Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iodine-mediated photoinduced tuneable disulfonylation and sulfinylsulfonylation of alkynes with sodium arylsulfinates†
Mandapati Bhargava
Reddy
 ab and
Eoghan M.
McGarrigle
ab and
Eoghan M.
McGarrigle
 *ab
*ab
aCentre for Synthesis & Chemical Biology, UCD School of Chemistry, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: eoghan.mcgarrigle@ucd.ie
bA2P CDT in Sustainable Chemistry and BiOrbic Bioeconomy SFI Research Centre, University College Dublin, Belfield, Dublin 4, Ireland
First published on 23rd May 2023
Abstract
An efficient transition-metal free iodine-mediated tuneable disulfonylation and sulfinylsulfonylation of alkynes with sodium arylsulfinates under visible light irradiation has been developed. This photochemical protocol offers broad substrate scope of 1,2-bissulfonylethenes with high functional group tolerance and good yields under mild reaction conditions. In addition, it was found that β-sulfinyl alkenylsulfones can be obtained in the absence of base. It is proposed that the reactions proceed via sulfonyl and sulfinyl radicals.
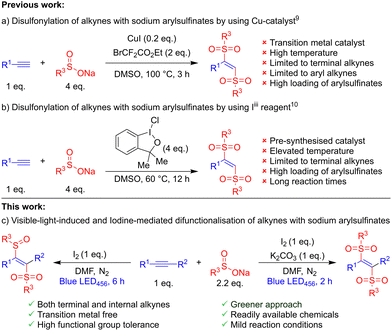
Organosulfur compounds are attractive scaffolds with numerous applications in organic chemistry,1 agrochemicals,2 material science,3 and medicinal chemistry.4 Among them, vinyl sulfones are an important class of organosulfur molecules as targets and versatile intermediates in organic chemistry.5 Recently, vinyl sulfones have been used in visible-light mediated reactions as radical acceptors.6 Due to their importance, there are many synthetic strategies to synthesise vinyl sulfones.7 Radical difunctionalisation of alkynes is one of the most efficient and direct approaches to access functionalised vinylsulfones with high step- and atom-economy.8 In 2020, Ning and Tang independently reported direct disulfonylation of terminal alkynes with sodium arylsulfinates using either copper(I) and bromodifluoroacetate or an iodine(III) reagent, respectively (Scheme 1a and b).9,10 Despite many advantages, these methods suffer from their scope being limited to terminal alkynes, harsh reaction conditions, use of copper or iodine(III), and high stoichiometric loading of arylsulfinates. To overcome these limitations, the development of a sustainable and simple method for disulfonylation of alkynes with readily available chemicals is highly sought-after.
Visible-light photocatalysis is playing an increasing role in organic synthesis because of demonstrated complex bond constructions under mild reaction conditions and visible light is environmentally benign.11 Recently, visible-light-initiated iodine mediated reactions have become attractive because these reactions avoid external transition-metal based photocatalysts and access the desired products by addition-elimination mechanisms.12E.g., Wang demonstrated photochemical iodine-catalysed electrophilic cyclisation reaction of alkynes for oxazole aldehydes.13 Separately, an environmentally friendly iodine-promoted synthesis of (E)-β-iodo vinylsulfones in water was developed by Liu14 and, recently, Bi reported the synthesis of β-sulfinyl alkenylsulfones by sulfinylsulfone radical additions to unsaturated hydrocarbons.15 Inspired by these, and related results16 herein we report the iodine-mediated tuneable synthesis of 1,2-bissulfonylethenes and β-sulfinyl alkenylsulfones under transition metal-free, mild photochemical conditions (Scheme 1c).
Table 1 shows optimisation results using phenylacetylene 1a and sodium p-toluenesulfinate 2a as model substrates for the photochemical difunctionalisation. After screening, it was found that 1 equiv. of I2 and 1 equiv. of K2CO3 in DMF under a nitrogen atmosphere for 2 h were the best conditions, yielding 3a in 88% (entry 1). We screened different solvents: MeCN was found to be less efficient than DMF (entry 2) and other solvents such as EtOH and H2O failed to afford 3a (entries 3 and 4). No significant change in yields was observed on replacing the K2CO3 with Cs2CO3, but organic bases DIPEA and DBU gave only 15% and 20% yield of 3a, respectively (entries 5–7). To our delight, in the absence of base we observed β-sulfinyl alkenylsulfones 6a in 30% yield (entry 8). Furthermore, increasing the reaction time to 6 h and doubling the dilution of the reaction mixture afforded 6a in 55% yield (entry 9). Decreasing the equivalents of iodine was found to lower the yield (entry 10). Air instead of a nitrogen atmosphere gave only 20% of the desired product 3a (entry 11). No product was observed in the absence of Iodine (entry 12) or blue light (35% of iodovinylsulfone 3a′ was obtained, entry 13).
| Entry | Variation from the standard conditions | Yieldb (%) | ||
|---|---|---|---|---|
| 3a | 6a | 3a′ | ||
| a Standard reaction conditions: 1a (0.1 mmol), 2a (0.22 mmol), I2 (0.1 mmol), and K2CO3 (0.1 mmol) in DMF (1 mL) was irradiated with a blue LED (456 nm, 40 W) under a N2 atmosphere. b Isolated yields. c Reaction mixture was irradiated for 6 h under blue LED and 2 mL DMF. n.r. = no reaction. | ||||
| 1 | None | 88 | 0 | 0 |
| 2 | MeCN instead of DMF | 75 | 10 | 0 |
| 3 | EtOH instead of DMF | Traces | 0 | 25 |
| 4 | H2O instead of DMF | 0 | 0 | 55 |
| 5 | Cs2CO3 instead of K2CO3 | 88 | 0 | 0 |
| 6 | iPr2EtN instead of K2CO3 | 15 | 0 | 22 |
| 7 | DBU instead of K2CO3 | 20 | 0 | 35 |
| 8 | Absence of base | 10 | 30 | 35 |
| 9c | Absence of base, 6 h, dilute | 15 | 55 | 20 |
| 10 | 0.5 equiv. of I2 | 66 | 0 | 0 |
| 11 | Air instead of N2 | 20 | 0 | 35 |
| 12 | Absence of I2 | n.r. | n.r. | n.r. |
| 13 | Absence of blue LED | 0 | 0 | 35 |
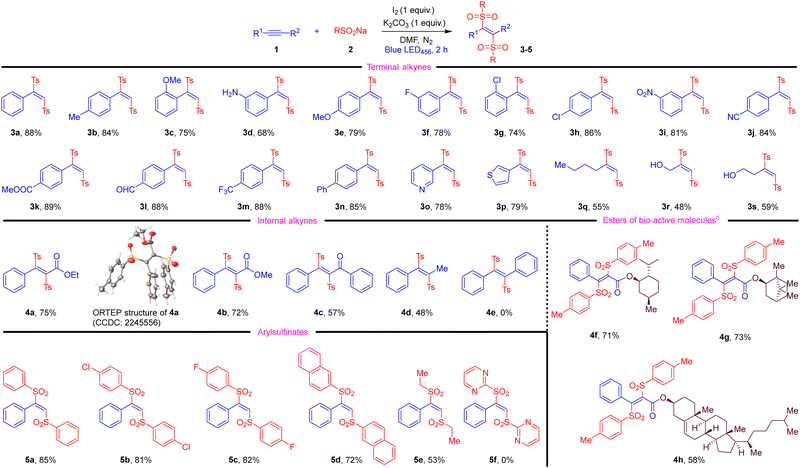
Having found suitable reaction conditions, we examined the generality of this photochemical difunctionalisation reaction (Table 2). Phenylacetylenes bearing electron-donating groups underwent the photochemical difunctionalisation with 2a smoothly, affording 1,2-bissulfonylethenes 3b–3e in 68–84% yield. Phenylacetylenes bearing halogens and stronger electron-withdrawing groups effectively reacted with 2a giving the products 3f–3n in 74–88%. Notably, aromatic heterocyclic pyridine and thiophene-substituted alkynes, and an aliphatic alkynes were also well tolerated and gave the corresponding products 3o–3s in good yields.
Next, we explored the scope of internal alkynes. To our delight, ethyl and methyl 3-phenylpropiolates smoothly reacted with 2a under this protocol and gave alkenes 4a and 4b in 75% and 72% yield, respectively. Ynone 2c was also well tolerated, giving 1,2-bissulfonylethene 4c in moderate yield. Remarkably, prop-1-yn-1-ylbenzene was also reactive under these conditions, giving the target 4d in 48% yield. In contrast, electronically symmetrical diphenylacetylene failed to produce alkene 4e. We extended the substrate scope to the ester derivates of bio-active menthol, borneol and cholesterol; these reacted smoothly affording alkenes 4f–4h in 58–73% yield.
We established the E-geometry from an X-Ray structure of 4a (see ESI†). To the best of our knowledge, this is the first time tetrasubstituted alkenes of this general structure have been reported. Finally, we varied the sulfinate. Aryl sulfinates gave high yields (5a–5d). However, pyrimidine-2-sulfinate failed to give the target 5f. Sodium ethane sulfinate also reacted to give 5e in 53% yield. Overall, our new method gives direct access from alkyne to disulfonylated alkene with excellent control of geometry, and uses milder conditions compared to previous methods. The substrate scope is broader, with amines tolerated, and especially access to tetra-substituted alkenes being noteworthy.
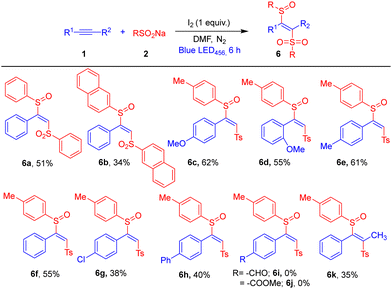
Next, we explored the observation that in the absence of base the major product was β-sulfinyl alkenylsulfone 6a (Table 1, entries 8–9). The scope of this tuneable synthesis of β-sulfinyl alkenylsulfones is shown in Table 3. Various alkynes and sodium arylsulfinates were screened under optimised conditions, β-sulfinyl alkenylsulfones 6a–6h were obtained in moderate-to-good yields. In contrast, phenylacetylenes bearing strong electron-withdrawing groups failed to give targets 6i and 6j; the competing pathway producing bissulfonylethenes dominated (see ESI† for other unsuccessful substrates). Tetrasubstituted 6k was obtained in low yield.
Next the reaction mechanism was investigated (Scheme 2a and b). Addition of the radical scavenger TEMPO stopped the production of 3a and 6a (Scheme 2a), and the tosyl radical-TEMPO adduct was detected (see ESI†). Addition of BHT also lowered the yield of 3a and the tosyl radical-BHT adducts were detected (see ESI†). Thus, both reactions are proposed to proceed via a tosyl radical intermediate. When the disulfonylation reaction mixture was quenched and isolated after 1 h, 55% of 3a′ and 20% of 3a were observed (Scheme 2ba).10 Subjecting 3a′ to the disulfonylation conditions gave 3a in 82% yield, suggesting that 3a′ may be an intermediate in the synthesis of 1,2-bissulfonylethenes (Scheme 2bb). In the presence of TEMPO 3a′ failed to produce 3a (Scheme 2bc), indicating that the conversion of 3a′ to 3a proceeds via a radical pathway. When 3a′ was subjected to the disulfonylation conditions without I2 the reaction failed to produce 3a (Scheme 2bd), while KI instead of I2 did give the target 3a in 80% yield (Scheme 2be), suggesting that I2 or iodide was needed for the second sulfonylation step. In contrast, subjecting 3a′ to the sulfinylsulfonylation conditions did not produce 6a; implying that 6a is not formed via intermediate 3a′.
Based on the above experiments and earlier literature,17 we propose the pathway shown in Scheme 3. Initially, sodium arylsulfinate 2 reacts with I2 to form intermediate A.18 Irradiation of A generates a sulfonyl radical B, which reacts with alkyne 1 to form a vinylsulfone radical C. Radical C could undergo radical–radical coupling with sulfonyl radical B to give the product 3,10 or it could react with iodine or iodine radical to give intermediate 3′ (which was isolated from quenched reactions).19 Intermediate 3′ can react with sulfonyl radical B in the presence of base and blue light to afford final compound 3 (Scheme 2bb). Given that 55% of 3a′ was isolated after 1 h of reaction (Scheme 2ba), it seems that this is likely to be the major pathway. The pathway for the formation of sulfinylsulfones 6 is less clear. The simplest explanation is that a sulfinyl radical forms and then follows the pathway proposed by Bi.15 One possibility is that in the absence of base, intermediate A reacts with iodide to generate intermediate D by elimination of IO−.20 Intermediate D could then form a sulfinyl radical on irradiation.20b Intermediate D or the sulfinyl radical could react with intermediate C to give the sulfinylvinylsulfone 6.
In conclusion, we have demonstrated a sustainable iodine-mediated tuneable disulfonylation and sulfinylsulfonylation of alkynes with sodium arylsulfinates. This photochemical difunctionalisation has a broad substrate scope and high functional group tolerance, and mild reaction conditions without transition-metal or external photocatalysts. A plausible mechanistic proposal was supported by control, quenching experiments and UV studies. We anticipate that this methodology will enable further applications of vinylsulfones, especially w.r.t. to the novel tetra-substituted alkene architectures that are accessible as a result of this work.
M. B. R. thanks the Irish Research Council for a Postdoctoral Fellowship (GOIPD/2022/576). We thank Julia Bruno-Colmenárez of the UCD X-Ray Diffraction Laboratory for the crystal structure of 4a. We thank SFI (18/RI/5702) for MS infrastructure, and the A2P CDT which is supported by SFI and EPSRC Grant No. 18/EPSRC-CDT/3582 and BiOrbic, the Bioeconomy SFI Research Centre, funded by Ireland's European Structural and Investment Programmes, SFI (16/RC/3889) and the European Regional Development Fund.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. Mellah, A. Voituriez and E. Schulz, Chem. Rev., 2007, 107, 5133–5209 CrossRef CAS PubMed; (b) T. M. Monos, R. C. McAtee and C. R. J. Stephenson, Science, 2018, 361, 1369–1373 CrossRef CAS PubMed; (c) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701–8780 CrossRef CAS PubMed.
- (a) P. Devendar and G.-F. Yang, Top. Curr. Chem., 2017, 82, 35–78 Search PubMed; (b) C. Lamberth, H. Walter, F. K. Murphy, L. Quaranta, R. Beaudegnies, S. Trah, A. Jeanguenata and F. Cederbaum, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 1225–1235 CrossRef CAS.
- (a) W. Guo, D.-Y. Wang, Q. Chen and Y. Fu, Adv. Sci., 2022, 9, 2103989 CrossRef CAS PubMed; (b) X. Zhang, K. Chen, Z. Sun, G. Hu, R. Xiao, H.-M. Cheng and F. Li, Energy Environ. Sci., 2020, 13, 1076–1095 RSC.
- (a) C. Jacob, Nat. Prod. Rep., 2006, 23, 851–863 RSC; (b) Y. Shen, C. A. Zificsak, J. E. Shea, X. Lao, O. Bollt, X. Li, J. G. Lisko, J. P. Theroff, C. L. Scaife, M. A. Ator, B. A. Ruggeri, B. D. Dorsey and S. K. Kuwada, J. Med. Chem., 2015, 58, 1140–1158 CrossRef CAS PubMed; (c) R. Ahmadi and S. Emami, Eur. J. Med. Chem., 2022, 234, 114255 CrossRef CAS PubMed.
- (a) A. Quintard and A. Alexakis, Org. Biomol. Chem., 2011, 9, 1407–1418 RSC; (b) A. P. Schaffner, V. Darmency and P. Renaud, Angew. Chem., Int. Ed., 2006, 45, 5847–5849 CrossRef CAS PubMed.
- (a) M. Ociepa, J. Turkowska and D. Gryko, ACS Catal., 2018, 8, 11362–11367 CrossRef CAS; (b) Q. Q. Zhou, S. J. S. Düsel, L. Q. Lu, B. König and W. J. Xiao, Chem. Commun., 2019, 55, 107–110 RSC; (c) A. Noble and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 11602–11605 CrossRef CAS PubMed.
- (a) D. B. Reddy, N. C. Babu, V. Padmavathi and R. P. Sumathi, Synthesis, 1999, 491–494 CrossRef CAS; (b) T. Kataoka, Y. Banno, S.-I. Watanabe, T. Iwamura and H. Shimizu, Tetrahedron Lett., 1997, 38, 1809–1812 CrossRef CAS; (c) C. Dai, J. Wang, S. Deng, C. Zhou, W. Zhang, Q. Zhu and X. Tang, RSC Adv., 2017, 7, 36112–36116 RSC.
- (a) X. Wenjiao, M. Pengju, Z. Yujun, X. Longyi, Q. Shengqi, H. Xuan, Y. Bo, G. Yuan and Z. Junmin, Org. Lett., 2022, 24, 6099–6104 CrossRef PubMed; (b) Z. Zhang, Q. Song, C. Feng, Z. Wang, Y. Zhao, W. Ning and Y. Wu, Chem. – Asian J., 2022, 17, e202200299 CAS.
- Z. Liu, L. Yang, K. Zhang, W. Chen, T. Yu, L. Wang, W. Gao and B. Tang, Org. Lett., 2020, 22, 2081–2086 CrossRef CAS PubMed.
- Y. Wang, K. Tang, Z. Liu and Y. Ning, Chem. Commun., 2020, 56, 13141–13144 RSC.
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (b) A. R. Nathan and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10116 CrossRef PubMed; (c) G. E. M. Crisenza and P. Melchiorre, Nat. Commun., 2020, 11, 803 CrossRef PubMed; (d) R. Cannalire, S. Pelliccia, L. Sancineto, E. Novellino, G. C. Tron and M. Giustiniano, Chem. Soc. Rev., 2021, 50, 766–897 RSC; (e) V. Srivastava, P. K. Singh and P. P. Singh, J. Photochem. Photobiol., C, 2022, 50, 100488 CrossRef CAS.
- (a) H. Zhang and K. Muñiz, ACS Catal., 2017, 7, 4122–4125 CrossRef CAS; (b) P. Li, J. Zhao, L. Shi, J. Wang, X. Shi and F. Li, Nat. Commun., 2018, 9, 1972 CrossRef PubMed; (c) M. Krumb, L. M. Kammer, R. Forster, C. Grundke and T. Opatz, ChemPhotoChem, 2020, 4, 101–104 CrossRef CAS; (d) J. Yang, D. Xie, H. Zhou, S. Chen, C. Huo and Z. Li, Org. Chem. Front., 2018, 5, 1325–1329 RSC.
- Y. Liu, B. Wang, X. Qiao, C.-H. Tung and Y. Wang, ACS Catal., 2017, 7, 4093–4099 CrossRef CAS.
- Y. Sun, A. Abdukader, D. Lu, H. Zhang and C. Liu, Green Chem., 2017, 19, 1255–1258 RSC.
- Z. Wang, Z. Zhang, W. Zhao, P. Sivaguru, G. Zanoni, Y. Wang, E. A. Anderson and X. Bi, Nat. Commun., 2021, 12, 5244–5255 CrossRef CAS PubMed.
- (a) M. B. Reddy and R. Anandhan, Chem. Commun., 2020, 56, 3781–3784 RSC; (b) M. B. Reddy, K. Prasanth and R. Anandhan, Org. Lett., 2022, 24, 3674–3679 CrossRef CAS PubMed; (c) J. Kumar, A. Ahmad, M. A. Rizvi, M. A. Ganie, C. Khajuria and B. A. Shah, Org. Lett., 2020, 22, 5661 CrossRef CAS PubMed; (d) M. Mohana Reddy, L. Jing and W. Jeh-Jeng, Chem. Commun., 2023 10.1039/D3CC00842H.
- (a) A. K. Sahoo, A. Dahiya, B. Das, A. Behera and B. K. Patel, J. Org. Chem., 2021, 86, 11968–11986 CrossRef CAS PubMed; (b) M. Y. Ansari, N. Kumar and A. Kumar, Org. Lett., 2019, 21, 3931–3936 CrossRef CAS PubMed; (c) F. Xiao, S. Chen, J. Tian, H. Huang, Y. Liu and G.-J. Deng, Green Chem., 2016, 18, 1538–1546 RSC.
- J. Gao, J. Lai and G. Yuan, RSC Adv., 2015, 5, 66723–66726 RSC.
- R. Kumar, V. Dwivedi and M. Sridhar Reddy, Adv. Synth. Catal., 2017, 359, 2847–2856 CrossRef CAS.
- (a) S. Samanta and S. Mondal, Asian J. Org. Chem., 2021, 10, 2503–2520 CrossRef CAS; (b) S. Hazra, M. Deb and A. J. Elias, Green Chem., 2017, 19, 5548–5552 RSC; (c) B. Li, J. Wang, G. Tu, G. Ju, K. Zhou and Y. Zhao, Eur. J. Org. Chem., 2023, e202201458 CAS; (d) F.-X. Wang and S.-K. Tian, J. Org. Chem., 2015, 80, 12697–12703 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterisation, X-ray data for 4a and copies of NMR spectra. CCDC 2245556. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc02011h |
| This journal is © The Royal Society of Chemistry 2023 |