Open Access Article
Open Access ArticleNanotubule inclusion in the channels formed by a six-fold interpenetrated, triperiodic framework†
Sotaro
Kusumoto
 a,
Youssef
Atoini
b,
Yoshihiro
Koide
a,
Kittipong
Chainok
a,
Youssef
Atoini
b,
Yoshihiro
Koide
a,
Kittipong
Chainok
 *c,
Shinya
Hayami
*c,
Shinya
Hayami
 *d,
Yang
Kim
*cd,
Jack
Harrowfield
*d,
Yang
Kim
*cd,
Jack
Harrowfield
 *e and
Pierre
Thuéry
*e and
Pierre
Thuéry
 *f
*f
aDepartment of Material & Life Chemistry, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686, Japan
bTechnical University of Munich, Campus Straubing, Schulgasse 22, Straubing 94315, Germany
cThammasat University Research Unit in Multifunctional Crystalline Materials and Applications (TU-MCMA), Faculty of Science and Technology, Thammasat University, Pathum Thani 12121, Thailand. E-mail: kc@tu.ac.th
dDepartment of Chemistry, Graduate School of Science and Technology, Institute of Industrial Nanomaterials (IINa), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan. E-mail: hayami@kumamoto-u.ac.jp; ykim@kumamoto-u.ac.jp
eUniversité de Strasbourg, ISIS, 8 allée Gaspard Monge, Strasbourg 67083, France. E-mail: harrowfield@unistra.fr
fUniversité Paris-Saclay, CEA, CNRS, NIMBE, Gif-sur-Yvette 91191, France. E-mail: pierre.thuery@cea.fr
First published on 25th July 2023
Abstract
When reacted together with uranyl ions under solvo-hydrothermal conditions, a bis(pyridiniumcarboxylate) zwitterion (L) and tricarballylic acid (H3tca) give the complex [NH4]2[UO2(L)2][UO2(tca)]4·2H2O (1). The two ligands are segregated into different units, an anionic nanotubule for tca3− and a six-fold interpenetrated cationic framework with lvt topology for L. The entangled framework defines large channels which contain the square-profile nanotubules. Complex 1 has a photoluminescence quantum yield of 19% and its emission spectrum shows the superposition of the signals due to the two independent species.
The use of zwitterionic dicarboxylates with a large spatial separation between the two complexing sites, in association with diverse anionic dicarboxylates, has provided an efficient way to synthesize uranyl ion complexes with original features, among which the more remarkable are mixed-ligand ring- or cage-like molecular species, and woven, interpenetrated or polycatenated polymeric structures.1,2 This strategy thus appears as a promising development in the area of uranyl–organic coordination polymers and frameworks.3 A complication, however, is that even where a single product with the desired mixed-ligand composition is obtained, true heteroleptic metal ion centres where both ligands are bound to any one cation are not necessarily present. This we have observed in our work concerning uranyl ion coordination polymers derived from mixtures of polyzwitterionic and polyanionic carboxylate-donor ligands which, on the basis of extensive studies of simpler species,4 are assumed to have essentially identical binding capacity of their carboxylate units. This work,2,5 although first seeming to confirm the validity of this assumption, subsequently provided examples of single crystals containing independent cationic polymer units involving principally or solely the polyzwitterion and anionic polymer units involving just the polycarboxylate.2b,d,5b While considerable progress has been made in the development of rational procedures providing mixed-ligand species with desired properties,6 the isolation of mixed-ligand complexes of labile metal ions is complicated by the fact that it depends not only on solution equilibria but also upon solubility. The present work provides a further example of the remarkable structures that can result from such “aberrant” behaviour.
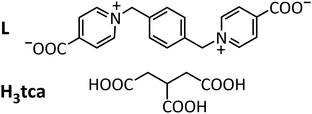
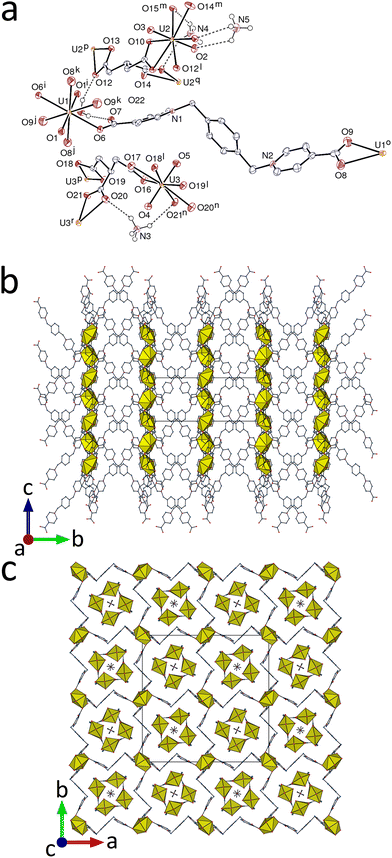
The complex [NH4]2[UO2(L)2][UO2(tca)]4·2H2O (1), where L is 1,1′-[(benzene-1,4-diyl)bis(methylene)]bis(pyridin-1-ium-4-carboxylate) and H3tca is tricarballylic acid (Scheme 1), has been synthesized (see ESI†) under solvo-hydrothermal conditions and its crystal structure determined.‡1 crystallizes in the tetragonal space group P42/n, with three independent uranium atoms, one of them on an inversion centre (Fig. 1). From previous work on uranyl ion complexes of tca3−,2b,7 it is known that this ligand has a tendency to generate tubular coordination polymers,2b,7b,d and that with the metalladizwitterion [Ni(tpyc)2] (tpyc− = 4′-carboxylato-2,2′;6′,2′′-terpyridine) it can form a true mixed-ligand species (in the sense that both of the inequivalent UVI centres are bound to both zwitterion and anionic carboxylate groups) which is tubular,2b though of a rather different profile to the tubular species formed with tca3− alone.7b,d In the present case, the trianion forms two inequivalent but very similar monoperiodic, tubular coordination polymer units directed along [001], containing atoms U2 and U3, which are both dimensionally almost identical with that found in [NH4][(UO2)2Pb(tca)2(NO3)(bipy)] (bipy = 2,2′-bipyridine),7b involving the ligand as a tris(κ2O,O′) chelate linking hexagonal-bipyramidal UVI centres [U–O(oxo), 1.769(3)–1.790(4) Å; U–O(carboxylato), 2.439(3)–2.478(3) Å in 1]. As in the U–Pb species, the tubes enclose ammonium cations, either one with two-fold rotation symmetry (N in Wyckoff position 4e) or two with four-fold rotoinversion symmetry (2a and 2b). All are involved in NH⋯O hydrogen bonding to carboxylato (and one oxo) oxygen atoms [N⋯O, 2.986(5)–3.242(3) Å; N–H⋯O, 125–152°]. N⋯N separations are close to 5.0 Å for all ammonium cations both here and in the previous complex. While these ammonium cations presumably arise from the complete oxidative decomposition of acetonitrile under solvothermal conditions in the former case, they are most probably a remainder of the NH4PF6 reactant used during the synthesis of the ligand here. That the square-profile, tubular {[UO2(tca)]−}n unit might act as an ammonium ion scavenger is a prospect to be investigated.
In the cationic part of the complex, [UO2(L)2]2+, the uranium atom U1 is in a centrosymmetric hexagonal-bipyramidal environment, being κ2O,O′-chelated by two carboxylate groups and bound to two more monodentate carboxylate donors [U–O(oxo), 1.775(4) Å; U–O(carboxylato), 2.324(3) Å for the monodentate group, 2.598(4) and 2.602(4) Å for the chelating group]. L adopts a divergent, S-shaped conformation and it bridges two uranyl cations, a situation most common with such flexible, zwitterionic dicarboxylates.2c,d,5b U1 is thus a four-coordinated (4-c) node and L is a simple edge in the uninodal, triperiodic framework formed, which has the point symbol {42·84} and the lvt topological type. The L edges are sufficiently elongated (separation of 20.3366(4) Å between the bridged uranium centres) to allow for six-fold interpenetration to occur (Fig. 2). The entanglement pertains to class Ia (one translation only), and the full interpenetration vector is [001].8 Framework interpenetration with high multiplicity is not frequent in uranyl chemistry, but another six-fold9 and one eight-fold10 case are known. When viewed down [100] (Fig. 1b), the structure displays alternate layers of uranyl cations and organic chains, with no π-stacking interactions being apparent in the latter. The presence of large voids in the interpenetrated structure is shown by the value of 0.34 for the Kitaigorodsky packing index (KPI) calculated for the cationic polymer alone.
The most original feature of this structure is related to the association of the anionic and cationic polymers. The six interpenetrated networks define two sets of slightly different channels running along [001], centered on four-fold rotoinversion or two-fold rotation axes, with the oxo atoms of the corner-defining uranyl ions either directed toward the centre of the channel or away from it (Fig. 1c). The section of both channels is roughly square, with a side length of ∼14 Å and a diagonal of ∼19.8 Å. One of the two independent anionic tubules is included in each of these two channels, with an orientation slightly different when viewed down [001] (Fig. 1c and 2d). Apart from electrostatic interactions, it is notable that two CH⋯π contacts involve one methylene proton in each of the two tca3− anions [H⋯centroid, 2.88 and 2.82 Å; C–H⋯centroid, 144° for both]; one U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π interaction may also be present [O5⋯centroid, 3.755(4) Å; U3–O5⋯centroid, 107.61(15)°]. As usual, several CH⋯O hydrogen bonds are also formed between the two polymeric motifs [C⋯O, 2.993(6)–3.455(7) Å; C–H⋯O, 114–156°]. The complete packing is quite compact, as shown by the KPI of 0.74.
O⋯π interaction may also be present [O5⋯centroid, 3.755(4) Å; U3–O5⋯centroid, 107.61(15)°]. As usual, several CH⋯O hydrogen bonds are also formed between the two polymeric motifs [C⋯O, 2.993(6)–3.455(7) Å; C–H⋯O, 114–156°]. The complete packing is quite compact, as shown by the KPI of 0.74.
Since the cationic and anionic polymers can in principle be separated without breaking of bonds, this arrangement is different from true 1D + 3D interpenetration, of which examples are known,11 and it may more properly be termed “semi-interpenetration”. Some simpler cases have previously been found in uranyl ion complexes, such as the inclusion of linear, dinuclear anions in the channels formed by the packing of diperiodic cations, both formed with a zwitterionic dicarboxylate,2d and that of uranyl citrate anionic chains in the channels formed by layers of uranyl complexes with the zwitterion [Ni(tpyc)2].2b The use of large zwitterionic dicarboxylates thus appears to be of interest for the synthesis of complexes displaying original entangled structures, as shown also by a case of 2D + 3D heterointerpenetration.2d
An interesting point concerns the presence of template effects in the formation of 1. Ammonium cations probably play a structure-directing role in the formation of the nanotubules with square cross-section as observed here and in [NH4][(UO2)2Pb(tca)2(NO3)(bipy)], as indicated by larger nanotubules, with a hexagonal cross-section, being formed in the presence of the [Co(en)3]3+ counterion, also an efficient hydrogen bond donor.7d In a second step, the nanotubules themselves could be necessary for the formation of the six-fold interpenetrated framework, although further investigation of uranyl ion complexes containing the ligand L will be needed to specify the possible range of different structures attainable (preliminary results show that a completely different structure is obtained with the ketopimelate coligand). If such a structure-directing effect were real, the formation of 1 would entail a nested, two-fold templating phenomenon, with ammonium as a sort of “second-sphere” structure-directing species for the framework.
The emission spectrum of 1 was measured in the solid state under excitation at 420 nm. The photoluminescence quantum yield (PLQY) reaches 19%, a high value for a carboxylate uranyl ion complex. The spectrum displays the broad envelope of several emission peaks, which can be separated by Gaussian deconvolution (Fig. 3). The first six intense peaks after deconvolution are easily identified as pertaining to two families (green and orange lines in Fig. 3), while the two weaker, very broad and most red-shifted peaks are presumed due to unresolved components. Each set shows the usual vibronic fine structure typical of uranyl ion emission (S11 → S00 and S10 → S0ν (ν = 0–4) electronic transitions),12 with average splitting energies of ∼800–900 cm−1. The first series (dashed green line) has maxima at 479, 501 and 521 nm, and the second (full orange line) at 489, 509 and 531 nm. Both sets of values are within the range usually observed for six-coordinate carboxylate uranyl ion complexes.13 Although the relative emissive powers of the cation and anion are unknown, it seems reasonable to attribute the most intense peaks to the nanotubules, which contain four times as many emitters as the framework; as further corroboration of this attribution, it can be remarked that the three main peaks of other uranyl tricarballylate complexes, measured under the same conditions, are at 484/495, 504/516 and 526/539 nm,7di.e. values which flank the present ones. If so, the nanotubules would correspond to the most red-shifted signals. This would indicate that the donor strength in the equatorial plane is greater in the anionic than in the zwitterionic complex, since it is known to induce a decrease in the bond order of oxo groups to uranyl.14 Evaluation of bond strengths from bond lengths as provided by calculation of bond valence parameters15 confirms this trend, with values for axial/equatorial components of 3.436/2.532 for U1, 3.406/2.694 for U2 and 3.393/2.726 for U3, indicating greater strength of the oxo bonds in zwitterion-bound U1 than in anionic carboxylate-bound U2 and U3 (overall bond valence parameters, 5.97, 6.10 and 6.12, respectively).
 | ||
| Fig. 3 Emission spectrum of complex 1 (red) and deconvoluted components, measured in the crystalline state upon excitation at 420 nm. | ||
The structure of complex 1 provides another remarkable example of the variations possible in mixed-ligand coordination polymers, one where the dizwitterion and the anionic carboxylate have independent roles. Although many interpenetrated or polycatenated uranyl ion-containing systems are presently known, with even instances of heterointerpenetration of motifs with different chemical nature and periodicity,2d the present inclusion of a monoperiodic nanotubular structure into the intricate scaffold formed by a six-fold interpenetrated framework is unprecedented. Another novel feature of the complex is the apparently clear distinction of the two emissive uranyl centres, raising the prospect of site-selective excitation given that only the interpenetrated polymers contain aromatic moieties possibly acting as antennae. More generally, this result is an example of the unusual supramolecular architectures which can be built through the use of the peculiar coordination preferences of the uranyl cation, other fascinating cases ranging from cages to porous frameworks and quasicrystals having been reported lately.16
This work was supported by Iketani Science and Technology Foundation, and KAKENHI Grant-in-Aid for Early-Career Scientists JP22K14698 for S. Kusumoto, and Thammasat University Research Fund, Contract No. TUFF02/2564 for K. Chainok.
Conflicts of interest
There are no conflicts of interest to declare.References
- (a) L. Mei, Z. N. Xie, K. Q. Hu, L. Y. Yuan, Z. Q. Gao, Z. F. Chai and W. Q. Shi, Chem. – Eur. J., 2017, 23, 13995 CrossRef CAS PubMed; (b) S. Wu, L. Mei, K. Q. Hu, Z. F. Chai, C. M. Nie and W. Q. Shi, J. Inorg. Mater., 2020, 35, 243 Search PubMed.
- (a) P. Thuéry and J. Harrowfield, CrystEngComm, 2021, 23, 7305 RSC; (b) P. Thuéry and J. Harrowfield, Inorg. Chem., 2022, 61, 9725 CrossRef PubMed; (c) S. Kusumoto, Y. Atoini, S. Masuda, J. Y. Kim, S. Hayami, Y. Kim, J. Harrowfield and P. Thuéry, Inorg. Chem., 2022, 61, 15182 CrossRef CAS PubMed; (d) S. Kusumoto, Y. Atoini, S. Masuda, Y. Koide, J. Y. Kim, S. Hayami, Y. Kim, J. Harrowfield and P. Thuéry, Inorg. Chem., 2023, 62, 3929 CrossRef CAS PubMed; (e) S. Kusumoto, Y. Atoini, S. Masuda, Y. Koide, K. Chainok, Y. Kim, J. Harrowfield and P. Thuéry, Inorg. Chem., 2023, 62, 7803 CrossRef CAS PubMed; (f) L. Baklouti and J. Harrowfield, Dalton Trans., 2023, 52, 7772 RSC.
- (a) M. B. Andrews and C. L. Cahill, Chem. Rev., 2013, 113, 1121 CrossRef CAS PubMed; (b) T. Loiseau, I. Mihalcea, N. Henry and C. Volkringer, Coord. Chem. Rev., 2014, 266–267, 69 CrossRef CAS; (c) J. Su and J. S. Chen, Struct. Bond., 2015, 163, 265 CrossRef CAS; (d) P. Thuéry and J. Harrowfield, Dalton Trans., 2017, 46, 13660 RSC; (e) K. Lv, S. Fichter, M. Gu, J. März and M. Schmidt, Coord. Chem. Rev., 2021, 446, 214011 CrossRef CAS.
- X. M. Chen and T. C. W. Mak, J. Crystallogr. Spectrosc. Res., 1993, 23, 291 CrossRef CAS.
- (a) P. Thuéry and J. Harrowfield, Eur. J. Inorg. Chem., 2022, e202200011 Search PubMed; (b) S. Kusumoto, Y. Atoini, S. Masuda, Y. Koide, J. Y. Kim, S. Hayami, Y. Kim, J. Harrowfield and P. Thuéry, CrystEngComm, 2022, 24, 7833 RSC.
- (a) Y. R. Zheng, Z. Zhao, M. Wang, K. Ghosh, J. B. Pollock, T. R. Cook and P. J. Stang, J. Am. Chem. Soc., 2010, 132, 16873 CrossRef CAS PubMed; (b) M. L. Saha, S. Neogi and M. Schmittel, Dalton Trans., 2014, 43, 3815 RSC; (c) H. Furukawa, U. Müller and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 3417 CrossRef CAS PubMed; (d) W. M. Bloch and G. H. Clever, Chem. Commun., 2017, 53, 8506 RSC; (e) S. Pullen and G. H. Clever, Acc. Chem. Res., 2018, 51, 3052 CrossRef CAS PubMed; (f) M. Viciano-Chumillas, X. Liu, A. Leyva-Pérez, D. Armentano, J. Ferrando-Soria and E. Pardo, Coord. Chem. Rev., 2022, 451, 214273 CrossRef CAS.
- (a) P. Thuéry, Chem. Commun., 2006, 853 RSC; (b) P. Thuéry and J. Harrowfield, Cryst. Growth Des., 2017, 17, 963 CrossRef; (c) P. Thuéry and J. Harrowfield, Eur. J. Inorg. Chem., 2018, 1016 CrossRef; (d) P. Thuéry, Y. Atoini and J. Harrowfield, Inorg. Chem., 2020, 59, 6953 CrossRef PubMed.
- V. A. Blatov, L. Carlucci, G. Ciani and D. M. Proserpio, CrystEngComm, 2004, 6, 377 RSC.
- P. Thuéry and J. Harrowfield, Cryst. Growth Des., 2020, 20, 262 CrossRef.
- X. Hou and S. F. Tang, Inorg. Chem., 2020, 59, 15824 CrossRef CAS PubMed.
- L. Carlucci, G. Ciani and D. M. Proserpio, Chem. Commun., 2004, 380 RSC.
- (a) A. Brachmann, G. Geipel, G. Bernhard and H. Nitsche, Radiochim. Acta, 2002, 90, 147 CrossRef CAS; (b) M. Demnitz, S. Hilpmann, H. Lösch, F. Bok, R. Steudtner, M. Patzschke, T. Stumpf and N. Huittinen, Dalton Trans., 2020, 49, 7109 RSC.
- P. Thuéry and J. Harrowfield, Inorg. Chem., 2017, 56, 13464 CrossRef PubMed.
- (a) M. P. Redmond, S. M. Cornet, S. D. Woodall, D. Whittaker, D. Collison, M. Helliwell and L. S. Natrajan, Dalton Trans., 2011, 40, 3914 RSC; (b) L. S. Natrajan, Coord. Chem. Rev., 2012, 256, 1583 CrossRef CAS.
- (a) N. E. Brese and M. O’Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef; (b) A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
- (a) P. C. Burns and M. Nyman, Dalton Trans., 2018, 47, 5916 RSC; (b) P. Li, N. A. Vermeulen, C. D. Malliakas, D. A. Gómez-Gualdrón, A. J. Howarth, B. L. Mehdi, A. Dohnalkova, N. D. Browning, M. O’Keeffe and O. K. Farha, Science, 2017, 356, 624 CrossRef CAS PubMed; (c) V. Smetana, S. P. Kelley, A. V. Mudring and R. D. Rogers, Sci. Adv., 2020, 6, eaay7685 CrossRef CAS PubMed; (d) J. C. Wu, E. C. Escudero-Adán, M. Martínez-Belmonte and J. de Mendoza, Front. Chem., 2023, 11, 1163178 CrossRef CAS PubMed.
- APEX3, ver. 2019.1-0, Bruker AXS, Madison, WI, 2019 Search PubMed.
- SAINT, ver. 8.40A, Bruker Nano, Madison, WI, 2019 Search PubMed.
- SADABS, ver. 2016/2, Bruker AXS, Madison, WI, 2016 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281 CrossRef PubMed.
- (a) M. N. Burnett and C. K. Johnson, ORTEPIII, Report ORNL-6895, Oak Ridge National Laboratory, TN, 1996; (b) L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849 CrossRef CAS.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272 CrossRef CAS.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, Fig. S1. CCDC 2266725. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc02636a |
‡ The data were collected at 100(2) K on a Bruker D8 Quest diffractometer using an Incoatec Microfocus Source (IμS 3.0 Mo) and a PHOTON III area detector, and operated with APEX3.17 The data were processed with SAINT,18 and empirical absorption corrections were made with SADABS.19 The structure was solved by intrinsic phasing with SHELXT,20 and refined by full-matrix least-squares on F2 with SHELXL,21 using the ShelXle interface.22 The hydrogen atoms of the ammonium cations were found on a residual electron density map, displaced to the proper distance, then treated as riding atoms since they were somewhat unstable upon refinement. A restraint was applied on the displacement parameter of the nitrogen atom of one of the three independent ammonium cations, which is possibly slightly disordered. Drawings were made with ORTEP-323 and VESTA,24 and the topological analysis with ToposPro.25 Crystal data for 1: C64H64N6O44U5, M = 2811.36, tetragonal, space group P42/n, a = 27.1130(9), c = 10.1061(3) Å, V = 7429.1(5) Å3, Z = 4. Refinement of 544 parameters on 7056 independent reflections out of 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845 measured reflections (Rint = 0.061) led to R1 = 0.026, wR2 = 0.059, Δρmin = −1.46, Δρmax = 1.09 e Å−3. 845 measured reflections (Rint = 0.061) led to R1 = 0.026, wR2 = 0.059, Δρmin = −1.46, Δρmax = 1.09 e Å−3. |
| This journal is © The Royal Society of Chemistry 2023 |