Open Access Article
Open Access ArticleTwo fluorinated thulium complexes as molecular temperature sensors in MR applications†
Felix
Mysegaes
*ab,
Pauline
Voigt
b,
Peter
Spiteller
b,
Isabell
Prediger
a,
Johannes
Bernarding
a and
Markus
Plaumann
 *a
*a
aInstitute of Biometry and Medical Informatics, Otto von Guericke University Magdeburg, Medical Faculty, Leipziger Str. 44, Magdeburg 39120, Germany. E-mail: markus.plaumann@med.ovgu.de
bInstrumental Analytics, University of Bremen, Leobener Str. 7, Bremen 28359, Germany. E-mail: s_zoc4hg@uni-bremen.de
First published on 5th July 2023
Abstract
19F-based magnetic resonance is a powerful tool to overcome several difficulties of standard 1H MR. We present the syntheses and characterization (including cell viability and stability tests) of two Tm3+ complexes. Both complexes allow the detection of temperature (ΔCT = −0.2319 ppm K−1 and −0.2122 ppm K−1) without a reference compound.
MR thermometry enables the detection of non-invasive temperature distributions within extended objects.1 Temperature plays an important role in various biochemical processes, where it can fluctuate within a range of 1–5 K over the course of a day.2 Therefore, medical applications such as discrimination of healthy and abnormal tissue or progressing of diseases are also of interest.2a,3 In heat treatments such as hypothermia, monitoring local temperature helps prevent damage to healthy tissue.3,4
There are some MR parameters of water that are temperature-sensitive, including T1 and T2 relaxation times and proton resonance frequency (PRF).2a,3,4b The PRF is currently the most widely used method for temperature detection and imaging. It involves measuring the resonance frequencies of water protons using a gradient-echo-based pulse sequence and calculating the phase coefficient from the measured phase shift as a function of temperature.3,5 However, when measuring the water protons using this technique, only a low temperature sensitivity of 0.01 ppm K−1 is measured.4b,5b–d,6
Therefore, the development of substances with highly temperature-sensitive MR signals is necessary. For this purpose, paramagnetic lanthanoid1b,6,7 (with CT-values up to 1.45 ppm K−1)7f and transition metal8 complexes are coming into focus. The lanthanoid complexes TmDOTMA− and TmDOTP− are probably the most promising examples, with temperature sensitivities of 0.57 ppm K−1 and 1.0 ppm K−1, respectively.1b,6,7 However, TmDOTP− has a high affinity towards Ca2+-ions and is strongly affected by pH value.1b,7c–e,9 Tsitovich et al. synthesized some Fe- and Co-complexes with temperature sensitivities up to 0.52 ppm K−1.8
These MR probes show a significant advantage in comparison to the PRF method, but are limited by the inherent Curie temperature dependence of chemical shift in paramagnetic complexes.319F NMR and MRI are gaining more and more interest for diagnostic studies due to the fact that they have some benefits compared to other nuclei. There is only one natural isotope of fluorine (19F). Additionally, it has a similar gyromagnetic ratio close to 1H. The most important advantage is the absence of fluorine signals in the body, which makes detection of 19F MR probes without background signals possible.2a,3,10
To the best of our knowledge, the list of molecular paramagnetic 19F MR temperature sensors is limited to only a few numbers of compounds.3,11 Other examples include perfluorocarbons or organofluorine compounds, but they are limited in their temperature sensitivity.2a,12 Lee et al. and Li et al. recently published a study in which they examined the temperature sensitivity of organofluorine compounds. The synthesized compounds contained several fluorine cores and use the difference between the strongest shifting signals while increasing temperature to determine the temperature sensitivity.2a,13 These compounds had a temperature sensitivity of 0.0195 ppm K−1, which is almost two times higher than that of PFCs.2a,13
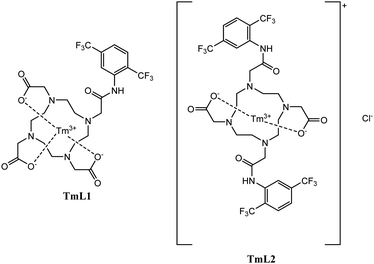
The spin-crossover complexes of Thorarinsdottir et al. showed much greater potential than 19F MR thermosensitive probes. These results showed a temperature sensitivity of up to 0.45 ppm K−1 in FBS (0.67 ppm K−1 in MeCN-d3) and, to our knowledge, are the highest values for 19F.3 In this paper, we present the synthesis of two Tm3+ complexes (TmL1 and TmL2, see Fig. 1) that have high temperature sensitivity. Both complexes possess two CF3 groups with different chemical shifts. Accordingly, the temperature is determined by the difference between the two signals, and no added reference substance is required.
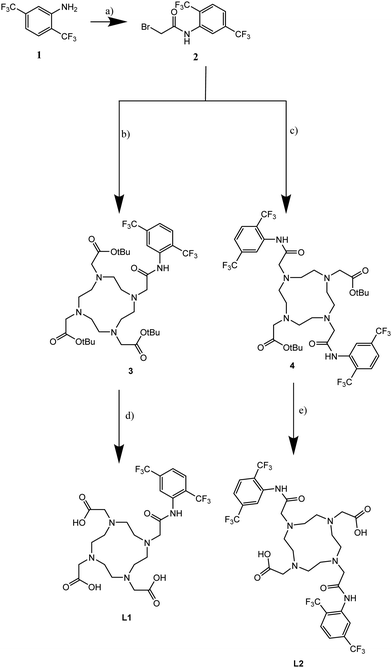
Both complexes were synthesized according to a previously published method.14 First, the tri- or bis-alkylated DO3A-tBu or DO2A-tBu and the bromoacetamide 2 were synthesized according to a literature procedure.15 The free amine(s) of DO3A-tBu and DO2A-tBu were substituted, following a slightly modified procedure from literature.14 The compounds were solved in CHCl3 and Na2CO3 was added, with stirring taking place over a period of 7 days. Deprotection can be carried out in two different ways: one way involves deprotection with TFA and the other involves a hydrolysis of the esters using formic acid. Due to difficulties in removing residues of TFA, hydrolysis with formic acid was the method of choice. Both complexes L1 and L2 were obtained in high yields (89% respectively 83%).
L2 had a higher hydrophobicity than L1, the usage of different complexation methods was necessary. Complexation with TmCl3 of L1 was carried out in water at 45 °C, stirred for one day, while complexation of L2 was carried out in MeOH at room temperature, stirred for three days. The purification step of the complexation started with precipitation of Tm(OH)3 at pH 10. After filtration of the salt, the solution was neutralized and the solvent was evaporated. The resulting solid was suspended in EtOH and centrifuged to remove NaCl, which yielded the pure complexes (92% for TmL1 and 85% for TmL2). Starting from DO3A-tBu or DO2A-tBu, both complexes were obtained with an overall yield of 75% for TmL1 and 34% for TmL2 (see Fig. 2).
Stability experiments were performed with TmL1, here, the complex was dissolved in D2O and two equivalents of ZnCl2 were added. Control of the pH-value showed a slight decrease after 48 h from 3.35 (before the addition of ZnCl2) to 2.83. The stability was verified by recording 19F NMR spectra after five minutes, thirty minutes, seven hours and twenty-four hours. For a long-term study, the probe was measured again after six months, and no transmetalation was observed. Additionally, the mixture was heated to 323.15 K and no changes in 19F NMR spectrum were observable. Finally, TmL1 showed a high stability towards Zn2+-ions (see Fig. 3).
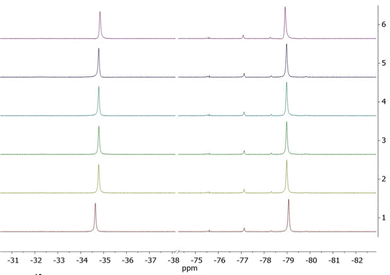
A solution of 0.1 mmol mL−1 of the complexes in 0.5 mL D2O was used in the experiments. The temperature range used was between 298.15 K and 323.15 K. The chemical shift changes of the two fluorine signals and the difference between these were determined. The difference between two fluorine signals allows the determination of the absolute temperature without the need for an internal or external reference. Complex TmL1 showed a decrease in the difference between the chemical shifts of both CF3-groups with rising temperature (see Fig. 4).
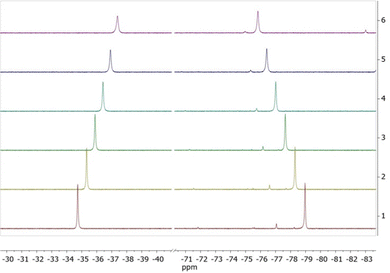 | ||
| Fig. 4 Variable-temperature 19F NMR spectra of TmL1 in D2O. The temperature range was chosen as 298 K to 323 K. 1: T = 298 K, 2: T = 303 K, 3: T = 308 K, 4: T = 313 K, 5: T = 318 K and 6: T = 323 K. | ||
The signal at −34.5 ppm (298.15 K) shifted to −37.5 ppm (323.15 K) at higher temperatures, resulting in a CT-value of −0.1058 ppm K−1. The other signal shifted from −78.7 ppm to −75.6 ppm, resulting in a CT-value of 0.1261 ppm K−1. This change in the difference between both signals resulted in a temperature coefficient ΔCT of −0.2319 ppm K−1, which is over 20 times higher than the temperature coefficient of water (CT = 0.01 ppm K−1).6b In the following step, the influence of Zn2+-ions on the temperature sensitivity of TmL1 was examined. A slight increase to CT = −0.2370 ppm K−1 was observed. We assumed that changes in the CT-value of ± 0.005 ppm K−1 were measurement errors. Thus, the presence of Zn2+-ions does not affect the temperature sensitivity.
Complex TmL2 showed a slightly lower overall temperature sensitivity compared to TmL1 (see ESI†). The CF3-groups displayed CT-values of 0.0904 ppm K−1 and −0.1218 ppm K−1, respectively. This resulted in a ΔCT between both signals of −0.2122 ppm K−1. Structural differences, such as the positive charge or higher hydrophobicity of TmL2, are suggested to have no significant influence on the sensitivity.
The work of Pujales-Paradela et al. proved that these types of complexes can be used for paraCEST (paramagnetic Chemical Exchange Saturation Transfer) imaging and fluorine imaging.14 In addition to their high temperature sensitivity, the synthesis of two multifunctional contrast agents was achieved.
Compared to the results of Thorarinsdottir et al., a lower temperature sensitivity (−0.2319 ppm K−1vs. 0.52 ppm K−1) was obtained.3 However, the use of two different CF3-groups for the determination of the temperature enables the calculation of the absolute temperature by determining the difference between the two signals. Also, the usage of one CF3-group gives these complexes an advantage for future imaging experiments. Furthermore, these complexes can be used as multifunctional contrast agents.
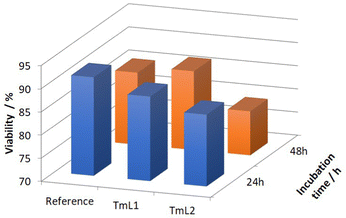
After conducting successful 19F VT NMR measurements, the toxicity of the synthesized complexes TmL1 and TmL2 was evaluated using fibroblasts (L929) in cell culture experiments. The method used is described in the experimental section. The complexes were dissolved in a cell culture medium at concentrations of 0.19 mM for TmL1 and 0.24 mM for TmL2 and added to the cells. The cell viability was determined after 24 h and 48 h of incubation, and the results were compared to a control sample containing only the cells and the cell culture medium. Both complexes showed no significant toxicity (Fig. 5). Complex TmL1 had a cell viability of 88.56% after 24 h and 87% after 48 h, while complex TmL2 had slightly lower viability at 85.5% and 79.5%, respectively. These results are comparable to the control samples (91.25% and 85.56%, respectively) and indicate that the complexes can be used in future in in vivo experiments (Fig. 5).
We presented the synthesis of two multifunctional complexes, TmL1 and TmL2, with a high 19F MR signal temperature sensitivity and no significant toxicity towards fibroblasts (L929). TmL1 had a slightly higher temperature sensitivity than TmL2, with a ΔCT of -02319 ppm K−1 (and -0.2122 ppm K−1), which is over 20 times higher than the temperature coefficient of water. The presence of Zn2+-ions did not affect the temperature sensitivity of TmL1. Based on the previously published complexes,14 these complexes could be used for paraCEST imaging and 19F imaging. Furthermore, the two different CF3-groups in each complex enables the determination of the absolute temperature without an internal or external reference. The promising complexes TmL1 and TmL2 will be examined in future MR imaging experiments.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) E. E. Turk, Forensic Sci., Med., Pathol., 2010, 6, 106–115 CrossRef PubMed; (b) S. K. Hekmatyar, R. M. Kerkhoff, S. K. Pakin, P. Hopewell and N. Bansal, Int. J. Hyperthermia, 2005, 21, 561 CrossRef CAS PubMed.
- (a) A. L. Lee, A. K. Pandey, S. Chiniforoush, M. Mandal, J. Li, C. J. Cramer, C. L. Haynes and W. C. K. Pomerantz, Anal. Chem., 2022, 94, 3782 CrossRef CAS PubMed; (b) D. Kalamida, I. V. Karagounis, A. Mitrakas, S. Kalamida, A. Giatromanolaki and M. I. Koukourakis, PLoS One, 2015, 10, e0116021 CrossRef PubMed.
- A. E. Thorarinsdottir, A. I. Gaudette and T. D. Harris, Chem. Sci., 2017, 8, 2448 RSC.
- (a) P. Wust, B. Hildebrandt, G. Sreenivasa, B. Rau, J. Gellermann, H. Riess, R. Felix and P. M. Schlag, Lancet Oncol., 2002, 3, 487 CrossRef CAS PubMed; (b) V. Rieke, Interventional Magnetic Resonance Imaging, ed. T. Kahn, H. Busse, Springer, Berlin, Heidelberg, 2011, p. 271 Search PubMed.
- (a) J. Yuan, C. S. Mei, L. P. Panych, N. J. McDannold and B. Madore, Quant. Imaging Med. Surg., 2012, 2, 21 Search PubMed; (b) K. Kuroda, Int. J. Hyperthermia, 2005, 21, 547 CrossRef CAS PubMed; (c) S. Roujol, M. Ries, B. Quesson, C. Moonen and B. Denis de Senneville, Magn. Reson. Med., 2010, 63, 1080 CrossRef PubMed; (d) L. Winter, E. Oberacker, K. Paul, Y. Ji, C. Oezerdem, P. Ghadjar, A. Thieme, V. Budach, P. Wust and T. Niendorf, Int. J. Hyperthermia, 2016, 32, 63 CrossRef CAS PubMed; (e) P. Wang, Quant. Imaging Med. Surg., 2017, 7, 259 CrossRef PubMed; (f) J. De Poorter, C. De Wagter, Y. De Deene, C. Thomsen, F. Stahlberg and E. Achten, Magn. Reson. Med., 1995, 33, 74 CrossRef CAS PubMed; (g) K. R. Gorny, C. P. Favazza, A. Lu, J. P. Felmlee, N. J. Hangiandreou, J. E. Browne, W. S. Stenzel, J. L. Muggli, A. G. Anderson, S. M. Thompson and D. A. Woodrum, Phys. Med., 2019, 67, 91 CrossRef CAS PubMed.
- (a) B. S. Park, M. J. Lizak, L. M. Angelone and S. S. Rajan, J. Electromagn. Anal. Appl., 2015, 7, 115 Search PubMed; (b) S. K. Pakin, S. K. Hekmatyar, P. Hopewell, A. Babsky and N. Bansal, NMR Biomed., 2006, 19, 116 CrossRef CAS PubMed.
- (a) J. R. James, Y. Gao, M. A. Miller, A. Babsky and N. Bansal, Magn. Reson. Med., 2009, 62, 550 CrossRef CAS PubMed; (b) S. K. Hekmatyar, P. Hopewell, S. K. Pakin, A. Babsky and N. Bansal, Magn. Reson. Med., 2005, 53, 294 CrossRef CAS PubMed; (c) D. Zhang, B. Itin and A. E. McDermott, J. Magn. Reson., 2019, 308, 106574 CrossRef CAS PubMed; (d) Y. Sun, M. Sugawara, R. V. Mulkern, K. Hynynen, S. Mochizuki, M. Albert and C. S. Zuo, NMR Biomed., 2000, 13, 460 CrossRef CAS PubMed; (e) C. S. Zuo, J. L. Bowers, K. R. Metz, T. Nosaka, A. D. Sherry and M. E. Clouse, Magn. Reson. Med., 1996, 36, 955 CrossRef CAS PubMed; (f) C. S. Zuo, A. Mahmood and A. D. Sherry, J. Magn. Reson., 2001, 151, 101–106 CrossRef CAS PubMed; (g) O. Y. Selyutina and S. P. Babailov, Molecules, 2022, 27, 6691 CrossRef CAS PubMed; (h) E. N. Zapolotsky, Y. Qu and S. P. Babailov, J. Incl. Phenom. Macrocycl. Chem., 2022, 102, 1 CrossRef CAS PubMed; (i) S. P. Babailov, Sens. Actuators, B, 2017, 251, 108 CrossRef CAS; (j) S. P. Babailov, Sens. Actuators, B, 2016, 233, 476 CrossRef CAS.
- (a) P. B. Tsitovich, T. Y. Tittiris, J. M. Cox, J. B. Benedict and J. R. Morrow, Dalton Trans., 2018, 47, 916 RSC; (b) P. B. Tsitovich, J. M. Cox, J. B. Benedict and J. R. Morrow, Inorg. Chem., 2016, 55, 700 CrossRef CAS PubMed.
- (a) C. S. Zuo, K. R. Metz, Y. Sun and A. D. Sherry, J. Magn. Reson., 1998, 133, 53 CrossRef CAS PubMed; (b) P. Konstanczak, P. Wust, B. Sander, S. Schründer, T. Frenzel, W. Wlodarczyk, T. Vogl, G. Müller and R. Felix, Strahlenther. Onkol. Organ Dtsch. Röntgengesellschaft, 1997, 173, 106 CrossRef CAS PubMed; (c) D. C. Buster, M. Margarida, C. A. Castro, C. F. G. C. Geraldes, C. R. Malloy, A. D. Sherry and T. C. Siemers, Magn. Reson. Med., 1990, 15, 25 CrossRef CAS PubMed.
- (a) K. L. Peterson, K. Srivastava and V. C. Pierre, Front. Chem., 2018, 6, 160 CrossRef PubMed; (b) I. Tirotta, V. Dichiarante, C. Pigliacelli, G. Cavallo, G. Terraneo, F. B. Bombelli, P. Metrangolo and G. Resnati, Chem. Rev., 2015, 115, 1106 CrossRef CAS PubMed; (c) J. Chen, G. M. Lanza and S. A. Wickline, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 2010, 2, 431 CrossRef CAS PubMed.
- (a) M. Plaumann, J. Willmann and D. Leibfritz, Influence of temperature, pH, metalion and ligand system of the 19F-chemical shifts of fluorinated contrast agents, European Society for Magnetic Resonance in Medicine and Biology (ESMRMB), Valencia, 2008 Search PubMed; (b) F. Mysegaes, P. Voigt, I. Prediger, J. Bernarding and M. Plaumann, Fluorinated Tm3+-complexes as molecular temperature sensors, International Society for Magnetic Resonance in Medicine (ISMRM), London, 2022 Search PubMed; (c) F. Mysegaes, P. Voigt, I. Prediger, J. Bernarding and M. Plaumann, Red Hot Fluorine 19F MRI and Small Animal MRI Symposiums (SAMS), Düsseldorf, 2022; (d) F. Mysegaes and M. Plaumann, 42nd FGMR Annual Discussion Meeting, GDCh Gesellschaft Deutscher Chemiker, online, 2021.
- (a) J. X. Yu, R. R. Hallac, S. Chiguru and R. P. Mason, Prog. Nucl. Magn. Reson. Spectrosc., 2013, 70, 25 CrossRef CAS PubMed; (b) B. A. Berkowitz, J. T. Handa and C. A. Wilson, NMR Biomed., 1992, 5, 65 CrossRef CAS PubMed; (c) A. G. Webb, N. B. Smith, D. S. Ellis and W. D. O’Brien, IEEE Ultrasonics Symposium. Proceedings. An International Symposium, 1995, 2, 1609.
- J. Li, T. F. Mundhenke, T. G. Smith, W. A. Arnold and W. C. K. Pomerantz, Anal. Chem., 2023, 95, 6071 CrossRef CAS.
- (a) R. Pujales-Paradela, T. Savic, D. Esteban-Gomez, G. Angelovski, F. Carniato, M. Botta and C. Platas-Iglesias, Chem. – Eur. J., 2019, 25, 4782 CrossRef CAS PubMed; (b) R. Pujales-Paradela, T. Savic, P. Perez-Lourido, D. Esteban-Gomez, G. Angelovski, M. Botta and C. Platas-Iglesias, Inorg. Chem., 2019, 58, 7571 CrossRef CAS PubMed.
- (a) E. Pitta, M. K. Rogacki, O. Balabon, S. Huss, F. Cunningham, E. M. Lopez-Roman, J. Joossens, K. Augustyns, L. Ballell, R. H. Bates and P. Van der Veken, J. Med. Chem., 2016, 59, 6709 CrossRef CAS; (b) H. S. Chong, S. Lim, K. E. Baidoo, D. E. Milenic, X. Ma, F. Jia, H. A. Song, M. W. Brechbiel and M. R. Lewis, Bioorg. Med. Chem. Lett., 2008, 18, 5792 CrossRef CAS PubMed; (c) F. Wan, M. Liu, J. Zhang, Y. Li and L. Jiang, Res. Chem. Intermed., 2014, 41, 5109 CrossRef; (d) C. Li and W.-T. Wong, Tetrahedron, 2004, 60, 5595 CrossRef CAS; (e) L. E. Hopper and M. J. Allen, Tetrahedron Lett., 2014, 55, 5560 CrossRef CAS PubMed; (f) S. N. M. Chilla, O. Zemek, J. Kotek, S. Boutry, L. Larbanoix, C. Sclavons, L. V. Elst, I. Lukes, R. N. Muller and S. Laurent, Bioorg. Med. Chem., 2017, 25, 4297 CrossRef CAS PubMed; (g) Z. Kovács and A. D. Sherry, Synthesis, 1997, 759 CrossRef; (h) L. M. De León-Rodríguez, Z. Kovacs, A. C. Esqueda-Oliva and A. D. Miranda-Olvera, Tetrahedron Lett., 2006, 47, 6937 CrossRef; (i) A. Rodriguez-Rodriguez, M. Regueiro-Figueroa, D. Esteban-Gomez, T. Rodriguez-Blas, V. Patinec, R. Tripier, G. Tircso, F. Carniato, M. Botta and C. Platas-Iglesias, Chem. – Eur. J., 2017, 23, 1110 CrossRef CAS PubMed; (j) M. Harris, L. Vander Elst, S. Laurent and T. N. Parac-Vogt, Dalton Trans., 2016, 45, 4791 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc02724d |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: