Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effect of S-alkylation on organocatalytic enamine activation through imidazolidine-4-thiones†
Magenta J.
Hensinger
a,
Anna C.
Closs
ab,
Oliver
Trapp
 *ab and
Armin R.
Ofial
*ab and
Armin R.
Ofial
 *a
*a
aDepartment Chemie, Ludwig-Maximilians-Universität München, Butenandtstr. 5-13, München 81377, Germany. E-mail: oliver.trapp@cup.uni-muenchen.de; ofial@lmu.de
bMax-Planck-Institute for Astronomy, Königstuhl 17, Heidelberg 69117, Germany
First published on 9th June 2023
Abstract
Imidazolidine-4-thiones have been suggested as potential prebiotic organocatalysts for light-driven α-alkylations of aldehydes by bromoacetonitrile. However, imidazolidine-4-thiones react with bromoacetonitrile to give S-cyanomethylated dihydroimidazoles. Kinetic studies show that enamines derived from these cyclic secondary amines and aldehydes are more nucleophilic than enamines derived from aldehydes and MacMillan organocatalysts.
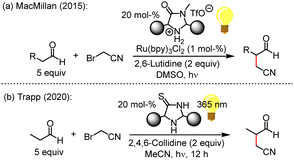
Five-membered cyclic secondary amine catalysts have proven their outstanding capacities in organocatalytic organic synthesis over the past two decades. For example, MacMillan's imidazolidine-4-ones or prolinol-based organocatalysts activate carbonyl compounds for subsequent transformations by enamine or iminium ion formation.1
Intermolecular α-cyanoalkylation of enolisable aldehydes was considered difficult because of the weak electrophilicity of alkyl halides, which hindered efficient reactions with nucleophilic enol derivatives. This issue has been overcome by MacMillan (Fig. 1a), who elegantly combined metal-based photoredox catalysis with enamine activation to α-alkylate aldehydes by bromoacetonitrile (BAN).2 The nitrile group in the product aldehydes beneficially introduced a wide range of options for further synthetic conversions.
Light-induced radical reactions of elementary bromine and acetonitrile3a,b may have led to BAN formation on the Early Earth. Motivated by this aspect, the Trapp group suggested a light-driven α-cyanomethylation of propanal by BAN that used imidazolidine-4-thiones (ITOs) as catalysts (Fig. 1b).4 ITOs are the thiolactam analogues5 of imidazolidine-4-ones (MacMillan catalysts), which feature a lactam moiety. Remarkably, ITOs assemble modularly from simple building blocks, that is, KCN, H2S, NH3, ketones or aldehydes.6 Thus, also the existence of ITOs under prebiotic conditions on the Early Earth seems likely,7 and ITO-catalysed α-alkylations of aldehydes by BAN may have led to the non-enzymatic formation of more complex, functionalized carbonyl compounds. The reagents and conditions used in Fig. 1b establish an organocatalytic version8 of the aldehyde α-alkylation,9 which is compatible with plausible prebiotic conditions.3,4,7
Most interestingly, ITO formation is reversible, and dynamic exchange of the carbonyl building blocks enables ITOs to adapt to environmental conditions. This process may provide a mechanism for molecular evolution toward structurally modified 2nd generation ITOs.7
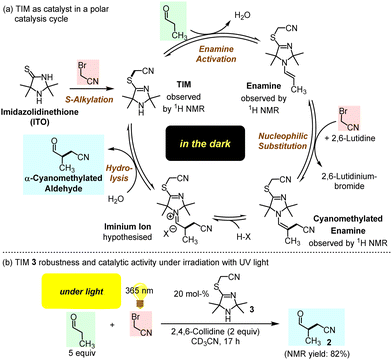
Yet, insight in the role of ITOs in the reaction shown in Fig. 1b has remained vague so far. On the one hand, it is known that the thiolactam group of ITOs is easily S-alkylated by alkyl halides or Michael acceptors to give 4-(alkylthio)-2,5-dihydro-1H-imidazoles (TIMs).10,11 On the other hand, it is widely accepted that the α-cyanomethylation of enolisable aldehydes requires enamine activation as a crucial step in the catalytic cycle. Given that enamine formation is slow and reversible but the competing S-alkylation is fast and irreversible, we suspected that ITOs are only the precursors of the effective species in the catalytic reactions shown in Fig. 1b.
We, therefore, set out to trace ITO-derived intermediates in the α-cyanomethylation of aldehydes by BAN. Potential enamine intermediates in the catalytic cycle were independently prepared and their nucleophilicity was determined by using Mayr's benzhydrylium methodology12 and the Mayr–Patz eqn (1), which is a linear free energy relationship.
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2(20 °C) = sN(N + E) k2(20 °C) = sN(N + E) | (1) |
In eqn (1), E represents the electrophilicity of the (reference) electrophile, while the nucleophilicity is characterised by two solvent-dependent parameters N and sN.13Eqn (1) has already been used to successfully quantify the nucleophilicities of prolinol-based and MacMillan organocatalysts14 as well as of enamines derived thereof.15 As a consequence, the reactivity of the enamines studied in this work can straightforwardly be compared with relevant species that occur in many, more commonly used, enamine-activated reactions.13e,15 Hence, a quantitative fundament for the understanding of both prebiotic relevance of ITO catalysts and their scope in organocatalytic reactions is provided in this work.
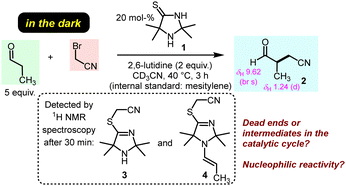
The previously discovered light-driven alkylation of propanal by BAN was re-investigated in the dark under otherwise identical conditions except for the use of 2,6-lutidine as the sterically hindered Brønsted base instead of 2,4,6-collidine (Fig. 2).
The 2,2,5,5-tetramethylated ITO 1 was selected because it had proven superior efficiency in the analogous light-driven α-alkylation compared to ITOs with other alkylation patterns.4 To simulate the temperature under intense irradiation, the reaction mixture was mildly heated at 40 °C. Interestingly, ITO 1 catalysed the α-alkylation of propanal with BAN without the need of excitation by light as evidenced by the occurrence of the characteristic resonances for the formyl and the methyl protons in 3-methyl-4-oxobutanenitrile (2) in the 1H NMR spectrum of the reaction mixture (Fig. 2 and ESI,† Fig. S1). Thus, light is not necessary to promote the α-alkylation of aliphatic aldehydes by BAN with ITO 1 as the organocatalyst.
Time-resolved 1H NMR spectroscopy enabled tracing of the ITO-derived intermediates over the course of the reaction. We observed that resonances of the initial ITO catalyst 1 decayed almost completely within 2 h. Meanwhile, further resonances were detected in the reaction mixture which were assigned to the S-alkylated ITO, that is TIM 3, and the TIM-derived enamine 4 (Fig. 2 and ESI,† Fig. S1).
We next investigated whether the TIM-derived enamine 4 is a dead end of the reaction or whether it is the crucial reactive intermediate that undergoes the C–C bond formation with BAN in ITO-driven α-alkylations of aliphatic aldehydes under the conditions of Fig. 2. We, therefore, prepared samples of the enamine 4 by irradiating a mixture of TIM 3, propanal and 2,6-lutidine in dry acetonitrile with light (420 nm). Additionally, we investigated the enamine 5 derived from 3 and phenylacetaldehyde. Enamine 5 allows one to assess the activating effect of TIM 3 by comparing it with a wide range of enamines derived from the same aldehyde and various types of cyclic secondary amines. Enamines derived from established organocatalysts have already been reported on the Mayr nucleophilicity scale and characterised by N and sN.13e,15
As indicated by the 3J coupling constants of the olefinic protons, the carbon–carbon double bonds in 4 and 5 are both in (E)-configuration. In the crystal structure, the phenylacetaldehyde-derived 5 adopts an s-cis conformation (Fig. 3). Given that both positions 2 and 5 at the heterocycles of 4 and 5 carry two methyl groups, we neglected to discuss the conformational orientation at the N–C(α) bond of the enamines in the subsequent reactivity studies.
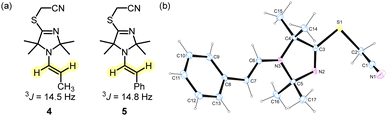 | ||
| Fig. 3 (a) Selected 3J coupling constants in enamines 4 and 5 (400 MHz, CD3CN). (b) X-Ray structure of enamine 5 (CCDC 2254388).† | ||
Under irradiation with UV light (365 nm, in MeCN with 2,6-lutidine as a base), the TIM-derived enamine 4 reacted cleanly with BAN to give the α-alkylated enamine product 6 (structure shown in Fig. 4, details are given in the ESI†).
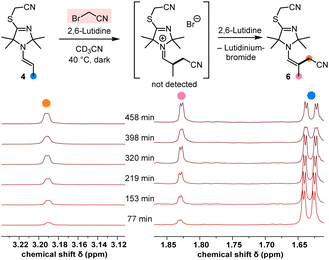 | ||
| Fig. 4 1H NMR spectroscopic monitoring of the α-alkylation of the enamine 4 by BAN in CD3CN at 40 °C (2.7 equiv. of 2,6-lutidine). | ||
Moreover, NMR spectroscopic monitoring demonstrated that BAN also alkylated the enamine 4 in the dark at 40 °C (Fig. 4), which underlined the reactive capacity of the nucleophilic TIM-derived enamine intermediate 4 in a polar catalytic cycle.
Since we started from the preformed enamine 4, the intermediate iminium bromide was not hydrolysed (as observed in the catalytic reaction, Fig. 2) but rather deprotonated by 2,6-lutidine to generate the α-cyanomethylated enamine 6. Owing to the fact that SN2 electrophiles cannot straightforwardly be used as references for integrating nucleophiles in Mayr's comprehensive reactivity scales, we decided to use the benzhydrylium methodology12 to calibrate the nucleophilicities of the TIM-derived enamines 4 and 5.
Benzhydrylium tetrafluoroborates 7 (Fig. 5) are widely used reference electrophiles with solvent-independent and reliably determined electrophilicity parameters E.12,13b,f Reactions of 7 with the enamines 4 and 5 and subsequent aqueous workup furnished the corresponding α-benzhydrylated aldehydes 9 in unoptimised yields of 63 to 74% (ESI†).
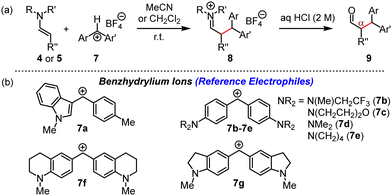 | ||
| Fig. 5 (a) Reactions of 4 or 5 with 7 furnished iminium salts 8, which were hydrolysed to α-alkylated aldehydes 9. (b) Structures of reference electrophiles 7a–7g (counterion: tetrafluoroborate). | ||
The kinetics of the reactions of 4 and 5 with 7a–7g in acetonitrile at 20 °C were monitored photometrically by following the consumption of 7 (stopped-flow method, 434 nm ≤ λmax ≤ 620 nm, 4 or 5 in >10-fold excess). First-order rate constants kobs (s−1) were derived by least squares fitting of the function At = A0 exp(−kobst) + C to the absorbance decays of 7. The second-order rate constants k2 (M−1 s−1) compiled in Table 1 were then obtained as the slopes of the linear correlations of kobs with the concentration of the enamines 4 or 5. Details of the individual kinetic measurements are given in the ESI.† Analogous kinetic studies on the nucleophilicity of 5 in dichloromethane showed that the reactivity of 5 did not differ significantly from that in acetonitrile, in accord with previously reported marginal solvent effects on reactions of enamines with cationic electrophiles.16
| Enamine | Electrophile | Mayr Ea | k 2 (M−1 s−1) | N, sN |
|---|---|---|---|---|
| a Electrophilicity parameters E (as defined in eqn (1) and reported in ref. 13). b In dichloromethane. With further data in ESI, N(sN) = 7.06 (1.11) was calculated for 5 in CH2Cl2. | ||||
| 4 | 7d | –7.02 | 7.06 × 101 | 9.25, 0.84 |
| 7e | –7.69 | 2.14 × 101 | ||
| 7f | –8.22 | 7.74 | ||
| 7g | –8.76 | 2.42 | ||
| 5 | 7a | –2.19 | 6.92 × 104 | 7.74, 0.87 |
| 7b | –3.85 | 1.96 × 103 | ||
| (1.75 × 103)b | ||||
| 7c | –5.53 | 8.84 × 101 | ||
| (5.49 × 101)b | ||||
Fig. S5 (ESI†) illustrates that the second-order rate constants (lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2) for the reactions of 7 with the enamines 4 and 5 correlate linearly with the reported Mayr electrophilicities E of the reference electrophiles 7. By using eqn (1), the electrophilicities E(7), and the experimentally determined second-order rate constants k2, we calculated the nucleophilicity parameters N (and sN) of the enamines 4 and 5, which are listed in Table 1.
k2) for the reactions of 7 with the enamines 4 and 5 correlate linearly with the reported Mayr electrophilicities E of the reference electrophiles 7. By using eqn (1), the electrophilicities E(7), and the experimentally determined second-order rate constants k2, we calculated the nucleophilicity parameters N (and sN) of the enamines 4 and 5, which are listed in Table 1.
The now available Mayr reactivity descriptors N for the enamines 4 and 5 facilitate a comparison in the context of previously studied enamines which are key species in organocatalytic reactions.‡ The TIM-derived enamines 4 and 5 are considerably more reactive than structurally analogous enamines derived from MacMillan catalysts (Fig. 6). However, enamines derived from the diphenylprolinol trimethylsilyl ether (Hayashi–Jørgensen catalyst) exceed 4 and 5 in nucleophilicity by one to three orders of magnitude.13e,15,17a
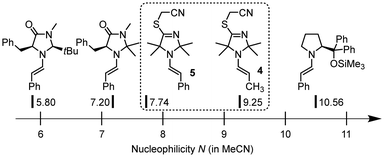 | ||
| Fig. 6 Comparison of nucleophilic reactivities of enamines 4 and 5 derived from TIM 3 with those of structural analogues derived from MacMillan's organocatalysts or the diphenylprolinol trimethylsilyl ether (with N parameters reported in ref. 13e and 15). | ||
In conclusion, we suggest TIMs as a new generation of organocatalytically active cyclic secondary amines, which are generated through S-alkylation of ITOs by BAN. Instead of the previously proposed ITOs,4 TIMs are the active catalysts in the α-alkylation of aldehydes (Fig. 7a). Without activation by light, the catalytic cycle starts with enamine activation of aldehydes by TIM, which generates a sufficiently nucleophilic enamine to undergo C–C bond-forming reactions with electrophiles, such as BAN. Previously studied ITO-catalysed reactions were carried out under conditions that could not benefit from TIM formation or the activating effect of S-alkylation on the TIM-derived enamine reactivity.5 Thus, the S-alkylation step opens the gate to a novel TIM generation of organocatalysts for organic synthesis.
Furthermore, S-cyanomethylation of ITOs to give TIMs may have been a beneficial step in molecular evolution under Early Earth conditions for the following reasons: (1) TIMs can assemble from prebiotically likely small molecules (KCN, H2S, NH3, short-chain carbonyl compounds, and BAN). (2) In the dark TIMs facilitate through enamine activation α-alkylations of aldehydes by electrophiles. (3) Once formed organic molecules should not degrade rapidly under the harsh conditions on the Early Earth. Remarkably, preliminary studies have shown that TIM 3 does not degrade when exposed to UV light (365 nm) but performs well as a catalyst (Fig. 7b and ESI†).9,17
We thank Dr Peter Mayer (Dept. Chemie, LMU München) for the X-ray structure determination and Nathalie Hampel for preparing the reference electrophiles 7. This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 101024710 “SImCat” (MSCA-IF-2020 to M. J. H.). We acknowledge financial support from the VolkswagenStiftung, “Initiating Molecular Life” (O.T.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) W. Notz, F. Tanaka and C. F. Barbas III, Acc. Chem. Res., 2004, 37, 580 CrossRef CAS PubMed; (b) S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef CAS PubMed; (c) D. W. C. MacMillan, Nature, 2008, 455, 304 CrossRef CAS PubMed; (d) P. Melchiorre, M. Marigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2008, 47, 6138 CrossRef CAS PubMed; (e) S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178 RSC; (f) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS PubMed; (g) B. S. Donslund, T. K. Johansen, P. H. Poulsen, K. S. Halskov and K. A. Jørgensen, Angew. Chem., Int. Ed., 2015, 54, 13860 CrossRef CAS PubMed; (h) Y.-Q. Zhou, F. M. Hörmann and T. Bach, Chem. Soc. Rev., 2018, 47, 278 RSC; (i) M. H. Aukland and B. List, Pure Appl. Chem., 2021, 93, 1371 CrossRef CAS.
- (a) D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77 CrossRef CAS PubMed; (b) E. R. Welin, A. A. Warkentin, J. C. Conrad and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2015, 54, 9668 CrossRef CAS PubMed.
- (a) N. F. W. Ligterink and M. Kama, Astron. Astrophys., 2018, 614, A112 CrossRef; (b) A. P. C. Mann and D. A. Williams, Nature, 1980, 283, 721 CrossRef CAS; (c) P. G. Stoks and A. W. Schwartz, Geochim. Cosmochim. Acta, 1982, 46, 309 CrossRef CAS.
- A. C. Closs, E. Fuks, M. Bechtel and O. Trapp, Chem. – Eur. J., 2020, 26, 10702 CrossRef CAS PubMed.
- The use of ITOs as organocatalysts has remained limited: (a) J. Li, X. Liang and W. Su, RSC Adv., 2015, 5, 106234 RSC; (b) X. Liang, J. Fan, F. Shi and W. Su, Tetrahedron Lett., 2010, 51, 2505 CrossRef CAS; (c) X. Liang, S. Li and W. Su, Tetrahedron Lett., 2012, 53, 289 CrossRef CAS; (d) X. Liang, N. Li, X. Chen and W. Su, RSC Adv., 2014, 4, 44039 RSC.
- (a) F. Asinger, W. Schäfer, H. Meisel, H. Kersten and A. Saus, Monatsh. Chem., 1967, 98, 338 CrossRef CAS; (b) M. Paventi and J. T. Edward, Can. J. Chem., 1987, 65, 282 CrossRef CAS.
- (a) A. C. Closs, M. Bechtel and O. Trapp, Angew. Chem., Int. Ed., 2022, 61, e202112563 CrossRef CAS PubMed; (b) O. Trapp, in Prebiotic Chemistry and the Origin of Life, ed. A. Neubeck and S. McMahon, Springer, Cham, 1st edn, 2021, pp. 165–182 Search PubMed.
- For reviews on organo photoredox catalysis, see: (a) A. Gualandi, P. G. Cozzi, G. Rodeghiero, T. P. Jansen and R. Perciaccante, Phys. Sci. Rev., 2019, 20180098 Search PubMed; (b) T. Bortolato, S. Cuadros, G. Simionato and L. Dell’Amico, Chem. Commun., 2022, 58, 1263 RSC.
- For related organocatalytic alkylations of aldehydes by direct photoexcitation of enamine intermediates, which starts a radical chain reaction of enamines with bromoalkanes, see: M. Silvi, E. Arceo, I. D. Jurberg, C. Cassani and P. Melchiorre, J. Am. Chem. Soc., 2015, 137, 6120 CrossRef CAS PubMed.
- (a) G. Nováková, P. Drabina, B. Frumarová and M. Sedlák, Adv. Synth. Catal., 2016, 358, 2541 CrossRef; (b) G. Nováková, P. Drabina, J. Svoboda and M. Sedlák, Tetrahedron Asymm., 2017, 28, 791 CrossRef.
- R. Mukkamala, US Pat., 2002/0198115A1, 2002 Search PubMed.
- H. Mayr, Tetrahedron, 2015, 71, 5095 CrossRef CAS.
- (a) H. Mayr and M. Patz, Angew. Chem., Int. Ed. Engl., 1994, 33, 938 CrossRef; (b) H. Mayr, T. Bug, M. F. Gotta, N. Hering, B. Irrgang, B. Janker, B. Kempf, R. Loos, A. R. Ofial, G. Remennikov and H. Schimmel, J. Am. Chem. Soc., 2001, 123, 9500 CrossRef CAS PubMed; (c) H. Mayr, B. Kempf and A. R. Ofial, Acc. Chem. Res., 2003, 36, 66 CrossRef CAS PubMed; (d) H. Mayr and A. R. Ofial, SAR QSAR Environ. Res., 2015, 26, 619 CrossRef CAS PubMed; (e) Database for N, sN, and E parameters and references to their origin: https://www.cup.lmu.de/oc/mayr/reaktionsdatenbank2/; (f) R. J. Mayer, N. Hampel, P. Mayer, A. R. Ofial and H. Mayr, Eur. J. Org. Chem., 2019, 412 CrossRef CAS.
- F. An, B. Maji, E. Min, A. R. Ofial and H. Mayr, J. Am. Chem. Soc., 2020, 142, 1526 CrossRef CAS PubMed.
- (a) S. Lakhdar, B. Maji and H. Mayr, Angew. Chem., Int. Ed., 2012, 51, 5739 CrossRef CAS PubMed; (b) H. Mayr, S. Lakhdar, B. Maji and A. R. Ofial, Beilstein J. Org. Chem., 2012, 8, 1458 CrossRef CAS PubMed; (c) H. Erdmann, F. An, P. Mayer, A. R. Ofial, S. Lakhdar and H. Mayr, J. Am. Chem. Soc., 2014, 136, 14263 CrossRef CAS PubMed.
- A. I. Leonov, D. S. Timofeeva, A. R. Ofial and H. Mayr, Synthesis, 2019, 1157 CAS.
- (a) Redox properties of enamines: Y. Li, D. Wang, L. Zhang and S. Luo, J. Org. Chem., 2019, 84, 12071 CrossRef CAS PubMed; (b) For photophysical properties of organocatalyst-derived enamines, see: K. B. Bergmann and R. L. Davis, Org. Lett., 2021, 23, 7033 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures and spectroscopic characterisation of products, and details of the kinetic experiments. CCDC 2254388. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc01912h |
| ‡ Given that the sN values of the enamines depicted in Fig. 6 are in a narrow range (0.84 to 1.14), we can directly compare their nucleophilic reactivity by only considering the nucleophilicity parameter N. |
| This journal is © The Royal Society of Chemistry 2023 |