Open Access Article
Open Access ArticleOptically-pure triptycene-based metallomacrocycles and homochiral self-sorting assisted by ladder formation†
Kosuke
Oki‡
 a,
Wei
Zheng‡§
a,
Wei
Zheng‡§
 a,
Eiji
Yashima
a,
Eiji
Yashima
 a and
Tomoyuki
Ikai
a and
Tomoyuki
Ikai
 *ab
*ab
aDepartment of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Chikusa-ku, Nagoya 464-8603, Japan. E-mail: ikai@chembio.nagoya-u.ac.jp
bPrecursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency (JST), Kawaguchi, Saitama 332-0012, Japan
First published on 23rd June 2023
Abstract
Optically-pure triptycene-based metallomacrocycles are, for the first time, synthesized by the coordination-driven self-assembly of enantiopure triptycene-derived ladder-type bis(benzo[f]isoquinoline) ligands with a cis-platinum(II) complex. A pair of enantiomeric homochiral metallomacrocycles is also produced by the coordination-driven homochiral self-sorting of the corresponding racemic ligands, which relies on the shape-persistent nature of the ladder-structured ligands.
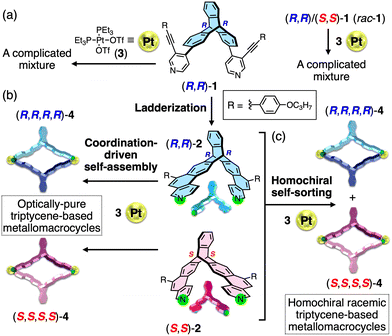
The pristine triptycene is a rigid and bulky achiral aromatic hydrocarbon with D3h-symmetry,1 but its derivatives become chiral when substituents are introduced in an asymmetric fashion as first reported by Sonoda et al. in 1962.2 In spite of the unique chiral framework of the substituted chiral triptycenes, however, the application of optically-active triptycenes as chiral functional materials has received little or no attention until the late 2010s. The groups of Chen, Fukushima, and Ikai have demonstrated the potential of optically-active triptycenes as a promising chiral scaffold for applications in chiral recognition,3 asymmetric catalysis,4 enantioseparation,5,6 and circularly polarized luminescence (CPL).7–9 Since then, the synthesis and applications of optically-active triptycenes have attracted ever-increasing attention.10–12 In terms of the rational design and construction of three-dimensional (3D) chiral nanostructures, the triptycene framework is very attractive, because its conformational freedom is highly restricted due to its robust 3D paddlewheel-like shape, which strictly defines the directionality of covalent bonds and overall spatial geometry of molecules, supramolecules, and polymers. In fact, covalently-bonded homochiral organic macrocycles/cages11,13 and one-handed helical polymers6 have been successfully synthesized using optically-pure triptycenes as a key chiral framework. However, despite their high structural designability, to the best of our knowledge, chiral triptycene-based optically-pure metallomacrocycles have never been reported. (For examples of triptycene-based optically-inactive metallomacrocycles composed of achiral or racemic building blocks, see: ref. 14–21.)
Homochiral self-sorting is a unique phenomenon probably related to the origin of biological homochirality, in which homochiral species spontaneously assemble with each other in preference to heterochiral assembly by stronger homochiral interactions primarily through metal coordination and hydrogen bonding, and has been observed in a variety of chiral molecules.22–27 However, there is no precedent for the homochiral self-sorting of racemic triptycenes.28
We now report the first synthesis of optically-pure triptycene-based metallomacrocycles ((R,R,R,R)- and (S,S,S,S)-4) formed by the coordination-driven self-assembly of optically-pure triptycene-derived ladder-type bis(benzo[f]isoquinoline) ligands ((R,R)- and (S,S)-2) with an equivalent amount of a 90° platinum(II) complex (cis-Pt(PEt3)2(OTf)2 (3))29 (Fig. 1b). We found that the shape-persistent ladder structure of the triptycene ligands ((R,R)- and (S,S)-2) obtained after versatile acid-promoted alkyne benzannulations of the corresponding precursor ligands ((R,R)- and (S,S)-1)6,30,31 enabled the quantitative and selective formation of the Pt2L2-type metallomacrocycles (Fig. 1a and b), whereas the precursor ligands bearing two flexible pyridyl groups as the metal-binding sites afforded a complicated oligomeric mixture upon mixing with 3. Moreover, we report a first homochiral self-sorting of a racemic triptycene ligand ((R,R)- and (S,S)-2 (rac-2)) with 3, thus producing a pair of enantiomeric homochiral triptycene-based metallomacrocycles ((R,R,R,R)/(S,S,S,S)-4 (rac-4)) (Fig. 1c).
We first synthesized the optically-pure ((R,R)- and (S,S)-1) and racemic (rac-1) triptycene-based precursors containing 2-[(p-propoxyphenyl)ethynyl]-4-pyridyl groups at the 2,6-positions of the triptycene unit by Suzuki–Miyaura coupling according to Scheme S1 (ESI†). The subsequent acid-promoted alkyne benzannulations6,30,31 with trifluoromethanesulfonic acid (TfOH) quantitatively produced (R,R)-, (S,S)-, and rac-2 in a complete regioselective manner, respectively (Scheme S1 and Fig. S1, ESI†).
When (R,R)-2 was allowed to react with an equimolar amount of 3 in dichloromethane at room temperature for 3 h (Fig. 2a and Scheme S2, ESI†), the complexation reaction completely proceeded, quantitatively affording the single (R,R)-2-platinum(II) complex with a highly symmetric structure, as supported by the simple 1H and 31P NMR spectra of the as-synthesized product (Fig. 2c and d(iii)); a new set of proton resonances and one phosphorus peak being significantly different from those of (R,R)-2 (Fig. 2c(i)) and 3 (Fig. 2d(ii)), respectively, were observed. In contrast, the 1H and 31P NMR spectra of the reaction products of the precursor ligand ((R,R)-1) with 3 were complicated, indicating the formation of various by-products including cyclic and non-cyclic oligomers (Fig. S3a, b(i, ii) and S4, ESI†). The high-resolution electrospray ionization mass (ESI-MS) analysis of the as-synthesized (R,R)-2-platinum(II) complex revealed the Pt2L2-type metallomacrocycle ((R,R,R,R)-4) formation, showing two characteristic molecular peaks at m/z of 577.7274 and 819.9560 corresponding to the [4–4OTf−]4+ and [4–3OTf−]3+ species, respectively (Fig. S5a(i, ii), ESI†). These peaks agreed well with the simulated ones. The (S,S)-2-platinum(II) complex ((S,S,S,S)-4) was also prepared in the same way (Scheme S2, ESI†).
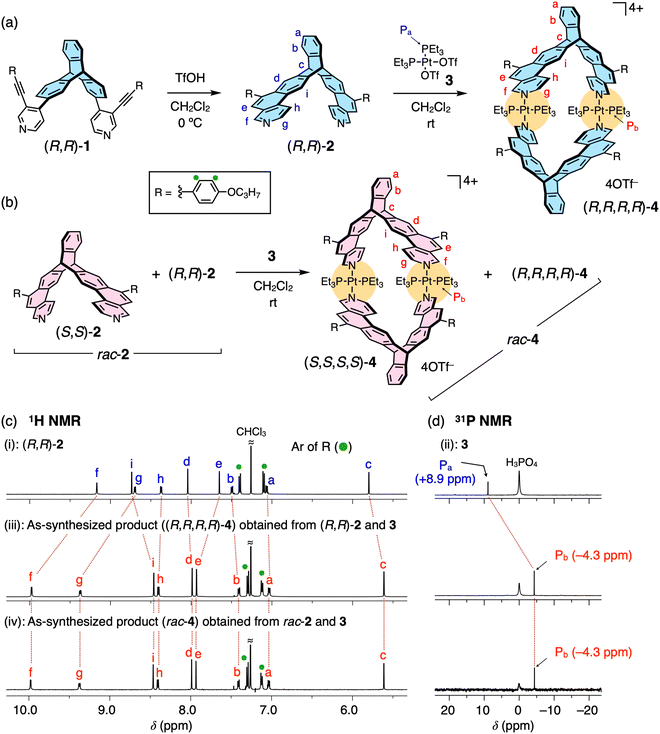 | ||
| Fig. 2 (a and b) Synthesis of optically-pure (a; (R,R,R,R)-4) and racemic (b; rac-4) homochiral triptycene-based metallomacrocycles by the coordination-driven self-assembly of (R,R)-2 and rac-2 with cis-Pt(PEt3)2(OTf)2 (3), respectively. (c and d) 1H (c; 500 MHz, CDCl3, 25 °C) and/or 31P (d; 203 MHz, CDCl3, room temperature) NMR spectra of (R,R)-2 (i), 3 (ii), and the as-synthesized products ((R,R,R,R)-4 (iii) and rac-4 (iv)) obtained through complexation reaction of (R,R)-2 and rac-2 with 3, respectively. For the signal assignments of (c), see Fig. S2 (ESI†). | ||
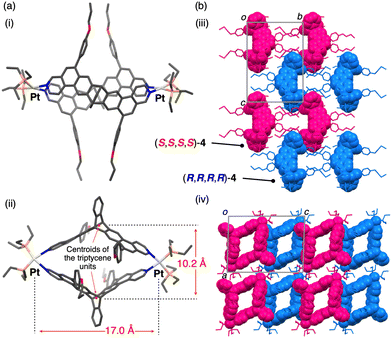
Single crystals of the (R,R)- and (S,S)-2-platinum(II) complexes suitable for X-ray analysis could not be obtained despite various attempts, but X-ray-quality crystals were successfully obtained from the reaction product of rac-2 (an equimolar mixture of (R,R)-2 and (S,S)-2) and 3 (Fig. 2b). The single-crystal X-ray crystallographic analysis of rac-4 unambiguously revealed the Pt2L2-type rhombic metallomacrocycle structure composed of two homochiral triptycene units, namely, (R,R,R,R)- and (S,S,S,S)-4 (Fig. 3), which were exclusively formed from rac-2 and 3via a complete homochiral self-sorting,22–28 as supported by the identical 1H and 31P NMR and ESI-MS spectra to those of the as-synthesized product ((R,R,R,R)-4) from (R,R)-2 and 3 (Fig. 1c, 2c, d, and Fig. S5, ESI†). The steric effect between the 4-propoxyphenyl (R) pendants most likely provides a crucial difference in the thermodynamic stability of (R,R,R,R)- and (R,R,S,S)-4 (for the DFT calculation results, see Fig S6, ESI†). In the crystal packing structure, (R,R,R,R)- and (S,S,S,S)-4 are separately stacked to form the corresponding homochiral columnar structures along the b axis (Fig. 3b). The distances between the two centroids of the triptycene units and between the two platinum atoms are 10.2 and 17.0 Å, respectively (Fig. 3a(ii)). As anticipated, such a self-sorting did not occur when rac-1 was mixed with 3 (Fig. S3a, b(iii, iv), ESI†). These results indicated the crucial role of the rigid ladder-structured ligand 2, in which the two metal-binding sites of 2 are preorganized to coordinate to the platinum(II) ions, leading to the quantitative and selective formation of the Pt2L2-type homochiral triptycene-based metallomacrocycles.
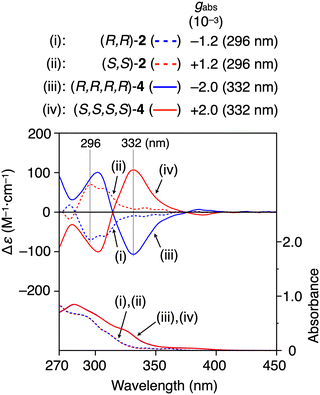
As shown in Fig. 4, the (R,R,R,R)- and (S,S,S,S)-4 metallomacrocycles exhibited absorption bands in a longer wavelength region than those of the free ligands, (R,R)- and (S,S)-2, due to the metal coordination. Hence, the homochiral metallomacrocycles showed mirror image circular dichroism (CD) signals more intense than those of the free ligands in the longer absorption region with the Kuhn's dissymmetry factor (|gabs|) of 2.0 × 10−3 at 332 nm (Fig. 4(iii and iv)) due to the unique chiral metallomacrocyclic structure, which was thermally stable in solution even at 80 °C (Fig. S7, ESI†). (R,R,R,R)-4 showed a green photoluminescence (PL) band centered at 500 nm in chloroform, which was >100 nm red-shifted from that of the blue emitting (R,R)-2, and their fluorescence quantum yields (ΦF) were determined to be 4 and 13%, respectively (Fig. S8, ESI†). The red-shifted emission band of 4 is most likely attributed to the intramolecular excimer formation between the benzo[f]isoquinoline moieties that is triggered by the close proximity of the two triptycene ligands in the metallomacrocycle (Fig. S9, ESI†). The enantiomeric pairs of 2 and 4 showed weak but mirror-imaged CPL signals with the luminescence dissymmetry factors (|glum|) up to ca. 0.4 × 10−3 in the corresponding PL regions (Fig. S8, ESI†).
In summary, we have succeeded in the first synthesis of optically-pure triptycene-based rhombic metallomacrocycles from enantiopure triptycene-derived ladder-type ligands with a high thermal stability by coordination-driven self-assembly with a cis-platinum(II) complex. In addition, we, for the first time, succeeded in the homochiral self-sorting of the racemic triptycene to exclusively produce a pair of enantiomeric homochiral metallomacrocycles. The rigid shape-persistent geometry of the ladder-structured ligand is of key importance for the quantitative and selective metallomacrocycle formation as well as the homochiral self-sorting. Therefore, the corresponding flexible non-ladder precursor ligands afforded a complicated oligomeric mixture upon mixing with a cis-platinum(II) complex. We believe that the present ligand design approach by ladderization of the flexible precursors can be applied to the selective synthesis of a further variety of discrete homochiral metallomacrocycles and cages with different geometry and ring sizes suitable for specific chiral or prochiral guest binding for chiral recognition/separation or asymmetric catalysis in a highly-enantioselective manner, respectively, based on the rational design of the chiral ladder-structured ligands and proper selection of metal ions.
We thank Professor Makoto Yamashita and Dr Ryo Nakano (Nagoya University) for their assistance in the X-ray crystallographic analysis of rac-4. This work was supported in part by JSPS KAKENHI (Grant-in-Aid for Specially Promoted Research, No. 18H05209 (E. Y. and T. I.) and Grant-in-Aid for Scientific Research (B), No. 21H01984 (T. I.)) and JST PRESTO (No. JPMJPR21A1 (T. I.)).
Conflicts of interest
There are no conflicts to declare.Notes and references
- P. D. Bartlett, M. J. Ryan and S. G. Cohen, J. Am. Chem. Soc., 1942, 64, 2649–2653 CrossRef CAS.
- A. Sonoda, F. Ogura and M. Nakagawa, Bull. Chem. Soc. Jpn., 1962, 35, 853–857 CrossRef CAS.
- G.-W. Zhang, P.-F. Li, Z. Meng, H.-X. Wang, Y. Han and C. F. Chen, Angew. Chem., Int. Ed., 2016, 55, 5304–5308 CrossRef CAS PubMed.
- F. K.-C. Leung, F. Ishiwari, Y. Shoji, T. Nishikawa, R. Takeda, Y. Nagata, M. Suginome, Y. Uozumi, Y. M. A. Yamada and T. Fukushima, ACS Omega, 2017, 2, 1930–1937 CrossRef CAS PubMed.
- T. Ikai, N. Nagata, S. Awata, Y. Wada, K. Maeda, M. Mizuno and T. M. Swager, RSC Adv., 2018, 8, 20483–20487 RSC.
- T. Ikai, T. Yoshida, K. Shinohara, T. Taniguchi, Y. Wada and T. M. Swager, J. Am. Chem. Soc., 2019, 141, 4696–4703 CrossRef CAS PubMed.
- T. Ikai, Y. Wada, S. Awata, C. Yun, K. Maeda, M. Mizuno and T. M. Swager, Org. Biomol. Chem., 2017, 15, 8440–8447 RSC.
- T. Ikai, T. Yoshida, S. Awata, Y. Wada, K. Maeda, M. Mizuno and T. M. Swager, ACS Macro Lett., 2018, 7, 364–369 CrossRef CAS PubMed.
- Y. Wada, K. Shinohara and T. Ikai, Chem. Commun., 2019, 55, 11386–11389 RSC.
- G. Preda, A. Nitti and D. Pasini, ChemistryOpen, 2020, 9, 719–727 CrossRef CAS PubMed.
- M. J. Gu, Y. F. Wang, Y. Han and C. F. Chen, Org. Biomol. Chem., 2021, 19, 10047–10067 RSC.
- M. N. Khan and T. Wirth, Chem. – Eur. J., 2021, 27, 7059–7068 CrossRef CAS PubMed.
- C. F. Chen and Y. Han, Acc. Chem. Res., 2018, 51, 2093–2106 CrossRef CAS PubMed.
- C. Azerraf, S. Cohen and D. Gelman, Inorg. Chem., 2006, 45, 7010–7017 CrossRef CAS PubMed.
- S. Chakraborty, S. Mondal, Q. J. Li and N. Das, Tetrahedron Lett., 2013, 54, 1681–1685 CrossRef CAS.
- A. Dubey, A. Mishra, J. W. Min, M. H. Lee, H. Kim, P. J. Stang and K. W. Chi, Inorg. Chim. Acta, 2014, 423, 326–331 CrossRef CAS PubMed.
- S. Chakraborty, S. Bhowmick, J. Q. Ma, H. W. Tan and N. Das, Inorg. Chem. Front., 2015, 2, 290–297 RSC.
- A. Vuillamy, S. Zebret, C. Besnard, V. Placide, S. Petoud and J. Hamacek, Inorg. Chem., 2017, 56, 2742–2749 CrossRef CAS PubMed.
- Y. Sakata, R. Yamamoto, D. Saito, Y. Tamura, K. Maruyama, T. Ogoshi and S. Akine, Inorg. Chem., 2018, 57, 15500–15506 CrossRef CAS PubMed.
- J. Plutnar, C. Givelet, C. Lemouchi, J. J. Dytrtová, I. Císařová, S. J. Teat and J. Michl, Organometallics, 2019, 38, 4633–4644 CrossRef CAS.
- S. Hasegawa, S. L. Meichsner, J. J. Holstein, A. Baksi, M. Kasanmascheff and G. H. Clever, J. Am. Chem. Soc., 2021, 143, 9718–9723 CrossRef CAS PubMed.
- M. M. Safont-Sempere, G. Fernández and F. Würthner, Chem. Rev., 2011, 111, 5784–5814 CrossRef CAS PubMed.
- H. Jędrzejewska and A. Szumna, Chem. Rev., 2017, 117, 4863–4899 CrossRef PubMed.
- H. Kar and S. Ghosh, Isr. J. Chem., 2019, 59, 881–891 CrossRef CAS.
- S. Hiraoka, Isr. J. Chem., 2019, 59, 151–165 CrossRef CAS.
- C. Li, Y. Zuo, Y.-Q. Zhao and S. Zhang, Chem. Lett., 2020, 49, 1356–1366 CrossRef.
- Q. Liu, B. Jin, Q. Li, H. Yang, Y. Luo and X. Li, Soft Matter, 2022, 18, 2484–2499 RSC.
- Das and co-workers reported that racemic 2,6-linked triptycene-based organoplatinum complexes self-assembled to form metallomacrocycles in the presence of disodium terephthalate, but their structures and self-sorting behaviors (homochiral or heterochiral) were not fully elucidated, see: ref 17.
- P. J. Stang, D. H. Cao, S. Saito and A. M. Arif, J. Am. Chem. Soc., 1995, 117, 6273–6283 CrossRef CAS.
- M. B. Goldfinger and T. M. Swager, J. Am. Chem. Soc., 1994, 116, 7895–7896 CrossRef CAS.
- M. B. Goldfinger, K. B. Crawford and T. M. Swager, J. Am. Chem. Soc., 1997, 119, 4578–4593 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterizations, and additional supporting data. CCDC 2258185. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc02259e |
| ‡ These authors contributed equally to this work. |
| § Present address: College of Materials Science and Engineering, Tongji University, Shanghai, 201804, China. |
| This journal is © The Royal Society of Chemistry 2023 |