated in low yield. Similarly, 3,5-dimethylbenzyl ether 5m gave the anticipated product 7m in 32% isolated yield together with biaryl 12 as a significant by-product (Scheme 3). Its formation implicates benzoisochromene cleavage to benzyl iodide 13 followed by a Ritter reaction with acetonitrile.8 Such side reactions were attenuated by an expeditious work-up and/or the addition of an equivalent of pyridine to the reaction mixture (e.g.Table 1).
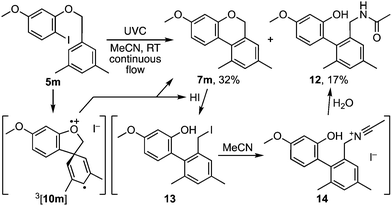 | ||
| Scheme 3 A side reaction promoted by HI involving benzoisochromene cleavage by HI and a Ritter reaction. | ||
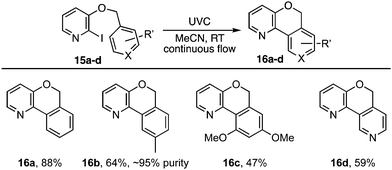
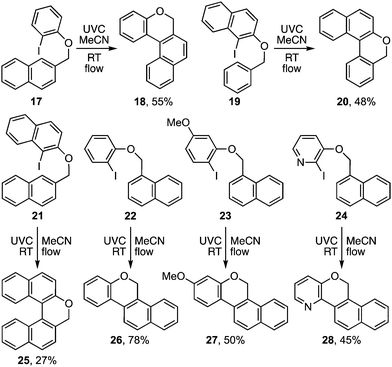
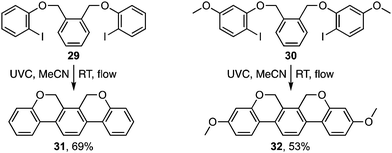
The photocyclisation was next extended to analogous pyridyl ethers 15a–d (Table 2) leading respectively to heterocycles 16a–d. It also proved effective for the synthesis of an array of polyaromatic ring systems through related photocyclisation (Scheme 4) and tandem photocyclisation reactions (Scheme 5). It is notable that lower yields were generally associated with products bearing substituents in the ‘fjord’ region (Scheme 4), indicating their increased sensitivity towards ethereal cleavage by HI due to steric strain. Nonetheless, the method was able to produce a myriad of polyaromatic ring systems, including oxahelicene 25 and terphenyls 31 and 32 (Schemes 4 and 5).
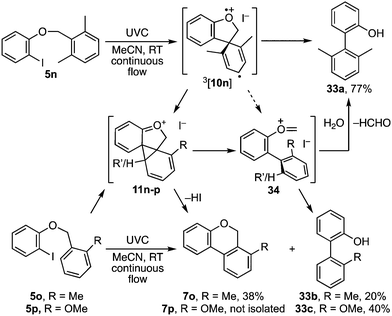
The presence of ortho-substituents on the benzyl ether was investigated next and revealed a further subtlety (Scheme 6). When both ortho sites carried methyl substituents, 5n, cyclisation of the spirocyclic intermediate 3[10n] to 11n is presumably followed by fragmentation to oxocarbenium ion 34, as the main product given was biaryl 33a.9 The rearrangement pathway to 7 and fragmentation pathway to 33 compete when the benzyl residue carries a single ortho-substituent, with the o-methyl analogue 5o favouring benzoisochromene 7o while the o-methoxy analogue 5p favoured biaryl 33c. The extent to which the spirocyclic triplet aryl cation intermediate 3[10] follows each of these pathways is presumably determined by a combination of steric and electronic factors.
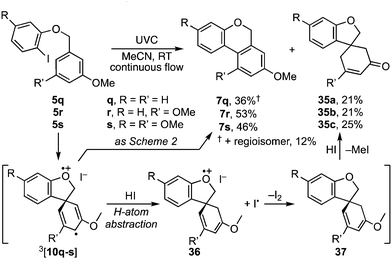
A further curiosity arose when the benzyl residue carried a meta-methoxy substituent, e.g.5q–s (Scheme 7). Photolysis under the aforementioned conditions principally followed the pathway leading to benzoisochromenes 7q–s respectively. However, these products were each accompanied by a spirocyclic enone, 35a–c, which we presume arises through H-atom abstraction from HI by 3[10q–s] to radical cation 36, then single electron transfer to dienyl ether 37, and demethylation.
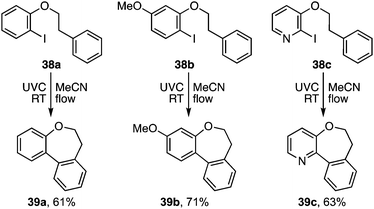
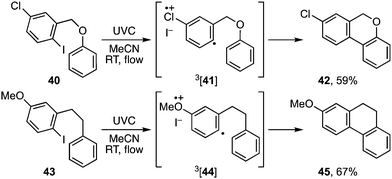
A more useful observation came through extension of the methodology to homobenzyl ortho-iodophenyl ethers 38a–c. Photocyclisation of these substrates proceeded smoothly and in good yield to give the corresponding 7-ring ethers 39a–c (Scheme 8). Additionally, we have established that p-chloro- and p-methoxy- substituents on the iodoarene, 40 and 43, can also facilitate triplet aryl cation formation to 3[41] and 3[44] respectively (Scheme 9). These extensions to regioisomeric benzoisochromenes (e.g.42) and dihydrophenanthrenes (e.g.45) demonstrate ways of extending the method to systems that do not carry an ethereal linkage to the iodoarene residue.
In conclusion, we have shown that intramolecular aryl–aryl coupling reactions of benzyl ortho-iodoaryl ethers and related substrates can be effected without reagents or catalysts by irradiation with UVC under flow. Reaction proceeds via triplet aryl cation intermediates such as 3[9] and 3[10] rather than by radical cyclisation. The method can be applied to systems bearing all manner of substituents including extended aromatic ring systems. The reaction works best when the arenes carry electron donating groups, yet also gives good to excellent yields with analogous pyridyl systems. The sensitivity of both starting materials and products towards benzyl ether cleavage by HI can be resolved, in part, by employing an expeditious work-up and/or a scavenger. ortho-Substituents are tolerated on either ring but do lead to diminished yields. When the benzyl residue carries two ortho-substituents, a biaryl is given. Notably, extensions to tandem cyclisation reactions gave terphenyl containing products and extensions to homobenzyl ethers gave 7-ring ethers. Importantly, para-chloro- and para-methoxy-substituents on the iodoarene have also been shown to facilitate triplet aryl cation formation, extending these photocyclisation reactions to systems devoid of an ethereal bond ortho- to the iodide.
Wei Sun and Luke Wilding-Steele performed the synthetic chemistry under the supervision of Richard Brown and the corresponding author David Harrowven. Wei Sun performed the TD-DFT analyses. We gratefully acknowledge financial support from EPSRC [EP/P013341/1 and EP/K039466/1] and the European Regional Development Fund [ERDF Interreg Va programme (Project 121)], Dr James Pearce for the gift of compound 15a and Dr Michael I. T. Nunn for some characterisation data presented in the ESI.†
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- Radical cyclisations of 2-iodoaryl benzyl ethers have been induced with (i) Bu3SnH: D. C. Harrowven, M. I. T. Nunn, N. A. Newman and D. R. Fenwick, Tetrahedron Lett., 2001, 42, 961 CrossRef CAS ; (ii) KOtBu and a nitrogen heterocycle: D. S. Roman, Y. Takahashi and A. B. Charette, Org. Lett., 2011, 13, 3242 CrossRef PubMed; S. Zhou, G. M. Anderson, B. Mondal, E. Doni, V. Ironmonger, M. Kranz, T. Tuttle and J. A. Murphy, Chem. Sci., 2014, 5, 476 RSC; S. Chen, W. Chen, Y. Chen, Z. Liu, T. Mu, P. Wang and Z. Yan, RSC Adv., 2020, 10, 14500 RSC; C.-L. Sun, Y.-F. Gu, W.-P. Huang and Z.-J. Shi, Chem. Commun., 2011, 47, 9813 RSC; H. Yang, L. Zhang and L. Jiao, Chem. – Eur. J., 2017, 23, 65 CrossRef PubMed ; (iii) KOtBu, DMSO and blue light: M. E. Budén, M. D. Heredia, M. Puiatti and R. A. Rossi, Org. Biomol. Chem., 2022, 20, 228 RSC ; (iv) with boron reagents: S. Pinet, V. Liautard, M. Debiais and M. Pucheault, Synthesis, 2017, 4759 CrossRef; A. B. Waghamare, R. K. Raut, N. Patel and M. Majumdar, Eur. J. Inorg. Chem., 2022, E202200089 Search PubMed ; (v) by photocatalysis: P. Herr, F. Glaser, L. A. Büldt, C. B. Larsen and O. S. Wenger, J. Am. Chem. Soc., 2019, 141, 14394 CrossRef PubMed ; (vi) with Me4NI: K. Nozawa-Kumada, K. Nakamura, S. Kurosu, Y. Iwakawa, C. Denneval, M. Shigeno and Y. Kondo, Chem. Pharm. Bull., 2019, 67, 1042 CrossRef PubMed.
- M. Kitamura, K. Ohmori, T. Kawase and K. Suzuki, Angew. Chem., Int. Ed., 1999, 38, 1229 CrossRef CAS PubMed; R. Grigg, V. Savic and V. Tambyrajah, Tetrahedron Lett., 2000, 41, 3003 CrossRef; T. Harayama, H. Yasuda, T. Akiyama, Y. Takeuchi and H. Abe, Chem. Pharm. Bull., 2000, 48, 861 CrossRef PubMed; K. Ohmori, M. Tamiya, M. Kitamura, H. Kato, M. Oorui and K. Suzuki, Angew. Chem., Int. Ed., 2005, 44, 3871 CrossRef PubMed; D. C. Harrowven, T. Woodcock and P. D. Howes, Angew. Chem., Int. Ed., 2005, 44, 3967 CrossRef; L.-C. Campeau, M. Parisien, A. Jean and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 581 CrossRef PubMed; M. Tamiya, K. Ohmori, M. Kitamura, H. Kato, T. Arai, M. Oorui and K. Suzuki, Chem. – Eur. J., 2007, 13, 9791 CrossRef PubMed; H. Kelgtermans, L. Dobrzanska, W. Dehaen and L. Van Meervelt, Org. Lett., 2012, 14, 5200 CrossRef PubMed; T. Matsukihira, T. Kida, K. Hidaka, S. Saga, M. Takemura, A. Yonoki, T. Nishimori, Y. Horino, T. Harayama, Y. Takeuchi and H. Abe, Heterocycles, 2013, 87, 2555 CrossRef; G. Bringmann, N. Manchala, T. Büttner, B. Hertlein-Amslinger and R. Seupel, Chem. – Eur. J., 2016, 22, 9792 CrossRef PubMed; R. Cano, J. M. Pérez, D. J. Ramón and G. P. McGlacken, Tetrahedron, 2016, 72, 1043 CrossRef; Y. Tanji, N. Mitsutake, T. Fujihara and Y. Tsuji, Angew. Chem., Int. Ed., 2018, 57, 10314 CrossRef PubMed; Y. An, B.-S. Zhang, Z. Zhang, C. Liu, X. Y. Gou, Y.-N. Ding and Y.-M. Liang, Chem. Commun., 2020, 56, 5933 RSC.
- F. H. Seubold Jr, J. Am. Chem. Soc., 1953, 75, 2532 CrossRef CAS; J. W. Wilt and W. W. Pawlikowski Jr, J. Org. Chem., 1975, 40, 3641 CrossRef; M. Weber and H. Fischer, J. Am. Chem. Soc., 1999, 121, 7381 CrossRef; Z.-M. Chen, X.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2015, 44, 5220 RSC; A. Behnia, M. A. Fard, J. M. Blacquiere and R. J. Puddephatt, Organometallics, 2017, 36, 4759 CrossRef; A. Navarro-Vázquez and D. Domínguez, Tetrahedron Lett., 2020, 61, 151871 CrossRef.
- For our work on flow photochemistry see: W. Sun, S. Kayal, W. A. T. Raimbach, X.-Z. Sun, M. E. Light, M. W. D. Hanson-Heine, M. W. George and D. C. Harrowven, Chem. Commun., 2022, 58, 1546 RSC; W. Sun, W. A. T. Raimbach, L. D. Elliott, K. I. Booker-Milburn and D. C. Harrowven, Chem. Commun., 2022, 58, 383 RSC; M. A. Manning, W. Sun, M. E. Light and D. C. Harrowven, Chem. Commun., 2021, 57, 4556 RSC; D. E. Collin, E. H. Jackman, N. Jouandon, W. Sun, M. E. Light, D. C. Harrowven and B. Linclau, Synthesis, 2021, 1307 Search PubMed; M. J. Ralph, D. C. Harrowven, S. Gaulier, S. Ng and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2015, 54, 1527 CrossRef CAS PubMed; D. C. Harrowven, M. Mohamed, T. P. Gonçalves, R. J. Whitby, D. Bolien and H. F. Sneddon, Angew. Chem., Int. Ed., 2012, 51, 4405 CrossRef PubMed.
- R. W. Taft, J. Am. Chem. Soc., 1961, 83, 3350 CrossRef CAS; W. E. Lee, J. G. Calvert and E. W. Malmberg, J. Am. Chem. Soc., 1961, 83, 1928 CrossRef; J. D. Dill, P. V. R. Schleyer and J. A. Pople, J. Am. Chem. Soc., 1977, 99, 1 CrossRef; H. Zollinger, Angew. Chem., Int. Ed. Engl., 1978, 17, 141 CrossRef; H. B. Ambroz and T. J. Kemp, Chem. Soc. Rev., 1979, 8, 353 RSC; H. B. Ambroz and T. J. Kemp, J. Chem. Soc., Perkin Trans. 2, 1980, 768 RSC; N. J. Bunce, J. P. Bergsma, M. D. Bergsma, W. De Graaf, Y. Kumar and L. Ravanal, J. Org. Chem., 1980, 45, 3708 CrossRef; H. B. Ambroz and T. J. Kemp, Chem. Commun., 1982, 172 RSC; H. B. Ambroz, T. J. Kemp and G. K. Przybytniak, J. Photochem. Photobiol., A, 1992, 68, 85 CrossRef; S. Gasper, C. Devadoss and G. B. Schuster, J. Am. Chem. Soc., 1995, 117, 5206 CrossRef; S. Steenken, M. Ashokkumar, P. Maruthamuthu and R. A. McClelland, J. Am. Chem. Soc., 1998, 120, 11925 CrossRef; P. S. J. Canning, K. McCrudden, H. Maskill and B. Sexton, J. Chem. Soc., Perkin Trans. 2, 1999, 2735 RSC; P. S. J. Canning, H. Maskill, K. McCrudden and B. Sexton, Bull. Chem. Soc. Jpn., 2002, 75, 789 CrossRef; S. Milanesi, M. Fagnoni and A. Albini, Chem. Commun., 2003, 216 RSC; M. Freccero, M. Fagnoni and A. Albini, J. Am. Chem. Soc., 2003, 125, 13182 CrossRef PubMed; M. Fagnoni and A. Albini, Acc. Chem. Res., 2005, 38, 713 CrossRef PubMed; S. Milanesi, M. Fagnoni and A. Albini, J. Org. Chem., 2005, 70, 603 CrossRef PubMed; M. De Carolis, S. Protti, M. Fagnoni and A. Albini, Angew. Chem., Int. Ed., 2005, 44, 1232 CrossRef PubMed; S. Protti, M. Fagnoni and A. Albini, Angew. Chem., Int. Ed., 2005, 44, 5675 CrossRef PubMed; M. Slegt, H. S. Overkleeft and G. Lodder, Eur. J. Org. Chem., 2007, 5364 CrossRef; V. Dichiarante and M. Fagnoni, Synlett, 2008, 787 Search PubMed; A. B. Penenory and J. E. Argüello, Handbook of Synthetic Photochemistry, ed. A. Albini and M. Fagnoni, Wiley-VCH, Weinheim, Germany, 2010, pp. 319–352 RSC; S. Protti, V. Dichiarante, D. Dondi, M. Fagnoni and A. Albini, Chem. Sci., 2012, 3, 1330 RSC; S. Protti, D. Ravelli, B. Mannucci, A. Albini and M. Fagnoni, Angew. Chem., Int. Ed., 2012, 51, 8577 CrossRef PubMed; V. Dichiarante, S. Protti and M. Fagnoni, J. Photochem. Photobiol. A: Chem., 2017, 339, 103 CrossRef; E. Abitelli, S. Protti, M. Fagnoni and A. Albini, J. Org. Chem., 2012, 77, 3501 CrossRef PubMed; H. Qrareya, C. Raviola, S. Protti, M. Fagnoni and A. Albini, J. Org. Chem., 2013, 78, 6016 CrossRef PubMed; S. V. Bondarchuk and B. F. Minaev, J. Phys. Chem. A, 2014, 118, 3201 CrossRef PubMed; S. Rayne and K. Forest, Theor. Chem. Acc., 2016, 135 Search PubMed; C. Raviola, F. Chiesa, S. Protti, A. Albini and M. Fagnoni, J. Org. Chem., 2016, 81, 6336 CrossRef PubMed; A. M. Mfuh, J. D. Doyle, B. Chhetri, H. D. Arman and O. V. Larionov, J. Am. Chem. Soc., 2016, 138, 2985 CrossRef PubMed; A. M. Mfuh, V. T. Nguyen, B. Chhetri, J. E. Burch, J. D. Doyle, V. N. Nesterov, H. D. Arman and O. V. Larionov, J. Am. Chem. Soc., 2016, 138, 8408 CrossRef PubMed; C. Pedroli, D. Ravelli, S. Protti, A. Albini and M. Fagnoni, J. Org. Chem., 2017, 82, 6592 CrossRef PubMed.
- A. Dreuw and M. Head-Gordon, Chem. Rev., 2005, 105, 4009 CrossRef CAS PubMed.
- K. Auwers, F. A. Traun and R. Welde, Chem. Ber., 1899, 32, 3317 CrossRef CAS.
- For reviews on the Ritter reaction see: L. I. Krimen and D. J. Cota, Org. React., 1969, 17, 213 Search PubMed; R. Bishop, Comp. Org. Synth., 2014, 6, 239 CrossRef CAS; D. Jiang, T. He, L. Ma and Z. Wang, RSC Adv., 2014, 4, 64936 RSC; J. Bolsakova and A. Jirgensons, Chem. Heterocycl. Compd., 2017, 53, 1167 CrossRef; G. M. Ziarani, F. S. Hasankiadeh and F. Mohajer, Chem. Select, 2020, 5, 14349 Search PubMed; M.-E. Chen, X.-W. Chen, Y.-H. Hu, R. Ye, J.-W. Lv, B. Li and F.-M. Zhang, Org. Chem. Front., 2021, 8, 4623 RSC.
- For a related approach to encumbered biaryls by radical cyclisation see: P. Poonpatana, G. dos Passos Gomes, T. Hurrle, K. Chardon, S. Bräse, K.-S. Masters and I. Alabugin, Chem. – Eur. J., 2017, 23, 9091 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental accounts with spectral details and copies of NMR spectra are available as supplementary information. See DOI: https://doi.org/10.1039/d3cc03271j |
| This journal is © The Royal Society of Chemistry 2023 |