Open Access Article
Open Access ArticleSequential chiral induction between organic and inorganic supramolecular helical assemblies for the in situ formation of chiral carbon dots†
Piyanan
Pranee
 a,
Antoine
Scalabre
a,
Antoine
Scalabre
 a,
Christine
Labrugere
c,
Naoya
Ryu
a,
Christine
Labrugere
c,
Naoya
Ryu
 d,
Akira
Yano
e,
Nanami
Hano
d,
Akira
Yano
e,
Nanami
Hano
 ah,
David
Talaga
b,
Yutaka
Okazaki
ah,
David
Talaga
b,
Yutaka
Okazaki
 f,
Emilie
Pouget
f,
Emilie
Pouget
 a,
Sylvain
Nlate
a,
Sylvain
Nlate
 a,
Sébastien
Bonhommeau
a,
Sébastien
Bonhommeau
 b,
Makoto
Takafuji
b,
Makoto
Takafuji
 h,
Takehiko
Wada
h,
Takehiko
Wada
 e,
Hirotaka
Ihara
e,
Hirotaka
Ihara
 hi,
Thierry
Buffeteau
hi,
Thierry
Buffeteau
 b,
Dario M.
Bassani
b,
Dario M.
Bassani
 *b and
Reiko
Oda
*b and
Reiko
Oda
 *ag
*ag
aUniv. Bordeaux, CNRS, Bordeaux INP, CBMN, UMR 5248, Pessac F-33600, France. E-mail: reiko.oda@u-bordeaux.fr
bUniv. Bordeaux, CNRS, Bordeaux INP, ISM UMR 5255, Talence F-33400, France
cCNRS, Université de Bordeaux, PLACAMAT UMS 3626, Pessac F-33600, France
dMaterials Development Department, Kumamoto Industrial Research Institute, 3-11-38, Higashimachi, Higashi-ku, Kumamoto 862-0901, Japan
eInstitute of Multidisciplinary Research for Advance Materials, Department of Chemistry, Graduate School of Science, Tohoku University, Sendai, 980-8577, Japan
fGraduate School of Energy Science, Kyoto University, Yoshida-Honmachi, Sakyo-ku, Kyoto 606-8501, Japan
gWPI-Advanced Institute for Materials Research, Tohoku University, 2-1-1, Katahira, Aoba-Ku, Sendai 980-8577, Japan
hDepartment of Applied Chemistry and Biochemistry, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
iNational Institute of Technology, Okinawa College, Henoko, Nano 905-2192, Japan
First published on 21st July 2023
Abstract
Self-organised helical bilayers of dicationic gemini surfactants confined in helical silica nanospace were transformed in situ to carbon dots (CDots) via pyrolysis. These water-dispersible CDots exhibit electronic absorption spanning the UV and visible range and possess symmetrical circular dichroism (CD) signals, the sign of which depends on the handedness of the helices.
Carbon dots (CDots) are luminescent carbon materials that are less than 10 nm in size and are composed of an amorphous carbon cluster and π-conjugated domains stabilized by surface functional groups.1,2 CDots have recently attracted much attention for a wide range of applications due to their unique fluorescent properties, good biocompatibility, and easy functionalization.3,4 It has been previously reported that CDots can be synthesized from various organic compounds by a bottom-up approach through hydrothermal reaction, reflux or pyrolysis.5,6 The transformation of organic molecules to CDots occurs through the condensation of functional groups, followed by carbonization.7,8 Meanwhile, chiral CDots with different effects on biological phenomena9 have shown great potential and promising properties in sensing amino acids, inhibiting peptide assemblies,10 and enantioselective recognition.11 For example, cysteine-based L-CDots were effective in glycolysis during the treatment of bladder cancer cells, whereas D-CDots showed no such effects.12 On the other hand, cysteine and citric acid-based D-CDots were able to enhance root vigor and the enzymatic activity of bean sprouts more effectively than L-CDots.13 Ma et al. found that citric acid- and aspartic acid-based L-CDots worked as strong irreversible inhibitors of tyrosinase (almost 100%), while D-CDots had lower inhibition rates (∼30%).14 Thus, the synthesis and characterization of chiral CDots combining luminescence and chiroptical properties have been at the forefront of designing original optical nanomaterials.
In general, there are three approaches to endow chiroptical properties to CDots: (1) Incomplete carbonization of chiral precursors,15,16 (2) attaching chiral molecules after the synthesis of the CDots,17,18 (3) incorporating achiral CDots within chiral supramolecular architectures.19 For the first method, Deka et al. synthesized chiral CDots from different enantiomers of chiral precursors via pyrolysis. The CDots obtained presented chiroptical activity originating from the chiral precursor.15 Meanwhile, Wang et al. synthesized chiral CDots by surface modification of achiral carbon cores by D-/L-tryptophan. These CDots showed mirror-image CD spectra resulting from the attached chiral molecules and selectively inhibited the activity of laccase. L-CDots showed a higher inhibition rate than D-CDots under the same reaction condition.20 Chiral CDots have also been successfully prepared by the co-assembly of achiral CDots with a chiral D-/L-glutamic acid-based supramolecular gelator. The intermolecular bond between CDots and the chiral source induced both CD and Circularly Polarized Luminescence (CPL) activity in the CDots.19
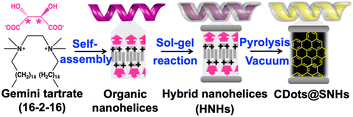
Previously, we have reported the self-organisation of an enantiopure tartrate with a gemini-type cationic surfactant, N,N′-dihexadecyl-N,N,N′,N′-tetramethylethylene diammonium (hereafter abbreviated as 16-2-16). 16-2-16 L- or D-tartrate in water self-assembles to form right- or left-handed (RH and LH, respectively) double-bilayer helical structures (Scheme 1).21 The chiral nanohelices can be used as templates for the sol–gel polycondensation of tetraethyl orthosilicate (TEOS) to obtain silica-organic hybrid nanohelices (HNHs), with 29.4 wt% of 16-2-16 and tartrate content.22 These HNHs show strong CD signals below 250 nm which originate from the tartrate anions (Fig. 1a).
 | ||
| Fig. 1 CD and absorption spectra of (a) LH/RH-HNHs and (b) CDots@LH/RH-SNHs in water. TEM images of (c) LH-HNHs and (d) CDots@LH-SNHs. | ||
When the HNHs are pyrolyzed under vacuum at 600 °C for 5 hr, they transform into a black powder (Fig. S1, ESI†) that gives rise to a broad absorption band spanning throughout the UV and visible range, with two strong peaks centred at 223 and 247 nm (Fig. 1(b)). This material also clearly shows chiroptical properties as evidenced by symmetric CD spectra in the 200–700 nm wavelength range with a negative signal for the LH helices and a positive signal for the RH helices. The CD signal above 250 nm indicates the formation of a new structure after pyrolysis with an asymmetry factor (gabs) of −3.0 × 10−4 (LH) and 3.0 × 10−4 (RH) at 570 nm, as calculated from gabs = Δε/ε = θ(mdeg)/(32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980 A) where θ = ellipticity, A = absorbance, Δε = molar circular dichroism. This signal is very different from that of the HNHs. The transmission electron microscope (TEM) images did not show significant morphology modification of the silica helices before and after pyrolysis (Fig. 1(c) and (d)). The double-bilayer organic structure was thus in situ converted to CDots while confined inside the helical silica framework. To further confirm the in situ transformation, the silica framework was removed by dissolving it in 6 M NaOH at 60 °C and washing with water. High-resolution TEM (HR-TEM) images (Fig. 2) of the resulting solution confirm the presence of nanoparticles with an average size of 4.1 ± 2.0 nm. The selected area electron diffraction (SAED) observation confirmed the presence of graphitic structure (Fig S2, ESI†). From these results, we conclude that pyrolysis of the HNHs leads to the formation of CDots which are confined inside the silica helices (CDots@SNHs) (Scheme 1). The CDots without silica nanotemplate showed no CD signal while they retained similar absorbance signals. The origin of the chiroptical signal of these CDots is thus due to their chiral organization inside the chiral nanospace of the silica structure.
980 A) where θ = ellipticity, A = absorbance, Δε = molar circular dichroism. This signal is very different from that of the HNHs. The transmission electron microscope (TEM) images did not show significant morphology modification of the silica helices before and after pyrolysis (Fig. 1(c) and (d)). The double-bilayer organic structure was thus in situ converted to CDots while confined inside the helical silica framework. To further confirm the in situ transformation, the silica framework was removed by dissolving it in 6 M NaOH at 60 °C and washing with water. High-resolution TEM (HR-TEM) images (Fig. 2) of the resulting solution confirm the presence of nanoparticles with an average size of 4.1 ± 2.0 nm. The selected area electron diffraction (SAED) observation confirmed the presence of graphitic structure (Fig S2, ESI†). From these results, we conclude that pyrolysis of the HNHs leads to the formation of CDots which are confined inside the silica helices (CDots@SNHs) (Scheme 1). The CDots without silica nanotemplate showed no CD signal while they retained similar absorbance signals. The origin of the chiroptical signal of these CDots is thus due to their chiral organization inside the chiral nanospace of the silica structure.
In order to investigate if such CD signals originate from the incomplete pyrolysis of tartrate, a control experiment was carried out by pyrolyzing a sample of 16-2-16 gemini tartrate under the same conditions as above but without the helical silica template. Blue-emitting CDots were obtained after pyrolysis, but no CD signals were detected (Fig. S3, ESI†).
No visible chiral mesostructures remained from the carbonization of the gemini tartrate. These results indicate that the silica nanohelices are necessary for the induction of the chiroptical properties in the CDots.
The elemental contents of HNHs before and after pyrolysis were characterized using elemental analysis (EA, Table S1, ESI†). HNHs (before pyrolysis) contained C (20.9%), N (1.1%), and H (4.4%) with an N/C ratio of 0.05. This ratio corresponds to the composition of the gemini tartrate. The elemental contents of C, N, and H of CDots@SNHs was found to be 8.12%, 0.27%, and 0.65%, respectively, with an N/C ratio of 0.03. This shows that the organic content decreased significantly during pyrolysis with a higher loss of N compared to C.23 The decrease in N/C contents during the pyrolysis is due to their transformation into volatile molecules (NOx species), NH3, HCN and N224,25 whereas from the C contents, they transformed in majority to CDots with some transformation to CO2 gas.
X-ray photoelectron spectroscopy (XPS) was used to investigate the chemical compositions and structure of the CDots@SNHs. The spectra show four predominant peaks corresponding to 9.24% carbon (C1s), 0.21% nitrogen (N1s), 57.53% of oxygen (O1s), and 32.66% Si (Si2p) (Fig. 3(a) and (b)). As shown in Fig. 3(c), the deconvolution of the high-resolution analysis of the C1s peak indicates contributions at 283.8 eV (58.34%) assigned to sp2 graphitic structures in CDots@SNHs, along with peaks at 285.1 eV (28.42%), 286.6 eV (8.11%), 288.9 eV (5.13%) corresponding to C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH bonds, respectively. The Raman spectrum recorded using 633 nm excitation (Fig. 3(d)) shows two broad D and G bands at ∼1330 and ∼1590 cm−1, respectively. The G band is related to the E2g mode of graphite and the vibration of sp2-bond carbon atoms in hexagonal lattices, whereas the D band originates from the vibrations of carbon atoms in disordered graphite. Therefore, both Raman and XPS data confirm that the CDots are principally composed of sp2 and sp3 carbon atoms.26,27 The ID/IG ratio of chiral CDots which provides the disorder and crystallite size of graphitic layers in CDots was ∼1.28 The Raman spectrum is very similar to the one observed for graphene oxide materials.29
C–OH bonds, respectively. The Raman spectrum recorded using 633 nm excitation (Fig. 3(d)) shows two broad D and G bands at ∼1330 and ∼1590 cm−1, respectively. The G band is related to the E2g mode of graphite and the vibration of sp2-bond carbon atoms in hexagonal lattices, whereas the D band originates from the vibrations of carbon atoms in disordered graphite. Therefore, both Raman and XPS data confirm that the CDots are principally composed of sp2 and sp3 carbon atoms.26,27 The ID/IG ratio of chiral CDots which provides the disorder and crystallite size of graphitic layers in CDots was ∼1.28 The Raman spectrum is very similar to the one observed for graphene oxide materials.29
The 2D fluorescence spectra of CDots@SNHs dispersed in H2O are shown in Fig. S4 (ESI†). The excitation-dependent emission intensity and wavelength are typical of CDots.30 As for the chiral CDots@SNHs in H2O, a maximum emission at 352 nm could be observed under an excitation wavelength of 270 nm. The photoluminescence property of SNHs@CDots was strongly affected by the dispersion medium.31 Indeed, the emission peak of CDots@SNHs in dimethylformamide (DMF) was red-shifted to 425 nm (Fig. 3(e), (f), and Fig. S5, ESI†). The shift in fluorescence emission of CDots@SNHs may be related to the effect of medium polarity and/or to H-bonding with surface functional groups on the CDots.32 The stability of CDots@SNHs was studied in water and DMF. While the fluorescence intensity of CDots@SNHs in DMF remained stable, that in water decreased dramatically over 5 days (Fig. S6, ESI†) even though the CD spectra and absorbance in water did not change during this time (Fig. S7, ESI†). Although dynamic quenching of CDot emission by water has been speculated,33 the slow loss of emission upon addition of water is incompatible with an excited-state process. Therefore, we assign this to the formation of non-emissive trap states.
Finally, to completely exclude that the chiroptical properties result from the chirality of the tartrate starting material, we exchanged the tartrate anion with achiral azide (N3−) anions inside the HNHs before pyrolysis as reported previously (Fig. S8, ESI†).22,34 The azide ion was selected since it does not interfere with the CD signal by absorbing in the UV-visible spectral region. Thus, the loss of the CD signal in the UV-visible region confirms the complete exchange of tartrate by azide (Fig. S9, ESI†). After pyrolysis at 600 °C, the azide(N)-doped CDots@SNHs showed strong and mirror image CD signals (Fig. S10(a), ESI†), indicating that the chiroptical properties of the CDots are a result of chiral induction by the nanohelices and not from the chiral tartrate ions. Following the replacement of tartrate by azide anion, the proportion of N and C of the CDots with respect to Si obtained by HR-XPS after pyrolysis (Fig. S11 and S12, ESI†) was higher than that of tartrate-based CDot@SNHs (1.2% and 60% vs. 0.6% and 28% for N and C, respectively). N-doped CDots showed a blue-shift emission at ∼417 nm compared to CDots in Fig. S13 (ESI†). We then investigated whether the chiral induction is specific to the gemini or can be extended to a system in which achiral aromatic monomers are polymerized in situ inside HNHs prior to pyrolysis. To this end, 2,6-dihydroxy naphthalene (DHN) was introduced into the HNHs by ion exchange, followed by polymerization using 1,3,5-trimethyl-1,3,5-triazinane (TMTA) (Fig. 4).35,36 In addition to being compatible with the HNHs, this polymer was previously shown to be a promising precursor for nitrogen-rich carbon material. Following ion exchange from tartrate to DHN, the HNHs were pinkish and showed an absorption maximum at 228 nm (Fig. 4(a)) with a ratio of DHN:gemini-CI2− (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Surprisingly, the DHN-doped HNHs did not show CD signals initially. However, after 3 days at 4 °C, we observed the progressive increase of mirror image CD signals, which possibly originate from the self-organization of DHN inside the HNHs. The colour of HNHs gradually turned purple.
1). Surprisingly, the DHN-doped HNHs did not show CD signals initially. However, after 3 days at 4 °C, we observed the progressive increase of mirror image CD signals, which possibly originate from the self-organization of DHN inside the HNHs. The colour of HNHs gradually turned purple.
 | ||
| Fig. 4 The illustration of polymerization of DHN in HNHs. CD and absorption spectra of (a) DHN-doped HNHs, (b) polymer-doped HNHs, and (c) polymer-based CDots@SNHs. | ||
The in situ polymerization of DHN in HNHs was induced by addition of 1 equivalent of TMTA to the suspension which was kept at 4 °C for 12 hr. During this time, polymerization was accompanied by a colour change from purple to brown. After that, the excess TMTA was washed away with cold water, and the morphology of polymer-doped HNHs was characterized using TEM (Fig. S14, ESI†), confirming that the helical silica framework was unchanged after polymerization. The formation of the polymer was accompanied by an important modification in the absorbance which extended to >600 nm following polymerization.35 After polymerization, the CD signal of HNHs increased significantly compared to the DHN-doped HNHs (Fig. 4b). These observations suggest that the polymerization enhances the chiroptical properties of the system. However, fluorescence from DHN-doped HNHs and polymer-doped HNHs was not clearly observed due to its low intensity (Fig. S15, ESI†).
The polymer-doped HNHs were subsequently pyrolyzed to afford a black powder exhibiting mirror image CD signals similar to CDots@SNHs. The ID/IG ratio calculated from the integrated intensities of D and G Raman bands is ∼0.94. Along with the decrease in this value, the presence of a sharp and well-defined peak at 1590 cm−1 indicates that CDots@SNHs prepared from the DHN/TMTA polymer have a higher graphitic carbon content (Fig. S16, ESI†). The CD and absorbance signals of the three CDots@SNHs systems are compared in Fig. S10 (ESI†). They are all relatively similar and characterized by a progressive decrease in absorbance which spans from 200 nm to 700 nm spectral range. In addition, the CD signals were negative (positive) below 320 nm and switch signs to positive (negative) broad bands for the right (left) handed helices. Exchanging the counterions to achiral ones did not suppress the induced chirality in the final CDots systems. We have demonstrated the in situ formation of chiral CDots synthesized within the nanometric confinement of a helical silica framework. These represent the first examples of CDots showing CD signals in the entire UV-Vis wavelength range extending from 200 nm to 700 nm.
Importantly, we show that the chirality of a helical silica template was the origin of the chirality of the CDots. This represents a unique example in which a chiral organic molecule is used to template the formation of a helical inorganic object, which is in turn used to induce chirality in CDots prepared from organic guests. These results thus demonstrate the high potential for chiral induction from mesoscopic chiral frameworks to carbon-based nanoparticles.
This work was supported by the CNRS and Université de Bordeaux. We thank the financial support from the ANR-21-CE09-0012-01 and the expertise of Placamat platform UAR3626.
Conflicts of interest
There are no conflicts to declare.Notes and references
- V. Georgakilas, J. A. Perman, J. Tucek and R. Zboril, Chem. Rev., 2015, 115, 4744–4822 CrossRef CAS PubMed.
- I. Papagiannouli, M. Patanen, V. Blanchet, J. D. Bozek, M. de Anda Villa, M. Huttula, E. Kokkonen, E. Lamour, E. Mevel, E. Pelimanni, A. Scalabre, M. Trassinelli, D. M. Bassani, A. Lévy and J. Gaudin, J. Phys. Chem. C, 2018, 122, 14889–14897 CrossRef CAS.
- J. Liu, R. Li and B. Yang, ACS Cent. Sci., 2020, 6, 2179–2195 CrossRef CAS PubMed.
- Y. Yan, J. Gong, J. Chen, Z. Zeng, W. Huang, K. Pu, J. Liu and P. Chen, Adv. Mater., 2019, 31, 1808283 CrossRef PubMed.
- M. Otten, M. Hildebrandt, R. Kühnemuth and M. Karg, Langmuir, 2022, 38, 6148–6157 CrossRef CAS PubMed.
- K. Yin, D. Lu, L. Wang, Q. Zhang, J. Hao, G. Li and H. Li, J. Phys. Chem. C, 2019, 123, 22447–22456 CrossRef CAS.
- X. Li, S. Zhang, S. A. Kulinich, Y. Liu and H. Zeng, Sci. Rep., 2014, 4, 4976 CrossRef CAS.
- Y. Zhou, S. K. Sharma, Z. Peng and R. M. Leblanc, Polymers, 2017, 9, 67 CrossRef PubMed.
- H. Yan, M. Cacioppo, S. Megahed, F. Arcudi, L. Đorđević, D. Zhu, F. Schulz, M. Prato, W. J. Parak and N. Feliu, Nat. Commun., 2021, 12, 7208 CrossRef CAS PubMed.
- E. Arad, S. K. Bhunia, J. Jopp, S. Kolusheva, H. Rapaport and R. Jelinek, Adv. Ther., 2018, 1, 1800006 CrossRef.
- Y. Zhang, L. Hu, Y. Sun, C. Zhu, R. Li, N. Liu, H. Huang, Y. Liu, C. Huang and Z. Kang, RSC Adv., 2016, 6, 59956–59960 RSC.
- F. Li, Y. Li, X. Yang, X. Han, Y. Jiao, T. Wei, D. Yang, H. Xu and G. Nie, Angew. Chem., Int. Ed., 2018, 57, 2377–2382 CrossRef CAS PubMed.
- M. Zhang, L. Hu, H. Wang, Y. Song, Y. Liu, H. Li, M. Shao, H. Huang and Z. Kang, Nanoscale, 2018, 10, 12734–12742 RSC.
- Y. Ma, M. Zhang, Z. Deng, X. Wang, H. Huang, K. Yang, B. Yuan, Y. Liu and Z. Kang, Nanoscale, 2022, 14, 1202–1210 RSC.
- M. J. Deka and D. Chowdhury, RSC Adv., 2017, 7, 53057–53063 RSC.
- L. Đorđević, F. Arcudi, A. D’Urso, M. Cacioppo, N. Micali, T. Bürgi, R. Purrello and M. Prato, Nat. Commun., 2018, 9, 3442 CrossRef PubMed.
- N. Suzuki, Y. Wang, P. Elvati, Z.-B. Qu, K. Kim, S. Jiang, E. Baumeister, J. Lee, B. Yeom, J. H. Bahng, J. Lee, A. Violi and N. A. Kotov, ACS Nano, 2016, 10, 1744–1755 CrossRef CAS PubMed.
- M. Vázquez-Nakagawa, L. Rodríguez-Pérez, M. A. Herranz and N. Martín, Chem. Commun., 2016, 52, 665–668 RSC.
- A. Li, D. Zheng, M. Zhang, B. Wu and L. Zhu, Langmuir, 2020, 36, 8965–8970 CrossRef CAS PubMed.
- X. Wang, M. Zhang, Y. Ma, J. Wu, Y. Wang, H. Huang, Y. Liu and Z. Kang, Appl. Surf. Sci., 2022, 583, 152540 CrossRef CAS.
- R. Oda, S. J. Candau and I. Huc, Chem. Commun., 1997, 2105–2106 RSC.
- Y. Okazaki, N. Ryu, T. Buffeteau, S. Pathan, S. Nagaoka, E. Pouget, S. Nlate, H. Ihara and R. Oda, Chem. Commun., 2018, 54, 10244–10247 RSC.
- W.-J. Liu, W.-W. Li, H. Jiang and H.-Q. Yu, Chem. Rev., 2017, 117, 6367–6398 CrossRef CAS PubMed.
- H. Nan, Z. Xiao, L. Zhao, F. Yang, H. Xu, X. Xu and H. Qiu, ACS Sustainable Chem. Eng., 2020, 8, 12197–12207 CrossRef CAS.
- C.-Z. Li and L. Tan, Fuel, 2000, 79, 1899–1906 CrossRef CAS.
- X. Zhang, J. Gu, A. Pang and J. Yang, Nanotechnology, 2016, 27, 165704 CrossRef PubMed.
- I. M. Ferrer, J. C. Vinci, N. W. Guterry, V. M. Colón, J. F. Destino, F. V. Bright and L. A. Colón, Appl. Spectrosc., 2015, 69, 1082–1090 CrossRef PubMed.
- V. Lee, L. Whittaker, C. Jaye, K. M. Baroudi, D. A. Fischer and S. Banerjee, Chem. Mater., 2009, 21, 3905–3916 CrossRef CAS.
- M. Yoshikawa, M. Murakami and Y. Fujita, J. Raman Spectrosc., 2022, 53, 1394–1401 CrossRef CAS.
- Z. Ramezani, M. Qorbanpour and N. Rahbar, Colloids Surf., A, 2018, 549, 58–66 CrossRef CAS.
- D. Chao, W. Lyu, Y. Liu, L. Zhou, Q. Zhang, R. Deng and H. Zhang, J. Mater. Chem. C, 2018, 6, 7527–7532 RSC.
- P. Mohammad-Jafarieh, A. Akbarzadeh, R. Salamat-Ahangari, M. Pourhassan-Moghaddam and K. Jamshidi-Ghaleh, BMC Chem., 2021, 15, 53 CrossRef CAS PubMed.
- J. Wei, Y. Yuan, H. Li, D. Hao, C. Sun, G. Zheng and R. Wang, New J. Chem., 2018, 42, 18787–18793 RSC.
- A. Scalabre, Y. Okazaki, B. Kuppan, T. Buffeteau, F. Caroleo, G. Magna, D. Monti, R. Paolesse, M. Stefanelli, S. Nlate, E. Pouget, H. Ihara, D. M. Bassani and R. Oda, Chirality, 2021, 33, 494–505 CrossRef CAS PubMed.
- M. N. Khan, Y. Orimoto and H. Ihara, Chem. Commun., 2018, 54, 13204–13207 RSC.
- H. Noguchi, M. Sultana, N. Hano, Y. Kuwahara, M. Takafuji, S. Nagaoka, H. Qiu and H. Ihara, J. Nanomater., 2020, 10, 1882 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information on synthesis and characterization by absorption, fluorescence and Raman spectroscopies, CD, XPS, EA and TEM. See DOI: https://doi.org/10.1039/d3cc02057f |
| This journal is © The Royal Society of Chemistry 2023 |