Open Access Article
Open Access ArticleStatically and dynamically flexible hydrogen-bonded frameworks based on 4,5,9,10-tetrakis(4-carboxyphenyl)pyrene†
Taito
Hashimoto
a,
Ryusei
Oketani
 ab,
Asato
Inoue
a,
Kohei
Okubo
c,
Kouki
Oka
ab,
Asato
Inoue
a,
Kohei
Okubo
c,
Kouki
Oka
 cd,
Norimitsu
Tohnai
cd,
Norimitsu
Tohnai
 c,
Kazuhide
Kamiya
c,
Kazuhide
Kamiya
 ab,
Shuji
Nakanishi
ab,
Shuji
Nakanishi
 ab and
Ichiro
Hisaki
ab and
Ichiro
Hisaki
 *ab
*ab
aDivision of Chemistry, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan. E-mail: i.hisaki.es@osaka-u.ac.jp
bResearch Center for Solar Energy Chemistry, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
cDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
dCenter for Future Innovation (CFi), Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
First published on 18th May 2023
Abstract
Aperture shape and size of flexible hydrogen-bonded organic frameworks (HOF) were statically modulated using various aromatic solvents, and dynamically changed by desorption and adsorption of the solvent molecules.
Soft porous frameworks have been of substantial interest as the third generation of framework materials because of unique functionality provided by reversible structural changes of the frameworks.1 Particularly, porous crystalline frameworks formed through non-covalent interactions can intrinsically show structural flexibility due to the reversible intermolecular interactions.2 However, the structural changes often result in collapse of the periodic framework to give non-porous, less-crystalline materials.
In this context, a hydrogen-bonded organic framework (HOF)3 is the suitable platform for flexible frameworks,4 because of moderately directional and relatively strong H-bonding interactions.5 For example, H. Wang and co-workers reported HOF-5 composed of a 2,4-diaminotriazine-substituted tetraphenylethene (TPE) derivative with rhombic lattice contracted by 21% upon guest removal and the resultant activated framework showed encapsulation of CO2 with high density.6 Z. Chi and co-workers reported a nitrobenzene-substituted TPE derivative formed framework (8PN) which shows large-scale void regulation and wide range of fluorescence color changes upon included solvent molecules.7 More recently, some layered HOFs composed tetracarboxylic acid derivatives were reported to undergo drastic crystal-to-crystal structural changes accompanied by rearrangements of H-bonds.8 However, essential structural factors of flexible HOFs have remained unclear, compared to rigid HOFs.9
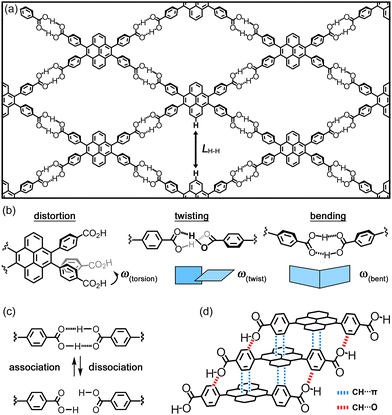
In this communication, we demonstrate that pyrene-based tetracarboxylic acid CP-Py forms statically and dynamically flexible HOFs with a layered structure of a sql-topological network (Fig. 1a). Desorption and adsorption of guest molecules triggers reversible structural transformation between CP-Py-1 and CP-Py-2, the latter of which further transformed irreversibly into CP-Py-3. Importantly, the structures of these three forms were characterized by single crystalline X-ray diffraction (SXRD) analysis, allowing us to identify the origin of structural flexibility of the present HOFs, that is, distortion of the peripheral carboxyphenyl arms and deformation (i.e. twisting and bending) of the H-bonded dimer (Fig. 1b), in addition to reversible H-bond formation and dissociation (Fig. 1c). Furthermore, the crystal-to-crystal transformation was crucially supported by robust 1D columnar assembly (Fig. 1d), which allows the regulated structural changes instead of random structural changes. These results provide fundamental insight to develop a new dynamic HOF.
CP-Py was synthesized according to procedures previously reported.10,11CP-Py was dissolved in a mixed solution of DMF and 1,2-dichlorobenzene (DCB) and left the solution at 120 °C to evaporate solvent slowly, giving crystals of solvated HOF CP-Py-1(DCB) suitable for SXRD analysis.
CP-Py-1(DCB) has a slip-stacked, layered structure composed of hydrogen-bonded sql-networked sheets as reported.11CP-Py-1(DCB) included DCB molecules with a host–guest ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
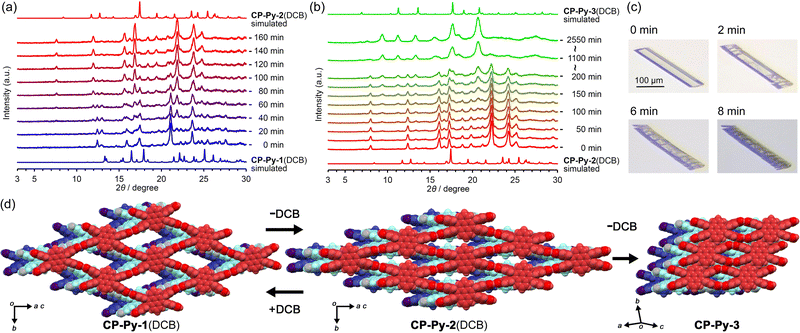
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Time-dependent powder X-ray diffraction (PXRD) measurements showed that diffraction peaks ascribable to CP-Py-1(DCB), such as those at 2θ of 12.4°, 12.9°, 17.4° and 21.1°, gradually disappeared, while new peaks at 7.6°, 12.0°, 16.9° and 21.9° appeared (Fig. 2a). This indicates that CP-Py-1(DCB) gradually transforms into the second crystalline form, i.e.CP-Py-2(DCB), upon spontaneous release of the solvent molecules under ambient conditions. It is noteworthy that addition of a drop of DCB on a bulk of CP-Py-2(DCB) immediately recovered the initial form CP-Py-1(DCB) (Fig. S1, ESI†), allowing reversible transformation between these two phases. When CP-Py-2(DCB) was further left under ambient or heated conditions, the diffraction peaks ascribable to CP-Py-2(DCB) decayed and new peaks appeared at 9.4°, 11.2°, 13.3°, 15.5°, and 20.6°, indicating further transformation into the third form CP-Py-3 (Fig. 2b). The thermal gravimetric curve indicates that weight loss started soon after heating and reached 45% till complete removal of the solvent at 116 °C, supporting the observed transformations accompanied by release of DCB molecules (Fig. S2, ESI†).
4. Time-dependent powder X-ray diffraction (PXRD) measurements showed that diffraction peaks ascribable to CP-Py-1(DCB), such as those at 2θ of 12.4°, 12.9°, 17.4° and 21.1°, gradually disappeared, while new peaks at 7.6°, 12.0°, 16.9° and 21.9° appeared (Fig. 2a). This indicates that CP-Py-1(DCB) gradually transforms into the second crystalline form, i.e.CP-Py-2(DCB), upon spontaneous release of the solvent molecules under ambient conditions. It is noteworthy that addition of a drop of DCB on a bulk of CP-Py-2(DCB) immediately recovered the initial form CP-Py-1(DCB) (Fig. S1, ESI†), allowing reversible transformation between these two phases. When CP-Py-2(DCB) was further left under ambient or heated conditions, the diffraction peaks ascribable to CP-Py-2(DCB) decayed and new peaks appeared at 9.4°, 11.2°, 13.3°, 15.5°, and 20.6°, indicating further transformation into the third form CP-Py-3 (Fig. 2b). The thermal gravimetric curve indicates that weight loss started soon after heating and reached 45% till complete removal of the solvent at 116 °C, supporting the observed transformations accompanied by release of DCB molecules (Fig. S2, ESI†).
It was also observed that a single crystal of CP-Py-1(DCB) started to get cracks after wiped off bulk solvent from surface of the crystal (Fig. 2c). The cracks are nearly parallel to the (1 0 − 4) plane which corresponds to the H-bonded 2D layers (Fig. S3, ESI†). This morphological change was brought from structural transformation triggered by the solvent release. Fortunately, the resultant platelet pieces remained single-crystallinity, allowing us to reveal crystal structures of CP-Py-2(DCB) and CP-Py-3 by SXRD analysis (Fig. 2d).
CP-Py-2(DCB) retains the space group of P21/c and the H-bonded network with sql-topology of the original framework, while the periodicity of framework was shrunk by 4.2 Å along the shorter diagonal direction parallel to the b axis and elongated by 1.7 Å along the longer diagonal direction of the rhombic aperture, resulting decrease of inclusion spaces from 51% to 35%. CP-Py-2(DCB) included DCB molecules with a host–guest ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. CP-Py-3 has a completely different molecular arrangement with the other two. H-bonded dimer of the carboxy groups expressing the graph set notation of R22(8) in the original rhombic framework were cleaved and re-organized by forming a distorted dimer with R22(8), resulting the generation of small cavity expressing the graph set notation of R22(30).12 The framework of CP-Py-3 has a narrow bottle-necked channel with aperture dimension of 8.6 Å × 3.4 Å (Fig. S4, ESI†). The void ratio was calculated to be 12% by PLATON.13CP-Py-3 was activated at 120 °C under vacuum condition and subjected to gas sorption experiments. CP-Py-3 showed almost no N2 adsorption, while adsorbed CO2 with the type-I sorption isotherm (Fig. S5, ESI†), indicating existence of micro pores. Brunauer–Emmett–Teller (BET) surface area was calculated based on the CO2 adsorption isotherm to be 172 m2 g−1 (Fig. S6, ESI†). It is not negligible that CO2 adsorption experiments reproducibly showed anomalous rapid uptake at the relative pressure larger than 0.4, indicating that sudden condensation of CO2 occurred irregularly on surface of the crystals (Fig. S7, ESI†). Since CP-Py-3 was formed through H-bond cleavage and rearrangements, single crystallinity was low and crystal data was not enough for detailed structural discussion.
2. CP-Py-3 has a completely different molecular arrangement with the other two. H-bonded dimer of the carboxy groups expressing the graph set notation of R22(8) in the original rhombic framework were cleaved and re-organized by forming a distorted dimer with R22(8), resulting the generation of small cavity expressing the graph set notation of R22(30).12 The framework of CP-Py-3 has a narrow bottle-necked channel with aperture dimension of 8.6 Å × 3.4 Å (Fig. S4, ESI†). The void ratio was calculated to be 12% by PLATON.13CP-Py-3 was activated at 120 °C under vacuum condition and subjected to gas sorption experiments. CP-Py-3 showed almost no N2 adsorption, while adsorbed CO2 with the type-I sorption isotherm (Fig. S5, ESI†), indicating existence of micro pores. Brunauer–Emmett–Teller (BET) surface area was calculated based on the CO2 adsorption isotherm to be 172 m2 g−1 (Fig. S6, ESI†). It is not negligible that CO2 adsorption experiments reproducibly showed anomalous rapid uptake at the relative pressure larger than 0.4, indicating that sudden condensation of CO2 occurred irregularly on surface of the crystals (Fig. S7, ESI†). Since CP-Py-3 was formed through H-bond cleavage and rearrangements, single crystallinity was low and crystal data was not enough for detailed structural discussion.
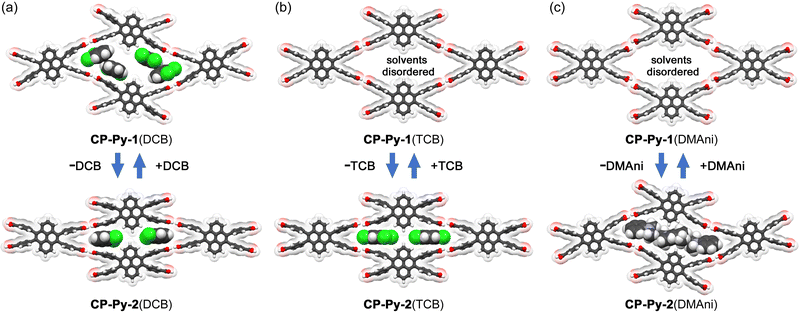
CP-Py was, furthermore, crystallized using other aromatic solvents, such as 1,2,4-trichlorobenzene (TCB) and N,N-dimethylaniline (DMAni) to yield the corresponding solvated HOFs CP-Py-1(TCB) and CP-Py-1(DMAni) with host/guest ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Fig. S8–S11, ESI†). They also transform into the second forms CP-Py-2(TCB) and CP-Py-2(DMAni) upon the solvent removal (Fig. S12 and S13, ESI†), and the transformation was revealed to be reversible (Fig. S14 and S15, ESI†).
4 (Fig. S8–S11, ESI†). They also transform into the second forms CP-Py-2(TCB) and CP-Py-2(DMAni) upon the solvent removal (Fig. S12 and S13, ESI†), and the transformation was revealed to be reversible (Fig. S14 and S15, ESI†).
To shed a light of flexibility of the framework, the size and shape of the rhombic aperture in the obtained HOFs were compared. The structures and structural parameters are shown in Fig. 3 and Table 1, respectively. Upon transformation from CP-Py-1(DCB) to CP-Py-2(DCB), a distorted rhombic aperture with LH–H distance of 7.10 Å and ω(torsion) of 2.6–5.8° changed to a much narrower one with LH–H of 2.65 Å and ω(torsion) of 10.1–13.1°, where the molecule of CP-Py is forced to be deformed (Fig. 3a). CP-Py-1(TCB) and CP-Py-1(DMAni), on the other hand, have stretched similar rhombic apertures with LH–H values of 6.93 Å and 8.32 Å, respectively, due to inclusion of larger solvent molecules (Fig. S16, ESI†). The rhombic apertures transformed into narrow and distorted ones with LH–H of 2.89 Å and 5.95 Å in CP-Py-2(TCB) and CP-Py-2(DMAni), respectively, upon the solvent release. The aperture shape and shrinking manners depend on the solvent molecules included in the framework, clearly showing static and dynamic flexibility of the present framework. The flexibility was realized by distortion of the peripheral carboxyphenyl groups. The distortion angle ω(torsion) of CP-Py-1 ranges from 2.6° to 5.8° in CP-Py-1(DCB) and is 4.3° in CP-Py- 1(DMAni). Upon guest release and shrinkage of the framework, the ω(torsion) values increased up to 13.1° in CP-Py-2(DCB). The ω(twist) and ω(bent), which characterize distortion in H-bonded dimer, also exhibit wide range of values depending on the aperture shape (Fig. S18, ESI†). For example, CP-Py-1(TCB) and CP-Py-1(DMAni) with stretched rhombic apertures exhibit small values of ω(twist) and ω(bent), while CP-Py-1(DCB) and CP-Py-2(DMAni) with distorted apertures show larger values (Table 1). It is noteworthy that addition of a drop of 1-methylnaphthalene (MeNaph) on the single crystal of CP-Py-2(DCB) resulted in exchange of the guest molecules from DCB to MeNaph and expansion of the framework from CP-Py-2 to CP-Py-1 (Fig. 4). This also clearly indicates the flexibility of the framework.
| Form | L H–H/Å | ω (torsion) /° | ω (twist) /° | ω (bent) /° |
|---|---|---|---|---|
| a Definition of the parameters are shown in Fig. S17 in ESI. | ||||
| CP-Py-1(DCB) | 7.10 | 5.8–2.6 | 5.1 | 13.7 |
| CP-Py-2(DCB) | 2.65 | 13.1–10.1 | 11.3 | 3.2 |
| CP-Py1(TCB) | 6.93 | 4.5–3.3 | 3.4 | 3.9 |
| CP-Py2(TCB) | 2.89 | 9.6 | 0 | 0 |
| CP-Py-1(DMAni) | 8.32 | 4.3 | 0 | 0 |
| CP-Py-2(DMAni) | 5.95 | 9.1–2.9 | 3.3 | 16.4 |
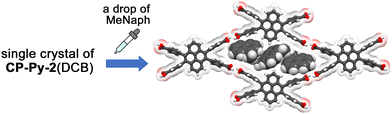 | ||
| Fig. 4 Crystal-to-crystal transformation from CP-Py-2(DCB) to CP-Py-1(MeNph) by adding a drop of MeNaph on a single crystal of CP-Py-2(DCB). | ||
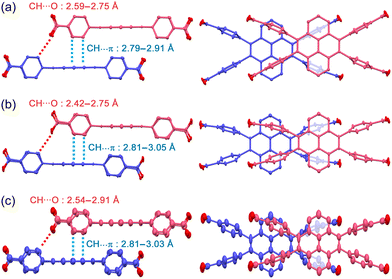
Not only the flexibility of the H-bonded network structure, but robustness of the 1D stacked columnar structure also plays a role on the crystal-to-crystal structural transformation. The robust 1D columnar structure enables the framework to undergo regulated structural changes instead of random structural changes. The 1D columnar structure is formed by complementary contacts of the accumulated molecules including edge-to-face contact (i.e. CH⋯π interactions) of the peripheral phenylene groups and the central pyrene ring and the CH⋯O contacts of the aromatic hydrogen and the carboxy oxygen atom (Fig. 1d and 5). This contact manner is observed in all forms, and plays role to keep crystallinity, although subtle differences in rotational and conformation of the phenylene rings were observed.
In this communication, we described static and dynamic flexibility of a HOF composed of tetratopic carboxylic acid CP-Py. Void spaces of the framework can be statically changed upon included guest molecules. Furthermore, guest desorption and adsorption makes the framework reversibly transform between two forms CP-Py-1 and CP-Py-2, the latter of which further changed into the third form CP-Py-3 possessing permanent porosity upon complete removal of solvent molecules. All forms were successfully characterized by SXRD analysis. The present system demonstrated that both deformation-tolerant parts and structurally robust stacking part are necessary for flexible HOFs that experiences crystal-to-crystal transformation.
This work was supported by KAKENHI (JP21H01919, JP21K18961, JP22H05461, JP23H04029) from MEXT and JSPS, Japan. I. H. thanks Hoansha Foundation and Iketani Science and Technology Foundation. I. H. also thanks MRL, Graduate School of Engineering Science, Osaka University. X-ray diffraction data of CP-Py-1(DMAni), CP-Py-2(DMAni) and CP-Py-3 were collected at BL40XU in SPring-8 with approval of JASRI (proposal No. 2022B1151 and 2023A1264).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. Kitagawa and K. Uemura, Chem. Soc. Rev., 2005, 34, 109 RSC; (b) S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695 CrossRef CAS; (c) S. Seth and S. Jhulki, Mater. Horiz., 2021, 8, 700 RSC.
- (a) L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000 CrossRef; (b) J. T. A. Jones, D. Holden, T. Mitra, T. Hasell, D. J. Adams, K. E. Jelfs, A. Trewin, D. J. Willock, G. M. Day, J. Bacsa, A. Steiner and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 749 CrossRef CAS PubMed; (c) H. Yamagishi, H. Sato, A. Hori, Y. Sato, R. Matsuda, K. Kato and T. Aida, Science, 2018, 361, 1242 CrossRef CAS PubMed; (d) F. Castiglioni, W. Danowski, J. Perego, F. K.-C. Leung, P. Sozzani, S. Bracco, S. J. Wezenberg, A. Comotti and B. L. Feringa, Nat. Chem., 2020, 12, 595 CrossRef CAS PubMed; (e) Y. Yakiyama, T. Fujinaka, M. Nishimura, R. Seki and H. Sakurai, Chem. Commun., 2020, 56, 9687 RSC.
- (a) R. B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362 RSC; (b) I. Hisaki, C. Xin, K. Takahashi and T. Nakamura, Angew. Chem., Int. Ed., 2019, 58, 11160 CrossRef CAS PubMed; (c) B. Wang, R.-B. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2020, 142, 14399 CrossRef CAS PubMed; (d) X. Song, Y. Wang, C. Wang, D. Wang, G. Zhuang, K. O. Kirlikovali, P. Li and O. K. Farha, J. Am. Chem. Soc., 2022, 144, 10663 CrossRef CAS PubMed; (e) P. Li, M. R. Ryder and J. F. Stoddart, Acc. Mater. Res., 2020, 1, 77–87 CrossRef CAS.
- (a) I. Hisaki, S. Nakagawa, Y. Suzuki and N. Tohnai, Chem. Lett., 2018, 47, 1143–1146 CrossRef CAS; (b) L. Chen, Z. Yuan, H. Zhang, Y. Ye, Y. Yang, F. Xiang, K. Cai, S. Xiang, B. Chen and Z. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202213959 CAS; (c) W. Yang, W. Zhou and B. Chen, Cryst. Growth Des., 2019, 19, 5184 CrossRef CAS; (d) Y. Suzuki, M. Yamaguchi, R. Oketani and I. Hisaki, Mater. Chem. Front., 2023, 7, 106 RSC.
- G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS.
- H. Wang, B. Li, H. Wu, T.-L. Hu, Z. Yao, W. Zhou, S. Xiang and B. Chen, J. Am. Chem. Soc., 2015, 137, 9963 CrossRef CAS.
- Q. Huang, W. Li, Z. Mao, L. Qu, Y. Li, H. Zhang, T. Yu, Z. Yang, J. Zhao, Y. Zhang, M. P. Aldred and Z. Chi, Nat. Commun., 2019, 10, 3074 CrossRef.
- (a) X.-Y. Gao, Y.-L. Li, T.-F. Liu, X.-S. Huang and R. Cao, CrystEngComm, 2021, 23, 4743 RSC; (b) Q. Ji, K. Takahashi, S. Noro, Y. Ishigaki, K. Kokado, T. Nakamura and I. Hisaki, Cryst. Growth Des., 2021, 21, 4656 CrossRef CAS; (c) H. Kubo, R. Oketani and I. Hisaki, Chem. Commun., 2021, 57, 8568 RSC.
- (a) F. Hu, C. Liu, M. Wu, J. Pang, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2017, 56, 2101 CrossRef CAS PubMed; (b) I. Hisaki, N. Ikenaka, E. Gomez, B. Cohen, N. Tohnai and A. Douhal, Chem. – Eur. J., 2017, 23, 11611 CrossRef CAS; (c) Q. Yin, P. Zhao, R.-J. Sa, G.-C. Chen, J. Lg, T.-F. Liu and R. Cao, Angew. Chem., Int. Ed., 2018, 57, 7691 CrossRef CAS; (d) I. Hisaki, Y. Suzuki, E. Gomez, B. Cohen, N. Tohnai and A. Douhal, Angew. Chem., Int. Ed., 2018, 57, 12650 CrossRef CAS.
- Z. Lu, R. Wang, Y. Liao, O. K. Farha, W. Bi, T. R. Sheridan, K. Zhang, J. Duan, J. Liu and J. T. Hupp, Chem. Commun., 2021, 57, 3571 RSC.
- T. Hashimoto, R. Oketani, M. Nobuoka, S. Seki and I. Hisaki, Angew. Chem., Int. Ed., 2023, 62, e202215836 CAS.
- J. Bernstein, R. E. Davis, L. Shimoni and N.-L. Chang, Angew. Chem., Int. Ed. Engl., 1995, 34, 1555 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detail of experiments, TG data, PXRD data, crystal structures CCDC 2255738–2255743 and 2262072. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc01877f |
| This journal is © The Royal Society of Chemistry 2023 |