Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective adsorption of polycyclic aromatic hydrocarbons by isostructural hydrogen-bonded organic frameworks†
Peng
Cui
 *abc,
Qiang
Zhu
b,
Fangfang
Zhang
a,
Dongni
Liu
ad and
Wenshuai
Zhu
*ac
*abc,
Qiang
Zhu
b,
Fangfang
Zhang
a,
Dongni
Liu
ad and
Wenshuai
Zhu
*ac
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, 212013, PR China
bDepartment of Chemistry and Materials Innovation Factory, University of Liverpool, Liverpool, L7 3NY, UK
cCollege of Chemical Engineering and Environment, State Key Laboratory of Heavy Oil Processing, China University of Petroleum-Beijing, Beijing, 102249, PR China. E-mail: P.Cui@ujs.edu.cn; zhuws@cup.edu.cn
dSchool of Materials Science and Engineering, Jiangsu University, Zhenjiang, 212013, PR China
First published on 18th September 2023
Abstract
Two isostructural hydrogen-bonded organic frameworks (HOFs) with 1-D hexagonal-shaped pores were crystallised using the molecules biphenyl-3,3′,5,5′-tetracarboxylic acid (BPTCA) and [1,1′:4′,1′′]terphenyl- 3,3′′,5,5′′-tetracarboxylic acid (TPTCA). The desolvated HOFs, named BPTCA-2 and TPTCA-2, exhibited selective adsorption towards naphthalene and anthracene, respectively, during competitive adsorption experiments.
Polycyclic aromatic hydrocarbons (PAHs) are organic environmental pollutants and by-products from the catalytic cracking of diesel.1 PAHs also play an important role in the chemical industry, such as the preparation of dyes, pesticides and resins.2 The removal of PAHs from diesel improves the purity and extracts industrially important feedstocks. Porous materials, such as metal organic frameworks (MOFs),3 porous organic cages,4 covalent organic frameworks (COFs),5–7 porous aromatic frameworks (PAFs),8 and hypercrosslinked polymers (HCPs)9 have been explored as PAHs adsorbents. However, porous adsorbents with selective adsorption abilities for different PAHs are less common.
Hydrogen-bonded organic frameworks (HOFs) are an exciting class of crystalline material that has more recently been explored as porous solids.10 HOFs are constructed from organic molecules and stabilised by non-covalent interaction, such as hydrogen bonding and π–π interactions.11 Due to the inherent flexibility of hydrogen bonds and the solubility of the building blocks, HOFs offer some advantages, such as easy purification and regeneration, solution processability, and synthetic tuneability.12 Early reports of HOFs include the interpenetrated form of trimesic acid (α-TMA).13 Several groups have recently explored the crystallisation of non- or low-interpenetrated porous HOFs, which have been used for gas storage,14 molecular separation,15 and enzyme encapsulation.16 Also, computational methods, such as crystal structure prediction (CSP) and high throughput (HT) crystallisation, have been used to accelerate the discovery of porous HOFs.17 However, a combination of weaker and flexible non-covalent interactions in HOFs can lead to poor structural stability, particularly for HOFs with low skeleton densities. This instability can lead to the collapse and rearrangement of HOFs during desolvation of the crystal pores.18
HOFs with permanent porosities have been reported to adsorb various organic molecules.19 They exhibit pores ranging from micropores (e.g., 3.4 × 5.3 Å)20 to mesopores (e.g., 2.8 nm),21 which has been achieved by adjusting the symmetry, size, and functionality of the building blocks.21,22 For example, two isostructural HOFs, T2-γ and T2E-α with 2 nm and 2.8 nm pores, respectively, were assembled using triptycene-based building blocks with benzimidazolone groups.21 Pyrene-based building blocks have been used to prepare isostructural HOFs, including HOF-102, which has been used for storing biomolecules and photochemically detoxifying mustard gas.23–25
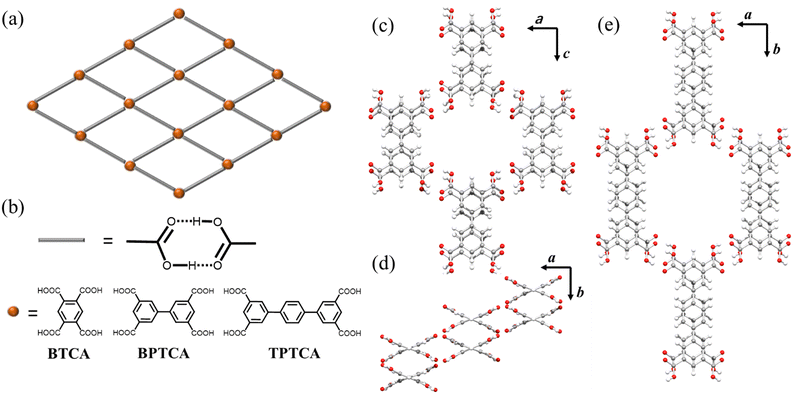
In this study, we used two building blocks containing biphenyl (BPTCA) and terphenyl (TPTCA) cores, each functionalised with four carboxylic acid groups, to construct isostructural HOFs with 1-D hexagonal-shaped pores (Fig. 1a). BPTCA and TPTCA are structurally related to 1,2,4,5-benzenetetracarboxylic acid (BTCA, Fig. 1b), which was reported to form a water solvate in 1971.26 Subsequently, BTCA was reported to form a square grid network that features R22 (8) hydrogen bonding interactions by Biradha Group,27 using phenol solvent that occupied the pores (Fig. 1b). Here, we focussed on the larger isostructural analogues BPTCA and TPTCA (Fig. 1b). BPTCA was reported to assemble into 3-fold interpenetrated HOF under hydrothermal conditions on cooling from 503 K.28 The surface assembly of BPTCA and TPTCA on Au(111)29 and nonanoic acid/graphite interfaces30 have also been explored. However, to our knowledge, the formation of non-interpenetrated porous HOF using BPTCA and TPTCA has not been reported.
Here, we attempted to crystallise BPTCA and TPTCA from a range of crystallisation solvents and obtained colourless needle shape crystals of BPTCA-1 and TPTCA-1 from DMF and CHCl3 solvent mixtures (see ESI,† Sections 2.1 and 2.3). The solvated single crystal X-ray diffraction (SC-XRD) structures showed that the phenyl groups of BPTCA in BPTCA-1 and TPTCA in TPTCA-1 adopt twisted conformations (Fig. 1). In BPTCA-1, the phenyl groups in BPTCA are twisted by 42.6° (Fig. 1d). In the BPTCA-1 structure, the BPTCA molecules hydrogen-bond to four other BPTCA molecules through R22 (8) interactions to generate distorted hexagonal-shaped pores (Fig. 1c). These pores pass through the offset, A-B-type, π–π stacked layers of BPTCA molecules, which are separated by ∼3.7 Å and an offset of ∼1.4 Å (Fig. 1c). The SC-XRD structure of TPTCA-1 showed that TPTCA adopts a non-planar conformation with the phenyl group twisted by 31.4° and 35.9°. In the extended TPTCA-1 structure, TPTCA, like BPTCA in BPTCA-1, hydrogen-bonds to four neighbouring TPTCA through R22 (8) hydrogen bonding interactions to assemble into a HOF with hexagonal-shaped pores (Fig. 1e). The TPTCA molecules also stack in offset, π–π stacked “A-B” layers in TPTCA-1 (Fig. 1e), which are separated by ∼3.7 Å and offset is ∼1.2 Å. Disordered solvent occupies the 1-D channels in BPTCA-1 and TPTCA-1, which we could not model accurately in the SC-XRD structures (Fig. S1 and S3, ESI†).
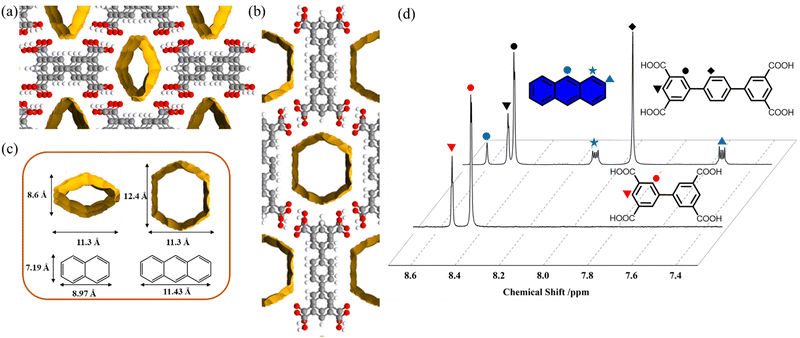
To evacuate the solvent-filled pores of BPTCA-1 and TPTCA-1, we first exchanged the crystallisation solvents by immersing the BPTCA-1 and TPTCA-1 crystals in n-pentane for five days. Then, we degassed the samples under a dynamic vacuum at room temperature for 2 hours to obtain BPTCA-2 and TPTCA-2. The SC-XRD structure showed that both BPTCA-2 and TPTCA-2 had non-interpenetrated porous frameworks after activation under these conditions. However, in BPTCA-2, the BPTCA molecules transformed into an A-B-C-type stacked structure, which we attribute to the slippage of the layers of BPTCA molecules along the c-axis (Fig. S2, ESI†). This structural transformation reduced the dihedral angle between the two-phenyl rings from 42.6° in BPTCA-1 to 35.1° in BPTCA-2. Despite the transformation, BPTCA-2 retained its 1-D pores, which we calculated had the dimensions 11.3 Å × 8.6 Å. The SC-XRD structure of TPTCA-2 (Fig. S4, ESI†) is comparable to TPTCA-1, and we calculated that the dimensions of the 1-D pores were 12.4 Å × 11.3 Å (Fig. 1e).
We recorded N2 sorption isotherms at 77 K to measure the surface areas of BPTCA-2 and TPTCA-2. Before the N2 sorption isotherms, we degassed the samples for 12 hours at RT. After activation under these conditions, the Brunauer–Emmett–Teller surface areas (SABET) of BPTCA-2 (15 m2 g−1, Fig. S5, ESI†) and TPTCA-2 (30 m2 g−1, Fig. S6, ESI†) were low. We attribute the low SABET of BPTCA-2 to a decrease in the crystallinity of the sample and a change in the structure observed by PXRD after degassing (Fig. S7, ESI†). We could not determine the SC-XRD of BPTCA after the sorption measurement. However, after the sorption measurement, we determined a new SC-XRD structure of TPTCA-2, named TPTCA-2_degas. The SC-XRD structure of TPTCA-2_degas showed a slippage of the 2-D sql nets, hydrogen-bonded TPTCA layers, in comparison to TPTCA-2, which led to the dimension of the pores shrinking to 5.9 Å × 3.4 Å (Fig. S8, ESI†). This sql nets, hydrogen-bonded TPTCA layers also changed from being planar in TPTCA-2 to a wave-like structure in TPTCA-2_degas (Fig. S9, ESI†). The structural transformation that we observed during degassing proved that TPTCA-2 is metastable, which agrees with the PXRD data (Fig. S10, ESI†). We likewise attribute the low SABET of TPTCA-2 to a decrease in the crystallinity of the sample and a change in structure during degassing.
To further explore the porosity of BPTCA-2 and TPTCA-2, we investigated the adsorption of different PAHs at room temperature. We selected naphthalene (NA, 9.0 Å × 7.2 Å, Fig. 2c) and anthracene (AN, 11.4 Å × 7.2 Å, Fig. 2c) as adsorbates because they have dimensions that are similar to the pore sizes in BPTCA-2 (11.3 Å × 8.6 Å, Fig. 2a and c) and TPTCA-2 (12.4 Å × 11.3 Å, Fig. 2b and c). We performed these measurements by immersing BPTCA-2 and TPTCA-2 solids (3 mg) in n-hexadecane solutions that contained NA or AN dissolved at concentrations of 200 ppm. Then, we collected the BPTCA-2 and TPTCA-2 solids by filtration and washed them with pure n-hexadecane before analysis. The crystallinity of BPTCA-2 and TPTCA-2 solids after immersion in the n-hexadecane solutions was insufficient for analysis with SC-XRD. Therefore, we used 1H NMR spectra to determine the NA and AN host: guest ratios in the BPTCA-2 and TPTCA-2 solids after dissolving the HOFs in deuterated dimethyl sulfoxide (DMSO-d6). We observed signals in the NMRs of the dissolved solids that indicated NA was adsorbed from the n-hexadecane solution by BPTCA-2 and TPTCA-2 (Fig. S11, ESI†). The host: guest ratio based on the NMR data was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.063 for BPTCA-2⊃NA (Fig. S11 and S12, ESI†) and 1
0.063 for BPTCA-2⊃NA (Fig. S11 and S12, ESI†) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 for TPTCA-2⊃NA (Fig. S11 and S12, ESI†). Fig. 2d shows the 1H NMR spectra of the dissolved BPTCA-2 and TPTCA-2 solids after immersion in the ANn-hexadecane solution. By comparison, we only observed AN signals in the 1H NMR spectrum of the dissolved TPTCA-2 solid, which indicates that AN was not adsorbed by the BPTCA-2 solid. The host: guest ratios based on this NMR data were, therefore, estimated to be 1
0.11 for TPTCA-2⊃NA (Fig. S11 and S12, ESI†). Fig. 2d shows the 1H NMR spectra of the dissolved BPTCA-2 and TPTCA-2 solids after immersion in the ANn-hexadecane solution. By comparison, we only observed AN signals in the 1H NMR spectrum of the dissolved TPTCA-2 solid, which indicates that AN was not adsorbed by the BPTCA-2 solid. The host: guest ratios based on this NMR data were, therefore, estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 for TPTCA-2⊃AN, and tentatively 1
0.2 for TPTCA-2⊃AN, and tentatively 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 for BPTCA-2⊃AN (Fig. 2d). Based on the relative integrals from the 1H NMR spectra, the adsorption capacities of BPTCA-2 for NA and AN are 24.3 mg g−1 and 0 mg g−1, respectively, and of TPTCA-2 for NA and AN are 35.0 mg g−1 and 87.8 mg g−1, respectively.
0 for BPTCA-2⊃AN (Fig. 2d). Based on the relative integrals from the 1H NMR spectra, the adsorption capacities of BPTCA-2 for NA and AN are 24.3 mg g−1 and 0 mg g−1, respectively, and of TPTCA-2 for NA and AN are 35.0 mg g−1 and 87.8 mg g−1, respectively.
We also used gas chromatography (GC) to investigate the adsorption properties of BPTCA-2 and TPTCA-2. The GC data showed a similar trend in absorption capacities (28.5 mg g−1 for BPTCA-2⊃NA; 41.2 mg g−1 for TPTCA-2⊃NA; 93.8 mg g−1 for TPTCA-2⊃AN), which indicates that BPTCA-2 and TPTCA-2 are promising PAHs adsorbents.
To explore the selective adsorption ability of TPTCA-2 towards NA and AN, we immersed TPTCA-2 crystal in n-hexadecane containing 200 ppm NA and AN. From the competitive adsorption experiment, we observed that TPTCA-2 exhibited the selective adsorption of AN, while no NA appeared to be adsorbed from the NA and AN mixture by the 1H NMR spectrum (Fig. S13, ESI†). We attribute this observation to the stronger CH⋯π interactions between AN and TPTCA-2.
We also investigated the selective adsorption of NA and AN by BPTCA-2 and TPTCA-2 using UV-vis spectroscopy (Fig. 3 and Fig. S14, ESI†). The UV-vis spectra showed that the absorbance intensity of NA and AN decreased over time after the addition of the BPTCA-2 and TPTCA-2 solids. The adsorption of AN appeared to reach equilibrium after 3 hours, which indicated that the PAHs were adsorbed by the HOFs (Fig. 3 and Fig. S14, ESI†).
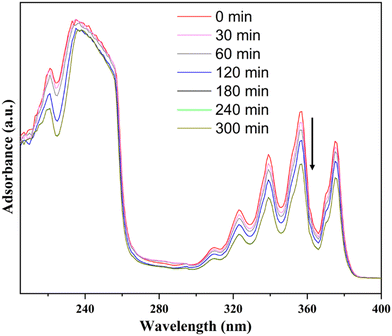 | ||
| Fig. 3 The UV-vis spectra of n-hexadecane solution containing 200 ppm of AN after the addition of TPTCA-2 solid. | ||
In summary, we have found two isostructural HOFs with hexagonal-shaped pores, BPTCA-1 and TPTCA-1, which crystallised from DMF and CHCl3 solvent mixtures. After degassing the activated crystals, BPTCA-2 and TPTCA-2, both HOFs had low SABET due to a combination of amorphous particles and structural transformations. However, the BPTCA-2 and TPTCA-2 showed good porosity for PAHs. Moreover, the BPTCA-2 exhibited exclusive perfect selectivity towards NA over AN, while TPTCA-2 showed selectivity towards AN from NA, which was determined by 1H NMR and UV-vis spectroscopy. The selective adsorption results provided an effective adsorption method towards to PAHs with different sizes using HOF adsorbents, demonstrating the potential of BPTCA-2 and TPTCA-2 as industrially relevant adsorbents for NA and AN in the petrochemical industry.
For funding, the authors acknowledge the Engineering and Physical Sciences Research Council (EPSRC) (EP/N004884/1), the National Natural Science Foundation of China (22308124), the Jiangsu University Funding (5501310018), the Leverhulme Trust via the Leverhulme Research Centre for Functional Materials Design. The authors acknowledge Dr Marc Little and Prof. Andrew Cooper for single crystal structure refinement and manuscript revisions. The authors thank Diamond Light Source for access to beamlines I19 (CY21726).
Conflicts of interest
There are no conflicts to declare.Notes and references
- X. Qin, L. Ye, L. Hou, T. Wang, M. Ma, X. Pu, X. Han, J. Liu, B. Luan and P. Liu, Chem. Eng. J., 2023, 466, 143078 CrossRef CAS.
- D. S. N. Parker and R. I. Kaiser, Chem. Soc. Rev., 2017, 46, 452–463 RSC.
- R. Sekiya and S. Nishikiori, Chem. Commun., 2012, 48, 5022 RSC.
- C. F. Espinosa, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2023, jacs.3c00661 Search PubMed.
- H. Zhou, C. Zhou, S. Tang, F. Zhang, S. Lei, Z. Li, J. Liu and M. Chen, Mater. Lett., 2022, 307, 131002 CrossRef CAS.
- S. Karak, K. Dey, A. Torris, A. Halder, S. Bera, F. Kanheerampockil and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 7572–7581 CrossRef CAS PubMed.
- H. S. Sasmal, A. Kumar Mahato, P. Majumder and R. Banerjee, J. Am. Chem. Soc., 2022, 144, 11482–11498 CrossRef CAS PubMed.
- Y. Yuan and G. Zhu, ACS Cent. Sci., 2019, 5, 409–418 CrossRef CAS PubMed.
- J. Li, B. Zhao, L. Guo, Z. Wang, C. Wang, Z. Wang, S. Zhang and Q. Wu, J. Chromatogr. A, 2021, 1653, 462428 CrossRef CAS PubMed.
- L. Chen, B. Zhang, L. Chen, H. Liu, Y. Hu and S. Qiao, Mater. Adv., 2022, 3, 3680–3708 RSC.
- Z.-J. Lin, S. A. R. Mahammed, T.-F. Liu and R. Cao, ACS Cent. Sci., 2022, 8, 1589–1608 CrossRef CAS PubMed.
- P. Li, M. R. Ryder and J. F. Stoddart, Acc. Mater. Res., 2020, 1, 77–87 CrossRef CAS.
- D. J. Duchamp and R. E. Marsh, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 5–19 CrossRef CAS.
- B. Wang, X.-L. Lv, J. Lv, L. Ma, R.-B. Lin, H. Cui, J. Zhang, Z. Zhang, S. Xiang and B. Chen, Chem. Commun., 2020, 56, 66–69 RSC.
- S. Feng, Y. Shang, Z. Wang, Z. Kang, R. Wang, J. Jiang, L. Fan, W. Fan, Z. Liu, G. Kong, Y. Feng, S. Hu, H. Guo and D. Sun, Angew. Chem., Int. Ed., 2020, 59, 3840–3845 CrossRef CAS PubMed.
- R.-B. Lin and B. Chen, Nat. Chem., 2019, 11, 1078–1080 CrossRef PubMed.
- P. Cui, D. P. McMahon, P. R. Spackman, B. M. Alston, M. A. Little, G. M. Day and A. I. Cooper, Chem. Sci., 2019, 10, 9988–9997 RSC.
- Y. Shi, S. Wang, W. Tao, J. Guo, S. Xie, Y. Ding, G. Xu, C. Chen, X. Sun, Z. Zhang, Z. He, P. Wei and B. Z. Tang, Nat. Commun., 2022, 13, 1882 CrossRef CAS PubMed.
- X. Song, Y. Wang, C. Wang, D. Wang, G. Zhuang, K. O. Kirlikovali, P. Li and O. K. Farha, J. Am. Chem. Soc., 2022, 144, 10663–10687 CrossRef CAS PubMed.
- Y. Chen, Y. Yang, Y. Wang, Q. Xiong, J. Yang, S. Xiang, L. Li, J. Li, Z. Zhang and B. Chen, J. Am. Chem. Soc., 2022, 144, 17033–17040 CrossRef CAS PubMed.
- A. Pulido, L. Chen, T. Kaczorowski, D. Holden, M. A. Little, S. Y. Chong, B. J. Slater, D. P. McMahon, B. Bonillo, C. J. Stackhouse, A. Stephenson, C. M. Kane, R. Clowes, T. Hasell, A. I. Cooper and G. M. Day, Nature, 2017, 543, 657–664 CrossRef CAS PubMed.
- Y. Suzuki, M. Gutiérrez, S. Tanaka, E. Gomez, N. Tohnai, N. Yasuda, N. Matubayasi, A. Douhal and I. Hisaki, Chem. Sci., 2021, 12, 9607–9618 RSC.
- K. Ma, P. Li, J. H. Xin, Y. Chen, Z. Chen, S. Goswami, X. Liu, S. Kato, H. Chen, X. Zhang, J. Bai, M. C. Wasson, R. R. Maldonado, R. Q. Snurr and O. K. Farha, Cell Rep. Phys. Sci., 2020, 1, 100024 CrossRef.
- Y. Wang, K. Ma, J. Bai, T. Xu, W. Han, C. Wang, Z. Chen, K. O. Kirlikovali, P. Li, J. Xiao and O. K. Farha, Angew. Chem., Int. Ed., 2022, 61, e202115956 CrossRef CAS PubMed.
- C. Wang, Y. Wang, K. O. Kirlikovali, K. Ma, Y. Zhou, P. Li and O. K. Farha, Adv. Mater., 2022, 34, 2202287 CrossRef CAS PubMed.
- F. Takusagawa, K. Hirotsu and A. Shimada, Bull. Chem. Soc. Jpn., 1971, 44, 1274–1278 CrossRef CAS.
- L. Rajput, N. Jana and K. Biradha, Cryst. Growth Des., 2010, 10, 4565–4570 CrossRef CAS.
- S. J. Coles, R. Holmes, M. B. Hursthouse and D. J. Price, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2002, 58, o626–o628 CrossRef CAS.
- I. Cebula, E. F. Smith, M. D. C. Gimenez-Lopez, S. Yang, M. Schröder, N. R. Champness and P. H. Beton, J. Phys. Chem. C, 2013, 117, 18381–18385 CrossRef CAS PubMed.
- R. Steeno, A. Minoia, M. C. Gimenez-Lopez, M. O. Blunt, N. R. Champness, R. Lazzaroni, K. S. Mali and S. De Feyter, Chem. Commun., 2021, 57, 1454–1457 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2277044 and 2277046–2277049. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc03131d |
| This journal is © The Royal Society of Chemistry 2023 |