Open Access Article
Open Access ArticleA sumanene-containing magnetic nanoadsorbent for the removal of caesium salts from aqueous solutions†
Artur
Kasprzak
 *a,
Magdalena
Matczuk
*a,
Magdalena
Matczuk
 b and
Hidehiro
Sakurai
b and
Hidehiro
Sakurai
 cd
cd
aChair of Organic Chemistry, Faculty of Chemistry, Warsaw University of Technology, Warsaw 00-664, Poland. E-mail: artur.kasprzak@pw.edu.pl
bChair of Analytical Chemistry, Faculty of Chemistry, Warsaw University of Technology, Warsaw 00-664, Poland
cDivision of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita 565-0871, Osaka, Japan
dInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ICSOTRI), Osaka University, Suita 565-0871, Osaka, Japan
First published on 6th July 2023
Abstract
Sumanene was covalently immobilised onto the surface of cobalt nanomagnets to obtain a magnetic nanoadsorbent. This nanoadsorbent was specifically designed to efficiently and selectively remove caesium (Cs) salts from aqueous solutions. The nanoadsorbent's application potential was evidenced by the removal of Cs from model aqueous solutions, simulating the concentrations of radioactive 137Cs in the environment. Additionally, Cs was effectively removed from aqueous wastes generated by routine chemical processes, including those used in drug synthesis.
The removal of caesium (Cs) salts from aqueous wastes is important from the viewpoint of human health and the protection of the natural environment. Cs contamination originates not only from organic syntheses wastes, where Cs salts like caesium fluoride (CsF) or caesium carbonate are commonly employed but also from the presence of the most dangerous radioactive 137Cs. Over the years, the major source of toxic 137Cs was the nuclear plant disasters, especially the Fukushima-Daiichi nuclear plant disaster in 2011, resulting in the uncontrolled release of significant amounts of radioactive 137Cs into groundwater and seawater.1–3 These incidents influenced the development of materials for removing Cs salts from liquid samples. The reported adsorbents primarily consist of mineral adsorbents like zeolites4,5 or graphene-family materials.5,6 However, these adsorbents featured low Cs-selectivity, leading to the unwanted, competitive removal of other salts present in the sample, such as sodium, potassium, or magnesium salts.
Sumanene (1; Fig. 1), firstly synthesised in 2003,7,8 is the polyaromatic subunit of fullerene C60, belonging to the group of the so-called buckybowls. The bowl shape of sumanene is a significant factor influencing interest in this molecule. This unique feature implies interesting properties of sumanene derivatives9–11 and is a powerful tool in synthesising highly organised structures.12,13 Consequently, sumanene and buckybowls, in general, are extensively discussed in terms of their applications. However, the reports on the practical applications of sumanene remain limited. Recently, from 2019 to 2023, we reported on the application of sumanene–ferrocene conjugates as highly selective and sensitive voltammetric sensors14–17 for detection of caesium cations (Cs+). The mechanism of action involved cation–π interactions and the perfect size match between the concave side of the sumanene bowl and the van der Waals radius of Cs+.18–20 Inspired by these studies, we further investigated the potential application of the sumanene molecule. In pursuit of discovering new practical applications of sumanene, we intended the design a new class of adsorbent dedicated to removing Cs salts from aqueous solutions. Combining the chemistries of sumanene and magnetic nanomaterials seemed particularly appealing. Magnetic nanomaterials show promise for various applications, including catalysis21–23 or adsorbent technologies.24,25 One of their key features is their ease of separation from the liquid phase using an external magnetic field (a permanent magnet) without the need for centrifugation or filtration. In this work, we present the design and synthesis of surface-functionalised cobalt nanomagnets with sumanene. These magnetic nanomaterials serve as the first magnetic nano-adsorbent 11 containing buckybowl structures, specifically designed for the selective removal of Cs salts from aqueous solutions.
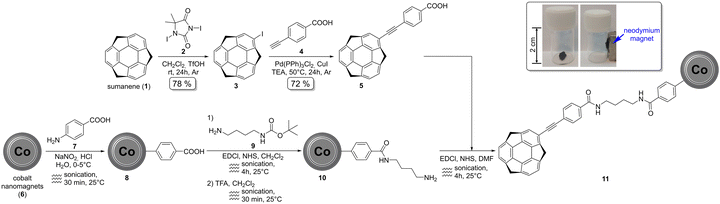 | ||
| Fig. 1 Synthesis of the target magnetic nanoadsorbent 11. The frame presents the response of 11 (50 mg) to a permanent magnet. | ||
The synthesis of the target magnetic nanoadsorbent 11 involved three steps, utilising commercially available carbon-coated cobalt nanomagnets266 (Fig. 1). Full experimental details can be found in ESI,† Section S1. In brief, firstly, carboxylic functionalities were introduced onto the surface of cobalt nanomagnets 6 using the diazotisation reaction with 4-aminobenzoic acid 7. As-obtained material 8 was functionalised to primary amino groups in two steps, i.e., by means of the carbodiimide-mediated amidation-type reaction with 9 followed by the Boc-deprotection reaction under acidic conditions. The target magnetic nanoadsorbent 11 was synthesised by means of the amidation-type reaction from material 10 and sumanene derivative 5, which was obtained in good yield employing the Sonogashira cross-coupling between 2-iodosumanene 3 and 4-ethynylbenzoic acid 4.
Confirmation of successful derivatisation of sumanene 1 to compound 5 was achieved through nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry. Photophysical properties of compound 5 were investigated using UV-vis and emission spectroscopies (data available in ESI,† Section S2).
To comprehensively analyse the target magnetic nanoadsorbent 11, various spectroscopic and materials characterisation techniques were employed. These included Fourier-transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy, Raman spectroscopy, powder X-ray diffraction, thermogravimetric analysis (TGA), transmission electron microscopy (TEM) with energy-dispersive X-ray spectroscopy (EDS) using the high-angle annular dark-field (HAADF) technique, scanning electron microscopy (SEM), and elemental analysis. The detailed characterisation can be found in ESI,† Section S2. The analyses confirmed the successful introduction of the desired moieties on the nanomaterial's surface, such as the presence of amide-I bands at ca. 1645 cm−1 in the FT-IR spectra (Fig. 2b). Furthermore, the presence of each element (C, H, N, O Co) in resultant material 11 was confirmed. The content of sumanene moieties in the resultant material is ca. 9 wt% (estimated from TGA analysis, see data in ESI,† Section S2). Notably, microscopic analyses revealed the unharmed core–shell structure of the resultant cobalt nanomagnets-containing material 11 and the presence of a non-uniform thin layer covering the carbon surface of nanoparticles, attributed to the introduced moieties (Fig. 2c; for representative TEM and SEM images, see data in ESI,† Section S2). EDS–HAADF–TEM analyses also indicated relatively homogeneous surface modification of the carbon layer of the nanomagnets (for details, see ESI,† Section S2). The magnetic nanoadsorbent 11 featured promising magnetic properties, as illustrated in Fig. 1. The material could be easily dispersed in water by simple shaking and features a fast and effective response to the external magnetic field in dispersion (see Fig. 2a).
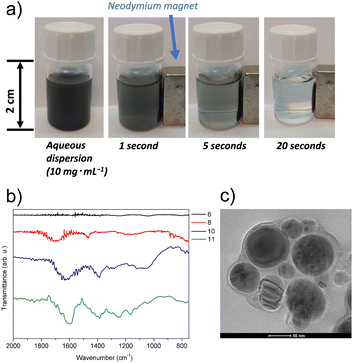 | ||
| Fig. 2 (a) Response of the aqueous dispersion of magnetic nanoadsorbent 11 to a permanent magnet; (b) FT-IR spectra of materials 6, 8, 10, 11; (c) TEM image of magnetic nanoadsorbent 11. | ||
The adsorption properties of magnetic nanoadsorbent 11 towards Cs were investigated using aqueous solutions of caesium chloride (CsCl), which served as a representative Cs salt simulating the concentrations of radioactive 137Cs in the environment. The concentrations of Cs, Na, and K in the samples were determined using inductively coupled plasma tandem mass spectrometry (ICP-MS/MS). Each adsorption experiment was repeated three times to ensure repeatability. After each adsorption trial, the magnetic properties of magnetic nanoadsorbent 11 allowed for easy and simple separation from the solution using a permanent magnet.
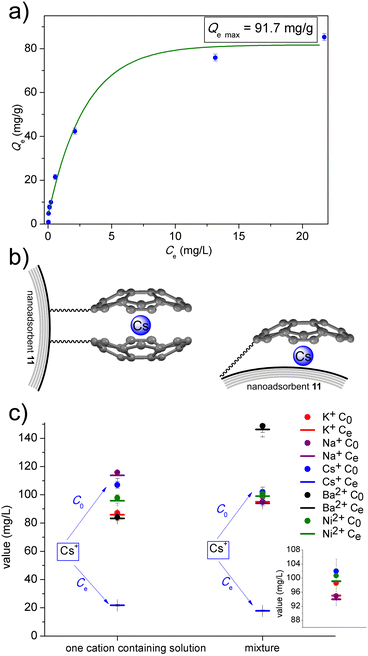
The adsorption experiments demonstrated the effective removal of Cs by magnetic nanoadsorbent 11 (Fig. 3a). Notably, SEM-EDS experiment confirmed the presence of adsorbed Cs on the surface of the magnetic nanoadsorbent 11 (the SEM image and EDS spectrum is provided in ESI,† Section S3). The adsorption process exhibited characteristics consistent with the Langmuir adsorption model,27 suggesting the formation of a monolayer on the surface (data available in ESI,† Section S3). Using the Langmuir model, the maximum adsorption capacity (Qemax) and Langmuir constant (KL) for the designed magnetic nanoadsorbent 11 were estimated to be 91.7 mg g−1 and 5.9 × 10−4 L g−1, respectively (details in ESI,† Section S3). In comparison, the Cs adsorption test using native cobalt nanomagnets 6 (surface area of native cobalt nanomagnets 6 is >15 m2 g−1 according to work24 and Merck, product no. 697745) revealed a Qemax value of approximately 4.4 mg g−1 (data in ESI,† Section S3). This value is consistent with literature data on the removal of other metal cations using similar carbon-encapsulated magnetic nanoparticles,25 and significantly lower than that of magnetic nanoadsorbent 11. This indicates the essential role of sumanene moieties on the surface of magnetic nanoadsorbent 11 in providing beneficial Cs adsorption properties. Zeta potential analyses revealed that the surface zeta potential of cobalt nanomagnets 6 (−9.0 ± 0.6 mV, in H2O) is ca 2-fold higher in comparison to the respective value for the nanoadsorbent 11 (−18.7 ± 1.0 mV). Materials comprising cobalt nanomagnets 6 are also highly magnetic, thus, their particles spontaneously agglomerate in dispersion. Interestingly, dynamic light scattering (DLS) measurements revealted the lower mean hydrodynamic diameter or particles of 11 in comparison to 6 (see size distribution histograms in ESI,† Section S3). This finding suggested that the designed functionalization method might limit the spontaneous aggregation of particles, thus, might influenced the improved dispersion stability. These findings form surface zeta potential and DLS measurements further supported the improved adsorption performance of 11 in comparison to 6.
Considering the estimated Qemax value for magnetic nanoadsorbent 11 (91.7 mg g−1) and the mass content of sumanene moieties in the resultant material (ca. 9 wt%), it can be concluded that the mass ratio of Cs to sumanene is ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, corresponding to a molar ratio of approximately 1
1, corresponding to a molar ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This suggests that the adsorption process may involve the formation of sandwich-type complexes between Cs and sumanene, as well as the potential involvement of the carbon layer of nanoparticles in the studied adsorption phenomena (see graphical representation of possible binding modes in Fig. 3b). Notably, the Cs adsorbption tests with 11 were also supported by the spectrofluorimetric investigation of sandwich-type complexes between sumanene (1) and caesium cations, which further supported the crucial role of sumanene moiety in 11 toward providing Cs binding property of the nanoadsorbent. (Apparent binding constant of 6.6 × 105 M−2 was concluded for this system, see data and discussion in ESI,† Section S7).
2. This suggests that the adsorption process may involve the formation of sandwich-type complexes between Cs and sumanene, as well as the potential involvement of the carbon layer of nanoparticles in the studied adsorption phenomena (see graphical representation of possible binding modes in Fig. 3b). Notably, the Cs adsorbption tests with 11 were also supported by the spectrofluorimetric investigation of sandwich-type complexes between sumanene (1) and caesium cations, which further supported the crucial role of sumanene moiety in 11 toward providing Cs binding property of the nanoadsorbent. (Apparent binding constant of 6.6 × 105 M−2 was concluded for this system, see data and discussion in ESI,† Section S7).
The Qemax value obtained for magnetic nanoadsorbent 11 demonstrates its superior adsorption capacity compared to previously reported adsorbents, including mineral adsorbents, graphene family materials, metal–organic frameworks (MOFs), crown ether or calixarene-containing materials, and iron oxide-based magnetic adsorbents (data available in ESI,† Section S3). Additionally, the magnetic nanoadsorbent 11 offers the advantage of easy magnetic separation from the liquid phase, making its removal from the treated aqueous solution the most facile and the fastest among all reported Cs adsorbents. Kinetic analyses revealed the most effective contact time to be 30 min (data in ESI,† Section S3).
Our experiments also revealed the possibility of reusing magnetic nanoadsorbent 11. After the adsorption process and magnetic separation, 11 could be easily recovered by washing with a small volume of water (see details in ESI,† Section S3). The magnetic nanoadsorbent 11 demonstrated reusability for at least four adsorption cycles. The results of each adsorption cycle were highly consistent, indicating the effectiveness of the washing process during the recovery stage. Regeneration of magnetic nanoadsorbent 11 after the adsorption process was also supported with the FT-IR spectroscopy, TEM microscopy and elemental analysis (see details in ESI,† Section S3). In particular, these analyses supported the elemental composition of the regenerated 11 sample, as well as confirmed no significant morphological differences between the samples of 11 before adsorption and after the regeneration process.
The high selectivity of magnetic nanoadsorbent 11 for Cs removal can be anticipated14–17 due to the presence of sumanene moieties on its surface. To investigate this selectivity, similar adsorption experiments were conducted using sodium (Na), potassium (K), barium (Ba), and nickel (Ni) salts, which are representative interferents or toxins, which might found in the environment, groundwater, and drinking water (Fig. 3c). The results demonstrated the high selectivity of magnetic nanoadsorbent 11 for Cs removal, even in the presence of interferents (see complete data in ESI,† Section S3). No interfering effect of Ba is essential, since Ba2+ features similar van der Waals radius as Cs+. This finding supports the hypothesis that the site-selective cation-π interactions between Cs+ and concave site of sumanene bowls14–17 shall play a vital role in the recognition phenomenon.
The application potential of magnetic nanoadsorbent 11 was further elucidated by utilizing it for the removal of Cs-containing aqueous wastes generated during the synthesis of (R,E)-N-(2,5-difluorobenzylidene)-2-methylpropane-2-sulfinamide 14 from (R)-2-methylpropane-2-sulfinamide 13 and 2,5-difluorobenzaldehyde 12 (for data and detailed information, refer to ESI,† Section S4). Compound 14 serves as a starting material in the synthesis of larotrectinib®, an anticancer drug recently approved by the FDA.28 The production of caesium-containing aqueous wastes in this synthesis is a result of the use of caesium carbonate (Cs2CO3). Our experiments demonstrated that the designed magnetic nanoadsorbent 11 effectively removes Cs2CO3 from the aqueous wastes generated in this process. Importantly, the effective action of magnetic nanoadsorbent 11 was confirmed by the detection of Cs on the surface of material 11 after the adsorption process. Note that this finding is constistent with the SEM-EDS experiment on model tests of CsCl adsorption with 11, in which the presence of adsorbed Cs on the surface of 11 was confirmed.
To further demonstrate the general application potential of magnetic nanoadsorbent 11, we focused on using this material for the removal of toxic caesium salts from aqueous wastes generated in commonly performed processes in organic chemistry laboratories. For this purpose, we selected the desilylation reaction, where caesium salts, such as CsF, are commonly used as desilylating agents.29,30 In our experiments, the CsF-containing aqueous wastes were generated in a representative CsF-mediated desilylation of (4-bromophenoxy)(tert-butyl)dimethylsilane 15 to 4-bromophenol 16 (for data and detailed information, refer to ESI,† Section S5). After treating the CsF-containing aqueous waste with the designed magnetic nanoadsorbent 11, we observed practically complete removal of caesium. This conclusion was further supported by detecting the presence of adsorbed Cs on the surface of magnetic nanoadsorbent 11.
In summary, we have presented the design and synthesis of a novel magnetic nanoadsorbent 11, incorporating sumanene for the efficient and selective removal of toxic Cs salts from aqueous solutions. The nanoadsorbent exhibited a high maximum adsorption capacity (Qemax = 91.7 mg g−1), easy magnetic separation, selectivity for Cs removal, and the potential for reusability. The effectiveness of the nanoadsorbent was demonstrated in the removal of Cs salts simulating radioactive 137Cs concentrations in the environment, as well as in the treatment of aqueous wastes generated (i) in the desilylation reaction, as the representative routine chemical process in organic chemistry laboratories, and (ii) in the process crucial in the pharmaceutical industry, namely the synthesis of starting material for larotrectinib® drug. This work paves the way for future research on practical applications of sumanene derivatives and buckybowls in general and represents a significant advancement in Cs salt removal technologies.
This research was funded by POB Technologie Materiałowe of Warsaw University of Technology through the Excellence Initiative: Research University (IDUB) programme, agreement no. 1820/335/Z01/POB5/2021 (A. K.), and JSPS KAKENHI grant no. JP19H00912 and JP21H05233 (H.S.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Kaeriyama, Fish. Oceanogr., 2017, 26, 99–113 CrossRef.
- T. Mizuno and H. Kubo, Sci. Rep., 2013, 3, 1742 CrossRef PubMed.
- T. J. Yasunari, A. Stohl, R. S. Hayano, J. F. Burkhart, S. Eckhardt and T. Yasunari, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 19530–19534 CrossRef CAS PubMed.
- S. Kwon, Y. Kim and Y. Roh, Sci. Rep., 2021, 11, 15362 CrossRef CAS PubMed.
- X. Liu, G.-R. Chen, D.-J. Lee, T. Kawamoto, H. Tanaka, M.-L. Chen and Y.-K. Luo, Bioresour. Technol., 2014, 160, 142–149 CrossRef CAS PubMed.
- P. Kaewmee, J. Manyam, P. Opaprakasit, G. T. Truc Le, N. Chanlek and P. Sreearunothai, RSC Adv., 2017, 7, 38747–38756 RSC.
- H. Sakurai, T. Daiko and T. Hirao, Science, 2003, 301, 1878 CrossRef CAS PubMed.
- H. Sakurai, Bull. Chem. Soc. Jpn., 2021, 94, 1579–1587 CrossRef.
- T. Amaya and T. Hirao, Chem. Rec., 2015, 15, 310–321 CrossRef CAS PubMed.
- T. Amaya and T. Hirao, Chem. Commun., 2011, 47, 10524 RSC.
- M. Saito, H. Shinokubo and H. Sakurai, Mater. Chem. Front., 2018, 2, 635–661 RSC.
- Y. Yakiyama, T. Hasegawa and H. Sakurai, J. Am. Chem. Soc., 2019, 141, 18099–18103 CrossRef CAS PubMed.
- I. Hisaki, H. Toda, H. Sato, N. Tohnai and H. Sakurai, Angew. Chem., Int. Ed., 2017, 56, 15294–15298 CrossRef CAS PubMed.
- A. Kasprzak and H. Sakurai, Dalton Trans., 2019, 48, 17147–17152 RSC.
- A. Kasprzak, A. Kowalczyk, A. Jagielska, B. Wagner, A. M. Nowicka and H. Sakurai, Dalton Trans., 2020, 49, 9965–9971 RSC.
- J. S. Cyniak, Ł. Kocobolska, N. Bojdecka, A. Gajda-Walczak, A. Kowalczyk, B. Wagner, A. M. Nowicka, H. Sakurai and A. Kasprzak, Dalton Trans., 2023, 52, 3137–3147 RSC.
- A. Kasprzak, A. Gajda-Walczak, A. Kowalczyk, B. Wagner, A. M. Nowicka, M. Nishimoto, M. Koszytkowska-Stawińska and H. Sakurai, J. Org. Chem., 2023, 88, 4199–4208 CrossRef CAS PubMed.
- S. N. Spisak, Z. Wei, A. Y. Rogachev, T. Amaya, T. Hirao and M. A. Petrukhina, Angew. Chem., Int. Ed., 2017, 56, 2582–2587 CrossRef CAS PubMed.
- U. D. Priyakumar and G. N. Sastry, Tetrahedron Lett., 2003, 44, 6043–6046 CrossRef CAS.
- D. Vijay, H. Sakurai, V. Subramanian and G. N. Sastry, Phys. Chem. Chem. Phys., 2012, 14, 3057 RSC.
- A. Schätz, T. R. Long, R. N. Grass, W. J. Stark, P. R. Hanson and O. Reiser, Adv. Funct. Mater., 2010, 20, 4323–4328 CrossRef PubMed.
- C. G. Tan and R. N. Grass, Chem. Commun., 2008, 4297 RSC.
- A. Kasprzak, M. Bystrzejewski and M. Poplawska, Org. Process Res. Dev., 2019, 23, 409–415 CrossRef CAS.
- R. Fuhrer, I. K. Herrmann, E. K. Athanassiou, R. N. Grass and W. J. Stark, Langmuir, 2011, 27, 1924–1929 CrossRef CAS PubMed.
- P. Strachowski, W. Kaszuwara and M. Bystrzejewski, New J. Chem., 2017, 41, 12617–12630 RSC.
- R. N. Grass, E. K. Athanassiou and W. J. Stark, Angew. Chem., Int. Ed., 2007, 46, 4909–4912 CrossRef CAS PubMed.
- H. Swenson and N. P. Stadie, Langmuir, 2019, 35, 5409–5426 CrossRef CAS PubMed.
- H. Mei, J. Han, S. Fustero, M. Medio-Simon, D. M. Sedgwick, C. Santi, R. Ruzziconi and V. A. Soloshonok, Chem. – Eur. J., 2019, 25, 11797–11819 CrossRef CAS PubMed.
- M. Fiorenza, A. Mordini, S. Papaleo, S. Pastorelli and A. Ricci, Tetrahedron Lett., 1985, 26, 787–788 CrossRef CAS.
- J. H. Clark, Chem. Rev., 1980, 80, 429–452 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, characterization data, adsorption experiments data. See DOI: https://doi.org/10.1039/d3cc02657d |
| This journal is © The Royal Society of Chemistry 2023 |