Open Access Article
Open Access ArticleWell-defined surface catalytic sites for solar CO2 reduction: heterogenized molecular catalysts and single atom catalysts
Peipei
Huang
,
Ehab
Shaaban
,
Esraa
Ahmad
,
Allison
St. John
,
Tianqi
Jin
and
Gonghu
Li
 *
*
Department of Chemistry, University of New Hampshire, Durham NH 03824, USA. E-mail: gonghu.li@unh.edu
First published on 4th July 2023
Abstract
Exciting progress has been made in the area of solar fuel generation by CO2 reduction. New photocatalytic materials containing well-defined surface catalytic sites have emerged in recent years, including heterogenized molecular catalysts and single atom catalysts. This Feature Article summarizes our recent research in this area, together with brief discussions of relevant literature. In our effort to obtain heterogenized molecular catalysts, a diimine-tricarbonyl Re(I) complex and a tetraaza macrocyclic Co(III) compound were covalently attached to different surfaces, and the effects of ligand derivatization and surface characteristics on their structures and photocatalytic activities were investigated. Single atom catalysts combine the advantages of homogeneous and heterogeneous catalysis. A single-site cobalt catalyst was prepared on graphitic carbon nitride, which demonstrated excellent activity in selective CO2 reduction under visible-light irradiation. Doping carbon nitride with carbon was found to have profound effects on the structure and activity of the single-site cobalt catalyst. Our research achievements are presented to emphasize how spectroscopic techniques, including infrared, UV-visible, electron paramagnetic resonance, and X-ray absorption spectroscopies, could be combined with catalyst synthesis and computation modeling to understand the structures and properties of well-defined surface catalytic sites at the molecular level. This article also highlights challenges and opportunities in the broad context of solar CO2 reduction.
1. Introduction
Carbon dioxide (CO2) is considered a renewable C1 feedstock for the production of valuable chemicals and fuels.1,2 Sufficient energy and catalysts are needed to activate CO2, which is a very stable molecule. Solar-driven CO2 reduction has been extensively investigated in recent years as it employs natural sunlight as a renewable energy source. Various photocatalysts capable of activating CO2 under light irradiation have been reported in the literature, including molecular complexes, inorganic semiconductors, and other systems.3–7In molecular systems, the photocatalyst could be a transition metal complex that can harvest light and activate CO2, or a molecular CO2-reduction catalyst combined with a photosensitizer.8–11 An example of the former is Re(bpy)(CO)3Cl (denoted “Re-bpy”), where bpy is 2,2′-bipyridine. This diimine-tricarbonyl Re(I) compound and its supramolecular complexes have demonstrated excellent reactivity and selectivity towards CO formation in photocatalytic CO2 reduction.12,13 Examples of the latter include [Co(cyclam)Cl2]Cl (denoted “Co-cyclam”), where cyclam is 1,4,8,11-tetraazacy-clotetradecane. This macrocyclic complex requires the use of photosensitizers, such as p-terphenyl, to harvest light and generate electrons needed for CO2 reduction.14,15 While these molecular photocatalysts are highly active and selective towards CO2 reduction, many suffer from poor stability under photochemical conditions. In addition, most molecular complexes require the use of non-renewable electron donors to maintain reactivity in CO2-reduction photocatalysis.
In comparison, inorganic semiconductors,16–21 most notably titanium dioxide (TiO2)-based materials, are robust heterogeneous photocatalysts for CO2 reduction since the early work by Inoue and co-workers.22 One attractive feature of such photocatalysts is that some semiconductors (e.g. TiO2) with appropriate band positions allow the use of water as a renewable electron donor for CO2 reduction. However, most semiconductor photocatalysts are inefficient in converting photonic energy into chemical energy due to recombination of photogenerated charge carriers (electrons and holes) that prevails in the bulk.23,24 Furthermore, such heterogeneous photocatalysts often lack well-defined surface sites for selective CO2 reduction.
Different strategies have been designed to prepare robust and efficient photocatalysts containing well-defined surface catalytic sites (Fig. 1). For instance, heterogenized molecular catalysts can be obtained by attaching molecular catalysts onto light-absorbing surfaces.25–31 Such photocatalysts generally show enhanced activity and improved photostability. Atomically dispersed catalysts or single-atom catalysts (SACs) are known to have well-defined active sites on a solid support.32–39 SACs have demonstrated distinct properties in a wide range of chemical reactions, including photocatalysis.40–43 Recently, there has been enormous interest in developing SACs for use in solar CO2 reduction.44–48
This Feature article summarizes our recent effort to construct well-defined catalytic sites on surface for use in solar CO2 reduction. In particular, Re-bpy and Co-cyclam were attached to different surfaces to obtain heterogenized molecular catalysts. These two complexes were chosen in our studies due to their well-known activity in CO2-reduction catalysis and their relatively simple molecular structures that allow facile ligand derivatization for surface attachment. Our research in this direction has been focused on the effects of ligand derivatization and surface characteristics on the photocatalytic activities of the heterogenized molecular catalysts. As will be discussed, heterogenization of the molecular catalysts on surfaces allowed us to investigate their structures and complexation using spectroscopic techniques, which could be less informative and more challenging in studying the molecular catalysts in homogeneous solutions. Inspired by the CO2-reduction activity of Co-cyclam and prior work by others in the area of SACs, we discovered a Co SAC on graphitic carbon nitride (C3N4) capable of catalyzing selective CO2 reduction under visible-light irradiation. The Co SAC structure was interrogated by using a combination of synthesis, spectroscopy, and modeling studies. Relevant research results by others are also discussed to highlight the progress and challenges in the broad context of solar CO2 reduction using innovative photocatalysts.
2. Solar CO2 reduction using Re-bpy covalently attached to SiO2
As a model molecular catalyst, Re-bpy has been covalently attached to semiconductor surfaces,49–57 including photoelectrodes,58,59 to achieve selective CO2 reduction under solar irradiation. Despite the fact that Re-bpy itself is capable of catalyzing CO2 reduction under light irradiation, the semiconductor support could further improve the photocatalytic performance of Re-bpy. For example, Windle and co-workers52 prepared a diimine-tricarbonyl Re(I) complex derivatized with phosphonate groups for immobilization on a TiO2 surface. The heterogenized Re(I) catalyst demonstrated significantly higher activity than Re-bpy in solar CO2 reduction under the same condition. Results obtained using transient absorption spectroscopy revealed that the enhancement in activity upon heterogenization was due to an increase in the lifetime of a reduced Re intermediate on the TiO2 surface. A later study by Abdellah and co-workers53 revealed the role of TiO2 as an electron reservoir, slowing down charge recombination and improving photocatalysis.We employ SiO2 as a model support for Re-bpy to carry out spectroscopic investigations since (i) SiO2 is almost “transparent” in studies using many spectroscopic techniques, and (ii) Re-bpy itself is capable of harvesting light and mediating CO2 reduction. Our research in this area has been focused on the effects of ligand derivatization and surface water on the complexation and activity of heterogenized Re-bpy.
2.1. Covalent attachment of Re-bpy onto SiO2via amide linkages
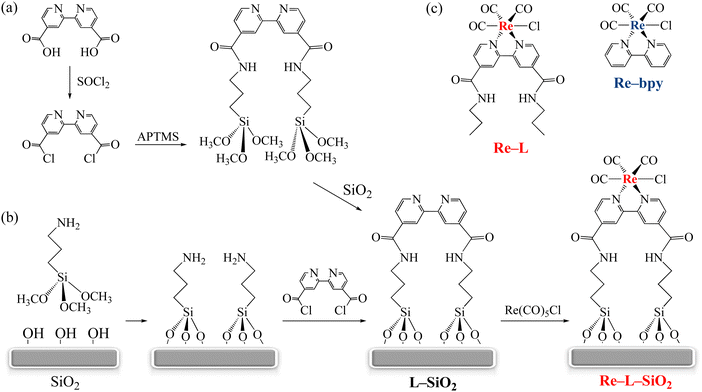
In our initial study, Re-bpy was physically adsorbed on a hierarchical mesoporous zeolite.60 Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was employed to examine the adsorption and complexation of Re-bpy upon light irradiation. In the presence of CO2 and an electron donor, the formation of important reaction intermediates, including Re-carboxylato and Re-formato species, was observed upon light irradiation.60 This initial work allowed us to establish experimental protocols for investigating molecular catalysts on surfaces in the absence of organic solvents.Covalent attachment of Re-bpy on SiO2 was achieved via two approaches (Scheme 1), both of which involved the derivatization of bpy with chlorocarbonyl groups.61,62 In the first approach (Scheme 1a), a bpy-functionalized SiO2 (denoted “L-SiO2”) was obtained in the reaction between a SiO2 surface and a bpy-containing silane coupling agent. In the second approach (Scheme 1b), the SiO2 surface was functionalized with an amino silane coupling agent prior to reacting with the derivatized bpy ligand. The reaction between L-SiO2 and Re(CO)5Cl led to the covalent attachment of Re-bpy on SiO2via amide linkages.
The heterogenized Re-bpy, denoted “Re-L-SiO2”, was characterized with DRIFTS to confirm the successful surface immobilization. Fig. 2 shows the DRIFTS spectra of SiO2, Re-bpy physically adsorbed on SiO2, L-SiO2, and Re-L-SiO2. An intense absorption at 3745 cm−1 featuring surface silanol (Si–OH) groups is present in the spectra of SiO2 and Re-bpy physically adsorbed on SiO2 (Fig. 2a and b). This peak is absent in the spectrum of L–SiO2 shown in Fig. 2c since the silanol groups were consumed upon functionalizing the SiO2 surface with the silane coupling agent. Further supporting this is the presence of infrared absorptions associated with the amide linkages at 1376 cm−1 (C–N stretch), 1548 cm−1 (N–H bend), and 1657 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch) as well as the methylene C–H stretching bands around 2800–3000 cm−1 (not labeled) shown in Fig. 2c. Compared to the spectrum of L-SiO2, the spectrum of Re-L-SiO2 contains two additional broad absorptions at 1903 cm−1 and 2026 cm−1 characteristic of surface Re(I)–carbonyl groups (Fig. 2d), indicating Re-bpy was successfully grafted on SiO2 through amide linkages.61
O stretch) as well as the methylene C–H stretching bands around 2800–3000 cm−1 (not labeled) shown in Fig. 2c. Compared to the spectrum of L-SiO2, the spectrum of Re-L-SiO2 contains two additional broad absorptions at 1903 cm−1 and 2026 cm−1 characteristic of surface Re(I)–carbonyl groups (Fig. 2d), indicating Re-bpy was successfully grafted on SiO2 through amide linkages.61
The characteristic carbonyl infrared bands have been utilized to probe the configurations of Re(I) complexes on surfaces. Anfuso and co-workers50 shown that a Re(I) complex bearing –COOH groups was bound to TiO2 through the carboxylate groups in bidentate or tridentate linkage motifs. The surface Re(I) complex had the bpy moiety nearly perpendicular to the TiO2 surface, exposing the Re(I) complex to the solution in a configuration suitable for catalysis.
2.2. Effects of derivatizing the bpy ligand with amide groups
Derivatization of the bpy ligand with amide groups allowed the successful surface immobilization of Re-bpy on SiO2. However, such ligand derivatization resulted in significantly changes in the photophysical and photochemical properties of Re-bpy. For instance, the ultraviolet-visible (UV-vis) spectrum of Re-bpy physically adsorbed on SiO2 (Fig. 3a, solid trace) features an intense absorption at 365 nm, which is associated with the metal-to-ligand charge transfer (MLCT) transition of Re-bpy on the surface.63 In comparison, the spectrum of the heterogenized Re(I) catalyst, Re-L-SiO2, has an MLCT band at 400 nm (Fig. 3b, solid trace).In order to explore the origin of this observed red shift, a homogeneous compound, Re-L (see Scheme 1c for its structure), was synthesized by derivatizing Re-bpy with amide groups. The MLCT transition of the homogeneous Re-L occurs around 395 nm in contrast with 371 nm for Re-bpy (Fig. 3a and b, dotted traces). Therefore, the red shift observed in Fig. 3(a and b) originated from the ligand derivatization. It is noteworthy that the MLCT bands of surface Re(I) complexes (Fig. 3a and b, solid traces) are much broader than those of the corresponding homogeneous complexes (Fig. 3a and b, dotted traces). This peak broadening is a strong indication of heterogeneity of the surface Re(I) complexes.
We further revealed that the ligand derivatization significantly altered the electrochemical and photocatalytic properties of Re-bpy.62 The presence of electron–withdrawing amide groups in Re-L shifted the redox potentials of Re-bpy to more positive values. Consequently, the activity of Re-L was found to be much lower than Re-bpy in photocatalytic CO2 reduction under simulated solar irradiation (Fig. 3c). Under the same experimental conditions, Re-L-SiO2 displayed slightly higher activity than Re-L. Similar effects of ligand derivatization were observed in our effort to covalently attach Co-cyclam onto SiO2, as will be described later in Section 3.1.
Effects of derivatizing Re-bpy with amide groups have been investigated in CO2-reduction electrocatalysis. Recently, Jia and co-workers64 prepared a series of heterogenized Re(I) catalysts on Si electrode surfaces. It was shown that covalent linkages containing the amide groups were not stable under highly reducing conditions, while alkyl linkages were more stable.
2.3. Effects of derivatizing the bpy ligand at different positions
Another effect of ligand derivatization was also discovered through studying heterogenized Re-bpy.65 In this case, the bpy ligand was derivatized at different positions prior to being coupled with a SiO2 surface through a dipodal silane coupling agent and being coordinated with Re(CO)5Cl. In particular, 2,2′-bipyridine-4,4′-dicarboxylic acid and 2,2′-bipyridine-5,5′-dicarboxylic acid were used to prepare surface-immobilized Re(I) catalysts following the synthesis described in Scheme 1b, resulting in the formation of Re-4-SiO2 and Re-5-SiO2, respectively (see structures in Fig. 4a). Both Re-4-SiO2 and Re-5-SiO2 exhibited a strong MLCT absorption band around 400 nm.65During photocatalysis testing in the presence of triethanolamine (TEOA) as an electron donor, it was observed that the reaction suspension of Re-5-SiO2 changed color from yellow to green. At room temperature, the green color gradually decayed over a few hours after the light was turned off, and re-appeared when light irradiation resumed.65 In addition, the green color immediately changed back to yellow as soon as the reaction suspension was exposed to air under continuous light irradiation. Interestingly, such color change was not observed for Re-4-SiO2. Spectroscopic studies indicated that this green color was associated with an absorption around 620 nm (Fig. 4b). We further synthesized corresponding homogeneous complexes by coordinating 2,2′-bipyridine-4,4′-dicarboxylic acid and 2,2′-bipyridine-5,5′-dicarboxylic acid with Re(CO)5Cl. A similar color change was observed for the Re(I) complex containing 2,2′-bipyridine-5,5′-dicarboxylic acid (Fig. 4c) upon light irradiation. Under the experimental conditions, the Re-bpy complex will be reduced, forming a one-electron reduced species. The green color observed for Re-5-SiO2 is likely due to the electron density in the reduced Re-bpy being more localized on the 5,5′-derivatized bpy ligand, whereas in reduced Re-4-SiO2 the electron density is more localized on the metal center.
This explanation was further supported by results obtained using electron paramagnetic resonance (EPR) spectroscopy.65 In EPR studies, the heterogenized Re(I) catalysts in powder form was mixed with TEOA and purged with CO2 prior to collecting EPR spectra. Fig. 4d shows EPR spectra of Re-4-SiO2 and Re-5-SiO2 collected at room temperature and under continuous light irradiation. The spectrum of Re-4-SiO2 shows some resonance features originated from hyperfine interactions between unpaired electrons with Re(I) nuclei (Fig. 4d, red trace). Less significant hyperfine coupling features are seen in the spectrum of Re-5-SiO2 (Fig. 4d, green trace). Therefore, the electron density in reduced Re-5-SiO2 is more localized on the bpy ligand while in reduced Re-4-SiO2 the electron density is more localized on the metal center.
Effects of ligand derivatization at different positions were observed in a study by Argazzi and co-workers,66 in which Ru(II) polypyridyl complexes were derivatized with –COOH groups for use in dye-sensitized solar cells. It was shown that moving the carboxylate moieties from the 4,4′-positions to the 5,5′-positions lowered the π* energy of the bpy ligand. Subsequently, the Ru(II) photosensitizer containing 2,2′-bipyridine-5,5′-dicarboxylic acid was less efficient in converting photons into electrons because of more significant nonradiative decay of the excited states of this Ru(II) sensitizer. In a study by Guyot and co-workers,67 carboxylic ester groups at the 5,5′-positions of the bpy ligand was found to have a drastic effect on the catalytic activity of Re-bpy toward electrochemical CO2 reduction since the reducing equivalents are mainly accumulated on the electron-withdrawing ester, preventing the formation of Re(0) species and its interaction with CO2. In comparison, alkyl-phosphonic ester substituents on the bpy ligand preserved the catalytic activity of Re-bpy. In our study, derivatizing the bpy ligand at different positions led to difference in charge density distribution within the reduced Re(I) complexes. This difference contributed to the higher activity of Re-4-SiO2 relative to Re-5-SiO2 in photocatalytic CO2 reduction.65
2.4. Effects of surface water
Some surface features could affect the structure, complexation, and behavior of heterogenized molecular catalysts. For example, Lian, Batista, and co-workers68 demonstrated that the binding structure of Re-bpy catalysts can be significantly influenced not only by the nature and length of the covalent linkages, but also by the crystallographic facet of the surface. For our heterogenized molecular catalysts, the presence of surface water was found to have profound effects on the complexation of Re-bpy, while the presence of surface –OH groups dedicated the structure and activity of surface Co-cyclam (see Sections 3.1 and 3.2).The infrared spectrum of Re-bpy in the molecular state contains three carbonyl bands in the 1900–2100 cm−1 range, corresponding to one high-energy, fully symmetric mode and two nearly degenerate lower-energy modes.56,69 The two lower-energy modes often coalesce to one broad feature, as seen in the DRIFTS spectrum of Re-L-SiO2 (Fig. 2d). These two modes can be resolved by exposing the heterogenized Re-bpy to TEOA and CO2.62 For example, three peaks at 2020, 1918, and 1896 cm−1 are present in the DRIFTS spectrum of Re-bpy covalently attached to a non-porous SiO2 (Fig. 5a). These absorption features are associated with the formation of a CO2-bound Re(I) adduct, Re(I)–OOC–OCH2CH2NR2 where R is CH2CH2OH, in the presence of TEOA and CO2.70 The Re(I)–OOC–OCH2CH2NR2 species was suggested to be the real catalyst in many CO2-reduction systems using diimine-tricarbonyl Re(I) complexes.
Interestingly, another group of three peaks at 1995, 1876, and 1849 cm−1 are seen in the DRIFTS spectrum of Re-bpy covalently attached to a mesoporous SiO2 (Fig. 5b). These three peaks are more prominent in the spectrum of Re-bpy covalently attached Kaolin, a hydrated aluminum silicate (Fig. 5c).71 These peaks are associated with a surface Re(I)–OH species, formed upon replacing the Cl− ligand on Re(I) with –OH, which could be produced by a reaction between TEOA and water (reaction (1)). The comparison shown in Fig. 5 highlights the effect of surface water on the complexation of surface-immobilized Re-bpy. It was suggested that the presence of water in the interlayer space of Kaolin and mesopores of the mesoporous SiO2 facilitated the formation of the Re(I)–OH species.71
| (HOCH2CH2)3N + H2O → (HOCH2CH2)3NH+ + OH− | (1) |
3. Heterogenization of Co-cyclam for visible-light CO2 reduction
In addition to Re-bpy, other molecular catalysts have been covalently attached onto semiconductor surfaces for use in light-driven CO2 reduction.72–79 Among these heterogenized molecular catalysts, many employed cobalt complexes because of their outstanding performance, low cost, and reasonable stability. Our research in this direction aims at achieving visible-light CO2 reduction using heterogenized Co-cyclam on semiconductor surfaces. Co-cyclam demonstrated excellent activity in selective CO2-to-CO conversion in the presence of a molecular photosensitizer, p-terphenyl, under UV irradiation (Scheme 2a). A serendipitous finding led to the deposition of Co-cyclam on SiO2via an oxo bridge (Scheme 2b). The resulting heterogenized Co-cyclam demonstrated better activity than the molecular Co-cyclam in CO2 reduction under the same conditions. Co-cyclam was then deposited on TiO2 (Scheme 2c) and C3N4 (Scheme 2d) which replaced p-terphenyl as the photosensitizers in CO2. Our research also revealed the effects of ligand derivatization and surface –OH groups on the structure and activity of heterogenized Co-cyclam. This line of research further inspired us to synthesize a Co SAC on C3N4 which showed excellent activity in visible-light CO2 reduction (see Section 4).3.1. Surface immobilization of Co-cyclam on SiO2
Sujandi and co-workers prepared Co-cyclam covalently attached onto mesoporous SiO2 through N-functionalization of the cyclam ligand.80 The resulting catalyst showed good activity in aerial oxidation of ethylbenzene. In our synthesis of heterogenized Co-cyclam, the SiO2 surface was initially functionalized with a bromo silane coupling agent (Scheme 3, left). Cyclam-functionalized SiO2 was then prepared by reacting bromo-functionalized SiO2 with cyclam in the presence of triethylamine (TEA). Heterogenized Co-cyclam, denoted “Co-L-SiO2”, was obtained as a green powder after reacting CoCl2 with cyclam-functionalized SiO2 in the presence of oxygen.81 The UV-vis spectrum of Co-L-SiO2 features a distinct peak around 630 nm (Fig. 7, solid trace), characteristic of the trans isomer of Co-cyclam (Fig. 7, dotted trace).82The synthesized Co-L-SiO2 was tested in photocatalytic CO2 reduction using p-terphenyl as a molecular photosensitizer. Both CO and H2 were detected as the major products in the presence of TEOA and methanol as electron donors. Under the same experimental condition, Co-L-SiO2 demonstrated significantly lower activity and selectivity towards CO formation than Co-cyclam.81 A turnover number (TON) of 12.5 for CO formation was obtained using Co-L-SiO2 after CO2 reduction for 4 h, compared to 141.8 for Co-cyclam. The selectivity towards CO formation, as measured by the CO/H2 ratio, was determined to be 7.8 and 15.1 for Co-L-SiO2 and Co-cyclam, respectively. These results indicate that heterogenization through N-functionalization (Scheme 3, left) was detrimental to the catalytic property of Co-cyclam. In a study by Froehlich and co-workers,83 a similar effect was observed in electrochemical CO2 reduction using homogeneous Ni(II) cyclam catalysts, where N-functionalization on cyclam with methyl groups drastically altered the redox and catalytic properties of Ni(II) cyclam. Therefore, the observed detrimental effect originated from derivatizing one of the amines on the cyclam ring. Such a detrimental effect could be avoided by derivatizing on one of the C atoms of the cyclam ligand for surface immobilization. Recently, Forget and co-workers84 prepared C-functionalized Ni(II) cyclam grafted onto a carbon electrode. The resulting catalyst demonstrated excellent activity in electrochemical CO2 reduction.
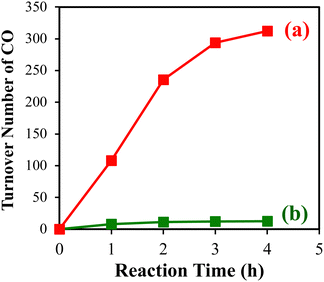
In another attempt to synthesize Co-L-SiO2, Co-cyclam was mixed directly with bromo-functionalized SiO2 in the presence of TEA (Scheme 3, right). Interestingly, this reaction resulted in the formation of a different catalyst, denoted “CoX/SiO2”, in which the Co(III) center is directly deposited on SiO2via an oxo bridge. Further characterization of CoX/SiO2 suggested that the chloro ligands in Co-cyclam was replaced with –OH ligands upon surface deposition.81 Likely, Co-cyclam reacted preferentially with surface silanol (Si–OH) groups, instead of the bromo groups, on the functionalized SiO2. Based on this discovery, we employed a microwave-assisted synthesis to achieve uniform deposition of Co-cyclam on an unfunctionalized mesoporous SiO2.85 The resulting CoX/SiO2 demonstrated superior activity in CO2 reduction using p-terphenyl as a photosensitizer. A TON more than 300 was obtained for CO after photocatalytic CO2 reduction for 4 h (Fig. 8).
In order to further probe the structure of CoX on surfaces, Co-cyclam was deposited via the microwave method on a mesoporous SiO2 with a surface area of 569 m2 g−1 and a non-porous SiO2 with a surface area of 200 m2 g−1. Under the same synthetic conditions, CoX deposited on the mesoporous SiO2 (cobalt loading 19 μmol g−1) displayed a green color, corresponding to three absorptions at 600 nm, ∼460 nm, and ∼380 nm in its UV-vis spectrum (Fig. 9a). In contrast, CoX deposited on the nonporous SiO2 (cobalt loading 13 μmol g−1) had a brown color, and the three peaks are not distinguishable in its UV-vis spectrum due to peak broadening and overlapping (Fig. 9b). Likely, CoX exists on the nonporous SiO2 as a peroxo Co(III) complex featuring a Co–O2–Co unit.86 In photocatalytic CO2 reduction using p-terphenyl as the photosensitizer, CoX on the mesoporous SiO2 demonstrated significantly higher activity than on the nonporous SiO2.
3.2. Deposition of Co-cyclam on TiO2
Following the same microwave synthesis, CoX was deposited on TiO2 (Scheme 2c) in order the eliminate the use of molecular photosensitizers such as p-terphenyl.87 Upon UV light irradiation, photoexcited electrons in TiO2 nanoparticles were transferred to the surface CoX for CO2 reduction. On the surface of a commercial TiO2 material, Evonik P25 consisting of ∼80% anatase and ∼20% rutile, a TON of 11.2 for CO production was obtained in photocatalytic CO2 reduction in the presence of TEOA as the electron donor (Fig. 10a). In comparison, no significant CO production was observed using a suspension containing P25 TiO2 nanoparticles and Co-cyclam.The presence of certain surface –OH groups on TiO2 was found to be essential for the molecular deposition of Co-cyclam.88 We prepared two photocatalysts, CoX/P25 and CoX/Rutile, which were prepared by depositing CoX on two commercial TiO2 materials, P25 and Rutile nanopowder (Sigma-Aldrich), respectively. In photocatalytic CO2 reduction, CoX/P25 exhibited very good activity, while negligible CO production was observed using CoX/Rutile (Fig. 10). DRIFTS studies indicated the presence of terminal Ti–OH groups on P25, while the surface of Rutile lacked such –OH groups. In a study by Petsi and co-workers,89 surface –OH groups were found to be important for the deposition of [Co(H2O)6]2+ on TiO2 nanoparticles. In our study, the terminal –OH groups are likely essential for the molecular deposition of catalytically active CoX on P25 via Ti–O–Co linkages. The lack of sufficient Ti–O–Co linkages in CoX/Rutile led to inefficient photoexcited electron transfer from Rutile to surface CoX, resulting in its poor activity in photocatalytic CO2 reduction (Fig. 10b).
Additional characterization confirmed that CoX exists in different forms on CoX/P25 than on CoX/Rutile. For example, the UV-vis spectrum of CoX/P25 contains two broad absorptions at ∼460 nm and 600 nm (Fig. 11a), indicating the presence of uniform, molecularly deposited CoX.88 However, these features are absent in the spectrum of CoX/Rutile (Fig. 11b). Microscopic images showed that amorphous, nano-sized aggregates of CoX are present in CoX/Rutile, but not in CoX/P25.
Based on the specific surface areas of the TiO2 materials and elemental analysis results, surface catalyst densities were estimated to be 6–7 Co per nm2 surface in CoX/P25 and ∼26 Co per nm2 surface in CoX/Rutile.88 These numbers further support our conclusion that CoX is molecularly deposited on P25. The absence of terminal –OH groups for uniform deposition of CoX on Rutile resulted in the formation of CoX in the aggregated form as observed in microscopic images.
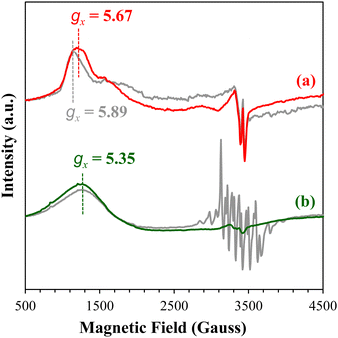
Characterization with EPR spectroscopy confirmed the fact that surface cobalt sites exist in different states on Rutile than on P25. Upon deposition on TiO2 surface in the presence of TEA, some Co(III) centers were reduced to Co(II), which served as a sensitive probe for EPR studies.88 As shown in Fig. 12, the spectrum of CoX/P25 under N2 (Fig. 12a, gray trace) contains resonances corresponding to mononuclear high-spin Co(II) (S = 3/2) centers at gx = 5.89, gy = 3.40, and gz = 2.22 (not labeled). The same spectrum also contains resonances near g = 2 (not labeled) that are associated with trapped electrons and holes in P2590,91 upon exposure to ambient light.
In the spectrum of CoX/Rutile (Fig. 12b, gray trace), however, a very different EPR spectrum was observed in the high-spin region featuring a broad Co(II) peak with g = 5.35 due to averaging of gx and gy components indicative of Co(II) in a broad range of coordination geometries. In addition, strong eight-line signals associated with oxygen-centered radicals were observed in the region of g = 2. Likely, some of the CoX exists as a peroxo complex featuring a Co–O2–Co unit on Rutile, similar to that on the nonporous SiO2 (Fig. 9).
Upon CO2 adsorption, the Co(II) sites in CoX/P25 displayed a high-spin signal at gx = 5.67 (Fig. 12a), indicating that the adsorbed CO2 on CoX/P25 took electron density away from the Co(II) centers. On CoX/Rutile (Fig. 12b), CO2 adsorption did not shift the high-spin Co(II) signal at gx = 5.35. Instead, the hyperfine splitting of the oxygen radicals vanished and an anisotropic signal appeared near g = 2 (not labeled). This observation implies that the electron density on the oxygen radicals adjacent to Co(II) was preferentially transferred to CO2 and adsorption of CO2, in fact, removed electron exchange of Co(II) with oxygen atoms on the Rutile surface. Based on these observations, it can be concluded that the Co(II) sites in CoX/Rutile are in a broad range of coordination geometries different than those in CoX/P25.
3.3. Deposition of Co-cyclam on C3N4
An inorganic polymeric semiconductor, C3N4 has attracted enormous interest due to its low cost, high thermal and chemical stability, nitrogen-rich structure, and suitable bandgap (2.7 eV).92–94 In CO2 reduction, C3N4-based materials have been employed as photocatalysts95–97 or coupled with metal-ligand complexes76–79,98–104 to achieve enhanced catalysis. For example, Roy and co-workers76 deposited cobalt phthalocyanine cyanine on mesoporous C3N4via a weak π–π stacking interaction for visible-light CO2 reduction. Wei and co-workers79 attached a cobalt quaterpyridine complex onto C3N4 through an amide linkage, which showed remarkable selectivity (98%) towards CO production under visible-light irradiation. In the recent work by Shang and co-workers,104 a cobalt phthalocyanine complex was deposited on C3N4 for photocatalytic CO2 reduction. The cobalt phthalocyanine complex contains –COOH groups, which enabled high surface coverage of the cobalt complex and promoted its catalytic property.In our study, Co-cyclam was deposited on C3N4 (Scheme 2d) in the presence of TEA, forming CoX/C3N4 as indicated by a broad peak around ∼600 nm in its UV-vis spectrum (Fig. 13). A TON of more than 160 for CO production was obtained using Co-cyclam deposited on a modified C3N4 after CO2 reduction for an extended period under visible-light irradiation (λ > 420 nm).105 Under the same reaction condition, no CO was produced using a physical mixture of Co-cyclam and C3N4.
In combination with computational modeling, X-ray absorption spectroscopy (XAS) was employed to investigate the structure of CoX on C3N4.105,106 For metal-based catalytic materials, the extended X-ray absorption fine structure (EXAFS) analysis can provide valuable insights into the local geometric structure and coordination environment of the metal sites,107 while the X-ray absorption near edge structure (XANES) spectroscopy is particularly sensitive to the electronic structure of the metal center and its local environment.108 As can be seen in Fig. 14a, the Co K-edge XANES spectrum of CoX/C3N4 has different features than that of Co-cyclam, confirming that the coordination environment of cobalt significantly changed upon deposition on C3N4. Based on prior studies,87,88,101 we hypothesize that the two axial Cl ligands of Co-cyclam were replaced with one OH group and one surface –O– or –NH– group upon deposition on C3N4 in the presence of TEA.
Our hypothesis was supported by the Co K-edge EXAFS spectra of Co-cyclam and CoX/C3N4 (Fig. 14b), which clearly indicate the absence of Co–Cl bonds in CoX/C3N4. Fitting the first peak of the EXAFS spectrum of Co-cyclam led to coordination numbers of 3.5 ± 1.2 and 2.0 ± 1.2 for Co–N and Co–Cl bonds, respectively. The Co–O scattering path was chosen to fit the first peak of CoX/C3N4, resulting in a coordination number of 6.3 ± 1.4 for Co–O nearest neighbors.105 Since the bond lengths of Co–N and Co–O are very close to each other, our experimental results were not able to distinguish between contributions from N and O atoms. We further carried out density functional theory (DFT) simulations, in which anchoring of CoX to C3N4 through either a surface –O– or –NH– group represents the most probable modes. Similar energies and distances between the Co center of CoX and C3N4 were obtained for these two modes. Therefore, it remains unclear whether CoX attaches to C3N4 through the –O– or –NH– group. Nevertheless, the structure shown in Scheme 2d represents a plausible model of CoX/C3N4. Fig. 14c shows a DFT optimized structure of CoX attached to C3N4 through an oxygen atom.
A similar macrocyclic complex, [Co(HMD)Cl2]Cl where HMD is 5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene, is also known for its catalytic activity in selective CO2 reduction.109 We have deposited this cobalt complex onto different semiconductor surfaces (TiO2, N-Ta2O5, and C3N4). The resulting heterogenized catalyst on TiO2 demonstrated lower activity than CoX/TiO2 in CO2 reduction under UV irradiation, while on C3N4 it showed significantly higher activity than CoX/C3N4 under visible-light irradiation.101
4. Single atom catalysts in C3N4 for visible-light CO2 reduction
Crystalline porous materials, particularly metal-organic frameworks (MOFs), are excellent support for constructing well-defined catalytic sites.110–112 A variety of molecular catalysts, including Re-bpy, have been incorporated in MOFs with demonstrated activity towards photocatalytic CO2 reduction.113–116 Other examples of such catalysts in porous materials include metal-to-metal charge transfer units117–119 and single atom catalysts (SACs).120–123 Porous materials represent an ideal platform for studying effects of chemical microenvironment on the activity of well-defined catalytic sites.124–126SACs have been extensively explored in a variety of applications including photocatalytic44–48 and electrocatalytic CO2 reduction.127–129 Two-dimensional materials, including N-doped graphene and C3N4, have been employed as the support for SACs.130–132 The N-rich framework of C3N4 enables it to serve as a photoactive support for SACs featuring metal (M)–N coordination.133–135 In 2018, we reported a Co SAC on C3N4 that demonstrated interesting activity in selective CO2 reduction under visible-light irradiation.136 Since then, many studies have examined SACs on C3N4 for solar CO2 reduction.137–151 For example, Wang and co-workers149 synthesized an Ni SAC anchored by boron–oxo species on C3N4 nanosheets. The Ni SAC was shown to possess a unique Ni–O6 coordination and demonstrated excellent activity towards CO and CH4 production in photocatalytic CO2 reduction. Sun and co-workers150 prepared two Cu SACs with different Cu configurations (Cu1N3 and Cu1P3) on phosphorus-doped C3N4. In visible-light CO2 reduction in the presence of H2O, the Cu SAC containing the Cu1N3 unit was found to be active towards CO production, while the Cu SAC containing the Cu1P3 unit preferentially produced H2 instead of CO. Ou and co-workers151 synthesized a SAC with two active centers, Mn and Co, on C3N4. Experimental results and DFT calculations showed an interesting synergy in photocatalysis using the Mn–Co SAC, where H2O oxidation occurred on the Mn center and CO2 reduction occurred on the Co center.
In 2016, Gao and co-workers152 predicted that efficient visible-light CO2 reduction can be achieved using single Pd and Pt atoms supported on C3N4. Inspired by this report and other prior work on SACs, we prepared a Co SAC on C3N4 (Fig. 15a) by mixing CoCl2 with C3N4 in acetonitrile, followed by microwave heating in the presence of TEA.136 The typical SAC behavior was observed in photocatalytic CO2 reduction using the synthesized material (Fig. 15b). At relatively low cobalt loadings, the presence of single Co2+ sites was confirmed with XAS (Fig. 15c, red trace). Catalytically inactive cobalt oxide nanoparticles were produced at high cobalt loadings (Fig. 15c, blue trace).
Different structural modes have been proposed for SACs on C3N4, including in-plane M–N3 and inter-layer M–N4 coordination,153 C–M–N2 coordination,137 M–N4 coordination in the presence of N vacancies,154 and other possibilities.155,156 Results from fitting the EXAFS spectrum shown in Fig. 15c indicated that our Co SAC has four equivalent Co–N bonds. However, computational modeling failed to reach such a Co–N4 structure inside the “pocket” of pristine C3N4 (Fig. 16a and b). Instead, a Co–N2+2 coordination at C3N4 edge sites appeared to be the most probable structure (Fig. 16c).157 For this structure, the calculated Co–N bond length is in the range of 2.07–2.10 Å, in excellent agreement with the value (dCo–N 2.08 Å) obtained by fitting the EXAFS spectrum. This mode of coordination was further supported by our experimental results obtained using a Co SAC in C3N4 treated with NH3, which contains more edge sites than untreated C3N4 and subsequently allows a significantly higher loading of single Co2+ sites. Similar SAC structures have been reported, such as the Fe–N2+2 sites of Fe SACs confined in N-doped carbon materials.129,158,159
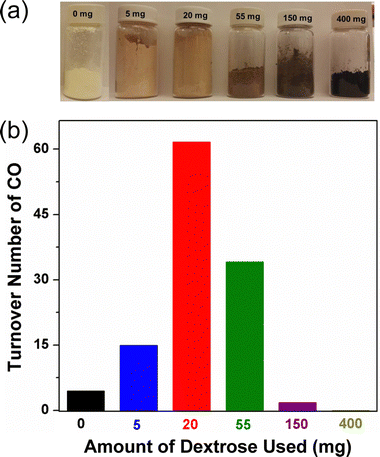
Doping C3N4 with an appropriate amount of carbon significantly enhanced the photocatalytic activity of the Co SAC on C3N4.160 In our study, carbon doping was achieved by adding a small amount of dextrose to urea (5–400 mg dextrose per 20 g urea) for synthesizing C3N4via pyrolysis. Fig. 17a shows the pictures of synthesized C3N4 containing varying amounts of carbon dopant. While pristine C3N4 synthesized from pure urea displays a light yellow color, C-doped C3N4 becomes darker in color with increasing amounts of dextrose used in synthesis. These C3N4 materials were used to prepare Co SACs following our established method, which was then tested in photocatalytic CO2 reduction to probe the effect of carbon doping. As can be seen from Fig. 17b, an optimal level of carbon doping exists since the highest activity was obtained using a Co SAC on a C-doped C3N4 material synthesized from 20 mg dextrose and 20 g urea. In subsequent studies, this particular C-doped C3N4 was utilized to prepare Co SACs.
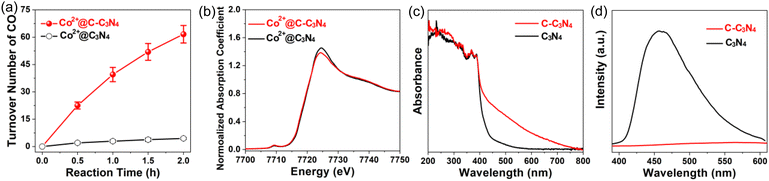
At a cobalt loading ∼0.014 μmol mg−1, TONs of 4.4 and 61.6 for CO production were obtained using a Co SAC on pristine C3N4 (denoted “Co2+@C3N4”) and a Co SAC on C-doped C3N4 (denoted “Co2+@C-C3N4”), respectively (Fig. 18a).157 The XANES spectrum of Co2+@C3N4 is almost identical to that of Co2+@C-C3N4 (Fig. 18b), suggesting that the two Co SACs have similar electronic and geometric structures. Additional characterization was done to explore possible reasons for the observed enhancement in photocatalysis. The synthesized C-C3N4 has greater absorption than pristine C3N4 in the visible-light region (400–800 nm, Fig. 18c). The different photoluminescence spectra of C-C3N4 and C3N4 (Fig. 18d) indicate that doping C3N4 with carbon significantly inhibited photoinduced charge recombination. It has been reported that appropriate carbon doping can enhance the photocatalytic properties of C3N4 materials by improving its photoresponse and inhibiting charge recombination.161 These factors should account for, at least partly, the observed higher activity of Co2+@C-C3N4 than Co2+@C3N4 in our study.
Our experimental results suggest that other factors also contributed to the enhanced activity of Co2+@C-C3N4 in photocatalytic CO2 reduction. In particular, Co-cyclam deposited on C-C3N4 displayed a 2-fold enhancement in activity in comparison with Co-cyclam deposited on C3N4 (CoX/C3N4 in Section 3.3).157 Under the same experimental condition, Co2+@C-C3N4 demonstrated ∼14 times higher activity than Co2+@C3N4 in CO2 reduction. Therefore, C doping likely also affected the coordination environment of Co2+ sites in C-C3N4. In our study, the Co–N2+2 coordination at C3N4 edge sites is also the most probable structure for Co2+@C-C3N4. A simplified model of C doping was proposed to explore possible additional effects of C doping. In this model, the presence of C dopant near the cobalt centers (Fig. 19, circled in red) results in shorter Co–N bond length and stronger Co–N binding energy. In addition to enhanced light absorption and charge separation in C-doped C3N4, the stronger Co–N binding upon C doping likely contributes to the improved catalytic properties of Co2+@C-C3N4.
It is much desired to obtain unified structure–property relationships for different catalytic materials.162 In our research, the Co–N4 unit is crucial for the CO2-reduction activity of both the heterogenized Co-cyclam and the Co SAC on C3N4. The calculated Co–N bond length in Co2+@C-C3N4 (1.96–1.97 Å) is very close to that in Co-cyclam (1.97–2.02 Å).157 It is possible that insights learned from studying such SACs could serve as inspiration for the design and development of innovative molecular catalysts, and vice versa.
5. Summary and outlook
Heterogenized molecular catalysts and SACs contain well-defined surface catalytic sites. They have demonstrated exciting activities in various applications, including solar CO2 reduction. Our recent research has focused on (i) covalently attaching two well-known molecular catalysts, Re-bpy and Co-cyclam, on different surfaces, and (ii) understanding the structures of Co SACs on C3N4. We have utilized catalyst synthesis, spectroscopic characterization, and computation modeling to interrogate the structures of well-defined surface catalytic sites and understand factors affecting their structures and photocatalytic activities.In particular, Re-bpy was covalently grafted onto SiO2 surfaces via amide linkages. Derivatization of the bpy ligand with electron-withdrawing amide groups significantly lowered the activity of Re-bpy in photocatalytic CO2 reduction. Such derivatization at different positions of the bpy ligand resulted in electron density being localized in different parts of Re-bpy upon photoreduction and subsequently different photocatalytic activity. The presence of surface water was found to have a strong impact on the complexation and even photoactivity of surface Re-bpy.
Similar effects of ligand derivatization and surface characteristics were observed in heterogenization of Co-cyclam on different surfaces. In the presence of p-terphenyl as a molecular photosensitizer, Co-cyclam grafted onto SiO2via one of the coordinating N atoms showed poor activity towards CO2 reduction. Under the same experimental conditions, Co-cyclam directly deposited on SiO2via an oxo bridge on the Co center demonstrated excellent activity. This oxo bridge strategy was employed to deposit Co-cyclam on TiO2, which functioned as a light absorber in photocatalysis, eliminating the need for p-terphenyl as a molecular photosensitizer. The presence of surface OH groups on SiO2 and TiO2 was found to be key for the effective surface deposition of Co-cyclam. Building on these results, visible-light CO2 reduction was achieved using Co-cyclam deposited C3N4, which has a narrower bandgap than TiO2 and can be activated under visible-light irradiation.
Inspired by the activity of Co-cyclam on C3N4 and prior work on SACs by others, we discovered a Co SAC featuring Co2+ coordinated to framework N atoms on C3N4. The Co SAC demonstrated excellent activity in visible-light CO2 reduction. Based on spectroscopic and modeling studies, a Co–N2+2 coordination at C3N4 edge sites was suggested to be the most probable structure. Doping C3N4 with an appropriate amount of carbon significantly enhanced the photocatalytic activity of the Co SAC. A model of carbon doping was also proposed, which involves the substitution of N atoms near the Co centers with C atoms.
In our research, spectroscopic techniques, including infrared, UV-visible, electron paramagnetic resonance, and X-ray absorption spectroscopies, were extensively utilized to understand the structures and properties of heterogenized molecular catalysts and SACs. Computational modeling further provided important insights regarding the structures of these well-defined surface catalytic sites at the molecular level. Our research has been largely inspired by exciting research results from other groups, some of which are briefly discussed in this article. Needless to say, many questions remain unanswered as we continue to investigate these photocatalytic materials.
Despite the tremendous progress made so far, major challenges and ample opportunities co-exist in this field, calling for collaborative efforts by experts from different disciplines. Many photocatalysts, especially those involving molecular photosensitizers, require the use of non-renewable, sacrificial electron donors in CO2 reduction. A sustainable photocatalytic system should use water as the sole electron donor. In this sense, photoelectrochemical synthesis, which often employ heterogenized molecular CO2-reduction catalysts on semiconductors with appropriate band structures, appear to be the ultimate solution to address this issue and huge progress has been made in this direction.163,164 Innovative design of SACs has allowed effective coupling between CO2 reduction and water oxidation.151 The development of photocatalysts based on earth-abundant metals165 is also important for achieving energy sustainability through solar CO2 reduction.
In some instances, heterogenization of molecular catalysts could lead to reduced catalytic activity due to detrimental ligand derivatization, in spite of enhanced stability upon surface attachment. This was observed in our studies using Re-bpy and Co-cyclam, as described in Sections 2 and 3. Therefore, effective heterogenization strategies should aim to preserve or even improve the catalytic activity of the metal centers. Research in this area could really benefit from existing knowledge on the effects of ligand derivatization on the activity of molecular catalysts.
It remains a challenge to reveal the exact structures of well-defined surface catalytic sites at the molecular level. Our research demonstrated that combining catalyst synthesis with advanced spectroscopy and theory is a powerful approach to study heterogenized molecular catalysts and SACs. Such an approach could generate critical information beyond just catalyst structures. For example, Lian, Batista, and co-workers50,166,167 employed phase-sensitive sum frequency generation spectroscopy and DFT calculations to interrogate the configuration of Re-bpy on TiO2 surfaces. The researchers investigated Re-bpy attached to TiO2via molecular linkages of various lengths, which could allow effective manipulation of the spatial orientation of Re-bpy on TiO2 surfaces.
Furthermore, elucidating the unique advantages of heterogenized molecular catalysts and SACs in photocatalysis requires mechanistic studies using ultrafast spectroscopies and in situ/operando techniques.168–171 As mentioned earlier, results obtained using transient absorption spectroscopy revealed underlying reasons for observed enhancement in the CO2-reduction activity of Re-bpy upon heterogenization on TiO2 surfaces.52,53 In this sense, one advantage of photocatalysts containing well-defined surface sites lies in the fact that they could utilize techniques from both homogeneous catalysis and heterogenous catalysis.
Conflicts of interest
There are no conflicts to delcare.Acknowledgements
This material is based upon work supported by the U.S. National Science Foundation under awards 1352437 and 2102655, and the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under award DE-SC0016417 to G. L. The authors acknowledge collaborations and discussions with Drs. Edward Wong, Richard Johnson, Tijana Rajh, Etsuko Fujita, Dunwei Wang, Anatoly Frenkel, N. Aaron Deskins, Christine A. Caputo, Jonathan Rochford, Gary Brudvig, Victor Batista, Robert Crabtree, and Charles Schmuttenmaer.References
- M. Mikkelsen, M. Jorgensen and F. C. Krebs, The Teraton Challenge. A Review of Fixation and Transformation of Carbon Dioxide, Energy Environ. Sci., 2010, 3(1), 43–81 RSC.
- A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. DuBois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer and G. L. Waldrop, Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- B. Kumar, M. Llorente, J. Froehlich, T. Dang, A. Sathrum and C. P. Kubiak, Photochemical and photoelectrochemical reduction of CO2, Annu. Rev. Phys. Chem., 2012, 63, 541–569 CrossRef CAS PubMed.
- J. L. White, M. F. Baruch, J. E. Pander, Y. Hu, I. C. Fortmeyer, J. E. Park, T. Zhang, K. Liao, J. Gu, Y. Yan, T. W. Shaw, E. Abelev and A. B. Bocarsly, Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes, Chem. Rev., 2015, 115(23), 12888–12935 CrossRef CAS PubMed.
- Y. Tamaki and O. Ishitani, Supramolecular Photocatalysts for the Reduction of CO2, ACS Catal., 2017, 7(5), 3394–3409 CrossRef CAS.
- Y. Wang, D. He, H. Chen and D. Wang, Catalysts in electro-, photo- and photoelectrocatalytic CO2 reduction reactions, J. Photochem. Photobiol. C: Photochem. Rev., 2019, 40, 117–149 CrossRef.
- K. E. Dalle, J. Warnan, J. J. Leung, B. Reuillard, I. S. Karmel and E. Reisner, Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes, Chem. Rev., 2019, 119(4), 2752–2875 CrossRef CAS PubMed.
- E. Fujita, Photochemical carbon dioxide reduction with metal complexes, Coord. Chem. Rev., 1999, 185–186, 373–384 CrossRef CAS.
- A. J. Morris, G. J. Meyer and E. Fujita, Molecular Approaches to the Photocatalytic Reduction of Carbon Dioxide for Solar Fuels, Acc. Chem. Res., 2009, 42, 1983–1994 CrossRef CAS PubMed.
- C. D. Windle and R. N. Perutz, Advances in molecular photocatalytic and electrocatalytic CO2 reduction, Coord. Chem. Rev., 2012, 256(21–22), 2562–2570 CrossRef CAS.
- E. Boutin, L. Merakeb, B. Ma, B. Boudy, M. Wang, J. Bonin, E. Anxolabéhère-Mallart and M. Robert, Molecular catalysis of CO2 reduction: recent advances and perspectives in electrochemical and light-driven processes with selected Fe, Ni and Co aza macrocyclic and polypyridine complexes, Chem. Soc. Rev., 2020, 49, 5772–5809 RSC.
- J. Hawecker, J.-M. Lehn and R. Ziessel, Efficient Photochemical Reduction of CO2 to CO by Visible Light Irradiation of Systems Containing Re(bipy)(CO)3X or Ru(bipy)32+-Co2+ Combinations as Homogeneous Catalysts, J. Chem. Soc., Chem. Commun., 1983, 536–538 RSC.
- G. Sahara and O. Ishitani, Efficient Photocatalysts for CO2 Reduction, Inorg. Chem., 2015, 54(11), 5096–5104 CrossRef CAS PubMed.
- E. Fujita, C. Creutz, N. Sutin and D. J. Szalda, Carbon dioxide activation by cobalt(I) macrocycles: factors affecting carbon dioxide and carbon monoxide binding, J. Am. Chem. Soc., 1991, 113(1), 343–353 CrossRef CAS.
- S. Matsuoka, K. Yamamoto, T. Ogata, M. Kusaba, N. Nakashima, E. Fujita and S. Yanagida, Efficient and selective electron mediation of cobalt complexes with cyclam and related macrocycles in the p-terphenyl-catalyzed photoreduction of carbon dioxide, J. Am. Chem. Soc., 1993, 115(2), 601–609 CrossRef CAS.
- M. R. Hoffmann, J. A. Moss and M. M. Baum, Artificial photosynthesis: semiconductor photocatalytic fixation of CO2 to afford higher organic compounds, Dalton Trans., 2011, 40, 5151–5158 RSC.
- H. He, C. Liu, K. D. Dubois, T. Jin, M. E. Louis and G. Li, Enhanced Charge Separation in Nanostructured TiO2 Materials for Photocatalytic and Photovoltaic Applications, Ind. Eng. Chem. Res., 2012, 51(37), 11841–11849 CrossRef CAS.
- K. Mori, H. Yamashita and M. Anpo, Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts, RSC Adv., 2012, 2, 3165–3172 RSC.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors, Angew. Chem., Int. Ed., 2013, 52, 7372–7408 CrossRef CAS PubMed.
- W. Tu, Y. Zhou and Z. Zou, Photocatalytic Conversion of CO2 into Renewable Hydrocarbon Fuels: State-of-the-Art Accomplishment, Challenges, and Prospects, Adv. Mater., 2014, 26(27), 4607–4626 CrossRef CAS PubMed.
- X. Li, J. Yu, M. Jaroniec and X. Chen, Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels, Chem. Rev., 2019, 119(6), 3962–4179 CrossRef CAS PubMed.
- T. Inoue, A. Fujishima, S. Konishi and K. Honda, Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders, Nature, 1979, 277, 637–638 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Environmental Applications of Semiconductor Photocatalysis, Chem. Rev., 1995, 95(1), 69–96 CrossRef CAS.
- G. Li and K. A. Gray, The solid–solid interface: Explaining the high and unique photocatalytic reactivity of TiO2-based nanocomposite materials, Chem. Phys., 2007, 339, 173–187 CrossRef CAS.
- P. D. Tran, L. H. Wong, J. Barber and J. S. C. Loo, Recent advances in hybrid photocatalysts for solar fuel production, Energy Environ. Sci., 2012, 5, 5902–5918 RSC.
- F. Wen and C. Li, Hybrid Artificial Photosynthetic Systems Comprising Semiconductors as Light Harvesters and Biomimetic Complexes as Molecular Cocatalysts, Acc. Chem. Res., 2013, 46(11), 2355–2364 CrossRef CAS PubMed.
- C. D. Windle and E. Reisner, Heterogenised Molecular Catalysts for the Reduction of CO2 to Fuels, Chimia, 2015, 69(7–8), 435–441 CrossRef CAS PubMed.
- M. E. Louis, T. G. Fenton, J. Rondeau, T. Jin and G. Li, Solar CO2 Reduction Using Surface-Immobilized Molecular Catalysts, Comments Inorg. Chem., 2016, 36(1), 38–60 CrossRef CAS.
- K. Maeda, Metal-Complex/Semiconductor Hybrid Photocatalysts and Photoelectrodes for CO2 Reduction Driven by Visible Light, Adv. Mater., 2019, 31(25), 1808205 CrossRef PubMed.
- A. Perazio, G. Lowe, R. Gobetto, J. Bonin and M. Robert, Light-driven catalytic conversion of CO2 with heterogenized molecular catalysts based on fourth period transition metals, Coord. Chem. Rev., 2021, 443, 214018 CrossRef CAS.
- M. Reguero, C. Claver, R. M. B. Carrilho and A. M. Masdeu-Bultó, Immobilized Molecular Catalysts for CO2 Photoreduction, Adv. Sustain. Sys., 2022, 6(6), 2100493 CrossRef CAS.
- B. C. Gates, M. Flytzani-Stephanopoulos, D. A. Dixon and A. Katz, Atomically dispersed supported metal catalysts: perspectives and suggestions for future research, Catal. Sci. Technol., 2017, 7(19), 4259–4275 RSC.
- X. Cui, W. Li, P. Ryabchuk, K. Junge and M. Beller, Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts, Nat. Catal., 2018, 1(6), 385–397 CrossRef CAS.
- A. Wang, J. Li and T. Zhang, Heterogeneous single-atom catalysis, Nat. Rev. Chem., 2018, 2(6), 65–81 CrossRef CAS.
- S. K. Kaiser, Z. Chen, D. Faust Akl, S. Mitchell and J. Pérez-Ramírez, Single-Atom Catalysts across the Periodic Table, Chem. Rev., 2020, 120(21), 11703–11809 CrossRef CAS PubMed.
- S. Ji, Y. Chen, X. Wang, Z. Zhang, D. Wang and Y. Li, Chemical Synthesis of Single Atomic Site Catalysts, Chem. Rev., 2020, 120(21), 11900–11955 CrossRef CAS PubMed.
- J. Xi, H. S. Jung, Y. Xu, F. Xiao, J. W. Bae and S. Wang, Synthesis Strategies, Catalytic Applications, and Performance Regulation of Single-Atom Catalysts, Adv. Funct. Mater., 2021, 31(12), 2008318 CrossRef CAS.
- S. Swain, A. Altaee, M. Saxena and A. K. Samal, A comprehensive study on heterogeneous single atom catalysis: Current progress, and challenges, Coord. Chem. Rev., 2022, 470, 214710 CrossRef CAS.
- J. Guo, H. Liu, D. Li, J. Wang, X. Djitcheu, D. He and Q. Zhang, A minireview on the synthesis of single atom catalysts, RSC Adv., 2022, 12(15), 9373–9394 RSC.
- C. Gao, J. Low, R. Long, T. Kong, J. Zhu and Y. Xiong, Heterogeneous Single-Atom Photocatalysts: Fundamentals and Applications, Chem. Rev., 2020, 120(21), 12175–12216 CrossRef CAS PubMed.
- B. Xia, Y. Zhang, J. Ran, M. Jaroniec and S.-Z. Qiao, Single-Atom Photocatalysts for Emerging Reactions, ACS Cent. Sci., 2021, 7(1), 39–54 CrossRef CAS PubMed.
- Z.-H. Xue, D. Luan, H. Zhang and X. W. Lou, Single-atom catalysts for photocatalytic energy conversion, Joule, 2022, 6(1), 92–133 CrossRef CAS.
- P. Zhou, M. Luo and S. Guo, Optimizing the semiconductor–metal-single-atom interaction for photocatalytic reactivity, Nat. Rev. Chem., 2022, 6(11), 823–838 CrossRef CAS PubMed.
- T. Xia, R. Long, C. Gao and Y. Xiong, Design of atomically dispersed catalytic sites for photocatalytic CO2 reduction, Nanoscale, 2019, 11(23), 11064–11070 RSC.
- Y. Zhang, B. Xia, J. Ran, K. Davey and S. Z. Qiao, Atomic-Level Reactive Sites for Semiconductor-Based Photocatalytic CO2 Reduction, Adv. Energy Mater., 2020, 10(9), 1903879 CrossRef CAS.
- Y. Lu, Z. Zhang, H. Wang and Y. Wang, Toward efficient single-atom catalysts for renewable fuels and chemicals production from biomass and CO2, Appl. Catal. B: Environ., 2021, 292, 120162 CrossRef CAS.
- C. B. Hiragond, N. S. Powar, J. Lee and S. I. In, Single-Atom Catalysts (SACs) for Photocatalytic CO2 Reduction with H2O: Activity, Product Selectivity, Stability, and Surface Chemistry, Small, 2022, 18(29), e2201428 CrossRef PubMed.
- L. Liu, M. Li, F. Chen and H. Huang, Recent Advances on Single-Atom Catalysts for CO2 Reduction, Small Struct., 2023, 4(3), 2200188 CrossRef CAS.
- J. Huang, D. Stockwell, Z. Huang, D. L. Mohler and T. Lian, Photoinduced Ultrafast Electron Transfer from CdSe Quantum Dots to Re-bipyridyl Complexes, J. Am. Chem. Soc., 2008, 130(17), 5632–5633 CrossRef CAS PubMed.
- C. L. Anfuso, R. C. Snoeberger III, A. M. Ricks, W. Liu, D. Xiao, V. S. Batista and T. Lian, Covalent Attachment of a Rhenium Bipyridyl CO2 Reduction Catalyst to Rutile TiO2, J. Am. Chem. Soc., 2011, 133, 6922–6925 CrossRef CAS PubMed.
- D. I. Won, J. S. Lee, J. M. Ji, W. J. Jung, H. J. Son, C. Pac and S. O. Kang, Highly Robust Hybrid Photocatalyst for Carbon Dioxide Reduction: Tuning and Optimization of Catalytic Activities of Dye/TiO2/Re(I) Organic-Inorganic Ternary Systems, J. Am. Chem. Soc., 2015, 137(42), 13679–13690 CrossRef CAS PubMed.
- C. D. Windle, E. Pastor, A. Reynal, A. C. Whitwood, Y. Vaynzof, J. R. Durrant, R. N. Perutz and E. Reisner, Improving the Photocatalytic Reduction of CO2 to CO through Immobilization of a Molecular Re Catalyst on TiO2, Chem. - Eur. J., 2015, 21, 3746–3754 CrossRef CAS PubMed.
- M. Abdellah, A. M. El-Zohry, L. J. Antila, C. D. Windle, E. Reisner, L. Hammarström and I. R. Time-Resolved, Spectroscopy Reveals a Mechanism with TiO2 as a Reversible Electron Acceptor in a TiO2–Re Catalyst System for CO2 Photoreduction, J. Am. Chem. Soc., 2017, 139(3), 1226–1232 CrossRef CAS PubMed.
- L. A. Faustino, B. L. Souza, B. N. Nunes, A.-T. Duong, F. Sieland, D. W. Bahnemann and A. O. T. Patrocinio, Photocatalytic CO2 Reduction by Re(I) Polypyridyl Complexes Immobilized on Niobates Nanoscrolls, ACS Sustain. Chem. Eng., 2018, 6(5), 6073–6083 CrossRef CAS.
- J. Huang, M. G. Gatty, B. Xu, P. B. Pati, A. S. Etman, L. Tian, J. Sun, L. Hammarström and H. Tian, Covalently linking CuInS2 quantum dots with a Re catalyst by click reaction for photocatalytic CO2 reduction, Dalton Trans., 2018, 47(31), 10775–10783 RSC.
- N. M. Orchanian, L. E. Hong, J. A. Skrainka, J. A. Esterhuizen, D. A. Popov and S. C. Marinescu, Surface-Immobilized Conjugated Polymers Incorporating Rhenium Bipyridine Motifs for Electrocatalytic and Photocatalytic CO2 Reduction, ACS Appl. Energy Mater., 2019, 2(1), 110–123 CrossRef CAS.
- Z.-C. Kong, H.-H. Zhang, J.-F. Liao, Y.-J. Dong, Y. Jiang, H.-Y. Chen and D.-B. Kuang, Immobilizing Re(CO)3Br(dcbpy) Complex on CsPbBr3 Nanocrystal for Boosted Charge Separation and Photocatalytic CO2 Reduction, Solar RRL, 2020, 4(1), 1900365 CrossRef CAS.
- M. Schreier, J. Luo, P. Gao, T. Moehl, M. T. Mayer and M. Grätzel, Covalent Immobilization of a Molecular Catalyst on Cu2O Photocathodes for CO2 Reduction, J. Am. Chem. Soc., 2016, 138(6), 1938–1946 CrossRef CAS PubMed.
- R. Kamata, H. Kumagai, Y. Yamazaki, G. Sahara and O. Ishitani, Photoelectrochemical CO2 Reduction Using a Ru(II)–Re(I) Supramolecular Photocatalyst Connected to a Vinyl Polymer on a NiO Electrode, ACS Appl. Mater. Interface, 2019, 11(6), 5632–5641 CrossRef CAS PubMed.
- K. D. Dubois, A. Petushkov, E. G. Cardona, S. C. Larsen and G. Li, Adsorption and Photochemical Properties of a Molecular CO2 Reduction Catalyst in Hierarchical Mesoporous ZSM-5: An In Situ FTIR Study, J. Phys. Chem. Lett., 2012, 3, 486–492 CrossRef CAS PubMed.
- K. D. Dubois, H. He, C. Liu, A. S. Vorushilov and G. Li, Covalent attachment of a molecular CO2-reduction photocatalyst to mesoporous silica, J. Mol. Catal. A: Chem., 2012, 363–364, 208–213 CrossRef CAS.
- C. Liu, K. D. Dubois, M. E. Louis, A. S. Vorushilov and G. Li, Photocatalytic CO2 Reduction and Surface Immobilization of a Tricarbonyl Re(I) Compound Modified with Amide Groups, ACS Catal., 2013, 3, 655–662 CrossRef CAS.
- H. Takeda, K. Koike, T. Morimoto, H. Inumaru and O. Ishitani, Photochemistry and photocatalysis of rhenium(I) diimine complexes, Adv. Inorg. Chem., 2011, 63, 137–186 CrossRef CAS.
- X. Jia, H. S. Nedzbala, S. R. Bottum, J. F. Cahoon, J. J. Concepcion, C. L. Donley, A. Gang, Q. Han, N. Hazari, M. C. Kessinger, M. R. Lockett, J. M. Mayer, B. Q. Mercado, G. J. Meyer, A. J. Pearce, C. L. Rooney, R. N. Sampaio, B. Shang and H. Wang, Synthesis and Surface Attachment of Molecular Re(I) Complexes Supported by Functionalized Bipyridyl Ligands, Inorg. Chem., 2023, 62(5), 2359–2375 CrossRef CAS PubMed.
- T. G. Fenton, M. E. Louis and G. Li, Effect of ligand derivatization at different positions on photochemical properties of hybrid Re(I) photocatalysts, J. Mol. Catal. A, 2016, 411, 272–278 CrossRef CAS.
- R. Argazzi, C. A. Bignozzi, T. A. Heimer, F. N. Castellano and G. J. Meyer, Enhanced Spectral Sensitivity from Ruthenium(II) Polypyridyl Based Photovoltaic Devices, Inorg. Chem., 1994, 33(25), 5741–5749 CrossRef CAS.
- M. Guyot, M.-N. Lalloz, J. S. Aguirre-Araque, G. Rogez, C. Costentin and S. Chardon-Noblat, Rhenium Carbonyl Molecular Catalysts for CO2 Electroreduction: Effects on Catalysis of Bipyridine Substituents Mimicking Anchorage Functions to Modify Electrodes, Inorg. Chem., 2022, 61(40), 16072–16080 CrossRef CAS PubMed.
- A. Ge, B. Rudshteyn, P. E. Videla, C. J. Miller, C. P. Kubiak, V. S. Batista and T. Lian, Heterogenized Molecular Catalysts: Vibrational Sum-Frequency Spectroscopic, Electrochemical, and Theoretical Investigations, Acc. Chem. Res., 2019, 52(5), 1289–1300 CrossRef CAS PubMed.
- J. M. Smieja and C. P. Kubiak, Re(bipy-tBu)(CO)3Cl-improved Catalytic Activity for Reduction of Carbon Dioxide: IR-Spectroelectrochemical and Mechanistic Studies, Inorg. Chem., 2010, 49(20), 9283–9289 CrossRef CAS PubMed.
- T. Morimoto, T. Nakajima, S. Sawa, R. Nakanishi, D. Imori and O. Ishitani, CO2 Capture by a Rhenium(I) Complex with the Aid of Triethanolamine, J. Am. Chem. Soc., 2013, 135(45), 16825–16828 CrossRef CAS PubMed.
- H. He, C. Liu, M. E. Louis and G. Li, Infrared studies of a hybrid CO2-reduction photocatalyst consisting of a molecular Re(I) complex grafted on Kaolin, J. Mol. Catal. A, 2014, 395, 145–150 CrossRef CAS.
- K. Maeda, K. Sekizawa and O. Ishitani, A polymeric-semiconductor-metal-complex hybrid photocatalyst for visible-light CO2 reduction, Chem. Commun., 2013, 49, 10127–10129 RSC.
- G. Neri, M. Förster, J. J. Walsh, C. M. Robertson, T. J. Whittles, P. Farras and A. J. Cowan, Photochemical CO2 reduction in water using a co-immobilised nickel catalyst and a visible light sensitiser, Chem. Commun., 2016, 52(99), 14200–14203 RSC.
- T. E. Rosser, C. D. Windle and E. Reisner, Electrocatalytic and Solar-Driven CO2 Reduction to CO with a Molecular Manganese Catalyst Immobilized on Mesoporous TiO2, Angew. Chem., Int. Ed., 2016, 55, 7388–7392 CrossRef CAS PubMed.
- M. F. Kuehnel, C. D. Sahm, G. Neri, J. R. Lee, K. L. Orchard, A. J. Cowan and E. Reisner, ZnSe quantum dots modified with a Ni(cyclam) catalyst for efficient visible-light driven CO2 reduction in water, Chem. Sci., 2018, 9(9), 2501–2509 RSC.
- S. Roy and E. Reisner, Visible-Light-Driven CO2 Reduction by Mesoporous Carbon Nitride Modified with Polymeric Cobalt Phthalocyanine, Angew. Chem., Int. Ed., 2019, 58(35), 12180–12184 CrossRef CAS PubMed.
- B. Ma, G. Chen, C. Fave, L. Chen, R. Kuriki, K. Maeda, O. Ishitani, T.-C. Lau, J. Bonin and M. Robert, Efficient Visible-Light-Driven CO2 Reduction by a Cobalt Molecular Catalyst Covalently Linked to Mesoporous Carbon Nitride, J. Am. Chem. Soc., 2020, 142(13), 6188–6195 CrossRef CAS PubMed.
- J.-W. Wang, M. Gil-Sepulcre, H.-H. Huang, E. Solano, Y.-F. Mu, A. Llobet and G. Ouyang, CH-π interaction boosts photocatalytic CO2 reduction activity of a molecular cobalt catalyst anchored on carbon nitride, Cell Rep. Phys. Sci., 2021, 2(12), 100681 CrossRef CAS.
- Y. Wei, L. Chen, H. Chen, L. Cai, G. Tan, Y. Qiu, Q. Xiang, G. Chen, T.-C. Lau and M. Robert, Highly Efficient Photocatalytic Reduction of CO2 to CO by In Situ Formation of a Hybrid Catalytic System Based on Molecular Iron Quaterpyridine Covalently Linked to Carbon Nitride, Angew. Chem., Int. Ed., 2022, 61(11), e202116832 CrossRef CAS PubMed.
- Sujandi, E. A. Prasetyanto, D.-S. Han, S.-C. Lee and S.-E. Park, Immobilization of Co(III) using tethered cyclam ligand on SBA-15 mesoporous silica for aerial oxidation of ethylbenzene, Catal. Today, 2009, 141(3–4), 374–377 CrossRef CAS.
- T. Jin, C. Liu and G. Li, Heterogenization of a macrocyclic cobalt complex for photocatalytic CO2 reduction, J. Coord. Chem., 2016, 69(11–13), 1748–1758 CrossRef CAS.
- B. Bosnich, C. K. Poon and M. L. Tobe, Complexes of cobalt(III) with a cyclic tetradentate secondary amine, Inorg. Chem., 1965, 4, 1102–1108 CrossRef CAS.
- J. D. Froehlich and C. P. Kubiak, Homogeneous CO2 Reduction by Ni(cyclam) at a Glassy Carbon Electrode, Inorg. Chem., 2012, 51(7), 3932–3934 CrossRef CAS PubMed.
- A. Forget, M. Regnacq, C. Orain, E. Touzé, E. Lelong, C. Brandily, H. Bernard, R. Tripier and N. Le Poul, Electrocatalytic reduction of CO2 in water by a C-functionalized Ni–cyclam complex grafted onto carbon, Chem. Commun., 2022, 58(48), 6785–6788 RSC.
- T. Fenton, S. Gillingham, T. Jin and G. Li, Microwave-assisted deposition of a highly active cobalt catalyst on mesoporous silica for photochemical CO2 reduction, Dalton Trans., 2017, 46(32), 10721–10726 RSC.
- B. Bosnich, C. K. Poon and M. L. Tobe, Peroxo complexes of cobalt(III) with a cyclic quadridentate secondary amine, Inorg. Chem., 1966, 5, 1514–1517 CrossRef CAS.
- T. Jin, C. Liu and G. Li, Photocatalytic CO2 reduction using a molecular cobalt complex deposited on TiO2 nanoparticles, Chem. Commun., 2014, 50(47), 6221–6224 RSC.
- C. Liu, T. Jin, M. E. Louis, S. A. Pantovich, S. L. Skraba-Joiner, T. Rajh and G. Li, Molecular deposition of a macrocyclic cobalt catalyst on TiO2 nanoparticles, J. Mol. Catal. A, 2016, 423, 293–299 CrossRef CAS.
- T. Petsi, G. D. Panagiotou, C. S. Garoufalis, C. Kordulis, P. Stathi, Y. Deligiannakis, A. Lycourghiotis and K. Bourikas, Interfacial Impregnation Chemistry in the Synthesis of Cobalt Catalysts Supported on Titania, Chem. – Eur. J., 2009, 15(47), 13090–13104 CrossRef CAS PubMed.
- D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh and M. C. Thurnauer, Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR, J. Phys. Chem. B, 2003, 107(19), 4545–4549 CrossRef CAS.
- D. C. Hurum, K. A. Gray, T. Rajh and M. C. Thurnauer, Recombination pathways in the Degussa P25 formulation of TiO2: Surface versus lattice mechanisms, J. Phys. Chem. B, 2005, 109(2), 977–980 CrossRef CAS PubMed.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Metal-Containing Carbon Nitride Compounds: A New Functional Organic-Metal Hybrid Material, Adv. Mater., 2009, 21(16), 1609–1612 CrossRef CAS.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Polymeric Photocatalysts Based on Graphitic Carbon Nitride, Adv. Mater., 2015, 27(13), 2150–2176 CrossRef CAS PubMed.
- W.-J. Ong, L.-L. Tan, Y. H. Ng, S.-T. Yong and S.-P. Chai, Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability?, Chem. Rev., 2016, 116(12), 7159–7329 CrossRef CAS PubMed.
- Y. Fang and X. Wang, Photocatalytic CO2 conversion by polymeric carbon nitrides, Chem. Commun., 2018, 54(45), 5674–5687 RSC.
- J. Liang, Z. Jiang, P. K. Wong and C.-S. Lee, Recent Progress on Carbon Nitride and Its Hybrid Photocatalysts for CO2 Reduction, Solar RRL, 2021, 5(2), 2000478 CrossRef CAS.
- R. Liu, Z. Chen, Y. Yao, Y. Li, W. A. Cheema, D. Wang and S. Zhu, Recent advancements in g-C3N4-based photocatalysts for photocatalytic CO2 reduction: a mini review, RSC Adv., 2020, 10(49), 29408–29418 RSC.
- R. Kuriki, H. Matsunaga, T. Nakashima, K. Wada, A. Yamakata, O. Ishitani and K. Maeda, Nature-Inspired, Highly Durable CO2 Reduction System Consisting of a Binuclear Ruthenium(II) Complex and an Organic Semiconductor Using Visible Light, J. Am. Chem. Soc., 2016, 138(15), 5159–5170 CrossRef CAS PubMed.
- C. Cometto, R. Kuriki, L. Chen, K. Maeda, T.-C. Lau, O. Ishitani and M. Robert, A Carbon Nitride/Fe Quaterpyridine Catalytic System for Photostimulated CO2-to-CO Conversion with Visible Light, J. Am. Chem. Soc., 2018, 140(24), 7437–7440 CrossRef CAS PubMed.
- J. Zheng, X. Li, Y. Qin, S. Zhang, M. Sun, X. Duan, H. Sun, P. Li and S. Wang, Zn phthalocyanine/carbon nitride heterojunction for visible light photoelectrocatalytic conversion of CO2 to methanol, J. Catal., 2019, 371, 214–223 CrossRef CAS.
- P. Huang, S. A. Pantovich, N. O. Okolie, N. A. Deskins and G. Li, Hybrid Carbon Dioxide Reduction Photocatalysts Consisting of Macrocyclic Cobalt(III) Complexes Deposited on Semiconductor Surfaces, ChemPhotoChem, 2020, 4(6), 420–426 CrossRef CAS.
- Z. Pan, P. Niu, M. Liu, G. Zhang, Z. Zhu and X. Wang, Molecular Junctions on Polymeric Carbon Nitrides with Enhanced Photocatalytic Performance, ChemSusChem, 2020, 13(5), 888–892 CrossRef CAS PubMed.
- W. Zhen and C. Xue, Atomic- and Molecular-Level Functionalizations of Polymeric Carbon Nitride for Solar Fuel Production, Solar RRL, 2021, 5(2), 2000440 CrossRef CAS.
- B. Shang, F. Zhao, C. Choi, X. Jia, M. Pauly, Y. Wu, Z. Tao, Y. Zhong, N. Harmon, P. A. Maggard, T. Lian, N. Hazari and H. Wang, Monolayer Molecular Functionalization Enabled by Acid–Base Interaction for High-Performance Photochemical CO2 Reduction, ACS Energy Lett., 2022, 7(7), 2265–2272 CrossRef CAS.
- J. Li, P. Huang, F. Guo, J. Huang, S. Xiang, K. Yang, N. A. Deskins, V. S. Batista, G. Li and A. I. Frenkel, X-ray Absorption Spectroscopy Studies of a Molecular CO2-Reduction Catalyst Deposited on Graphitic Carbon Nitride, J. Phys. Chem. C, 2023, 127(7), 3626–3633 CrossRef CAS.
- S. Xiang, P. Huang, J. Li, Y. Liu, N. Marcella, P. K. Routh, G. Li and A. I. Frenkel, Solving the structure of “single-atom” catalysts using machine learning – assisted XANES analysis, Phys. Chem. Chem. Phys., 2022, 24(8), 5116–5124 RSC.
- J. Timoshenko, Z. Duan, G. Henkelman, R. M. Crooks and A. I. Frenkel, Solving the Structure and Dynamics of Metal Nanoparticles by Combining X-Ray Absorption Fine Structure Spectroscopy and Atomistic Structure Simulations, Annu. Rev. Anal. Chem., 2019, 12(1), 501–522 CrossRef CAS PubMed.
- A. L. Ankudinov, B. Ravel, J. J. Rehr and S. D. Conradson, Real-space multiple-scattering calculation and interpretation of x-ray-absorption near-edge structure, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58(12), 7565–7576 CrossRef CAS.
- E. Fujita, L. R. Furenlid and M. W. Renner, Direct XANES Evidence for Charge Transfer in Co-CO2 Complexes, J. Am. Chem. Soc., 1997, 119(19), 4549–4550 CrossRef CAS.
- T. Zhang and W. Lin, Metal-organic frameworks for artificial photosynthesis and photocatalysis, Chem. Soc. Rev., 2014, 43(16), 5982–5993 RSC.
- C.-C. Wang, Y.-Q. Zhang, J. Li and P. Wang, Photocatalytic CO2 reduction in metal-organic frameworks: A mini review, J. Mol. Struct., 2015, 1083, 127–136 CrossRef CAS.
- Q. Wang, Y. Zhang, H. Lin and J. Zhu, Recent Advances in Metal-Organic Frameworks for Photo-/Electrocatalytic CO2 Reduction, Chem. – Eur. J., 2019, 25(62), 14026–14035 CrossRef CAS PubMed.
- H. Takeda, M. Ohashi, T. Tani, O. Ishitani and S. Inagaki, Enhanced Photocatalysis of Rhenium(I) Complex by Light-Harvesting Periodic Mesoporous Organosilica, Inorg. Chem., 2010, 49(10), 4554–4559 CrossRef CAS PubMed.
- C. Wang, Z. Xie, K. E. deKrafft and W. Lin, Doping Metal-Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis, J. Am. Chem. Soc., 2011, 133(34), 13445–13454 CrossRef CAS PubMed.
- S. Yang, W. Hu, X. Zhang, P. He, B. Pattengale, C. Liu, M. Cendejas, I. Hermans, X. Zhang, J. Zhang and J. Huang, 2D Covalent Organic Frameworks as Intrinsic Photocatalysts for Visible Light-Driven CO2 Reduction, J. Am. Chem. Soc., 2018, 140(44), 14614–14618 CrossRef CAS PubMed.
- J. Zhu, P. M. Usov, W. Xu, P. J. Celis-Salazar, S. Lin, M. C. Kessinger, C. Landaverde-Alvarado, M. Cai, A. M. May, C. Slebodnick, D. Zhu, S. D. Senanayake and A. J. Morris, A New Class of Metal-Cyclam-Based Zirconium Metal–Organic Frameworks for CO2 Adsorption and Chemical Fixation, J. Am. Chem. Soc., 2018, 140(3), 993–1003 CrossRef CAS PubMed.
- R. Nakamura, A. Okamoto, H. Osawa, H. Irie and K. Hashimoto, Design of All-Inorganic Molecular-Based Photocatalysts Sensitive to Visible Light: Ti(IV)-O-Ce(III) Bimetallic Assemblies on Mesoporous Silica, J. Am. Chem. Soc., 2007, 129(31), 9596–9597 CrossRef CAS PubMed.
- W. Kim, G. Yuan, B. A. McClure and H. Frei, Light Induced Carbon Dioxide Reduction by Water at Binuclear ZrOCoII Unit Coupled to Ir Oxide Nanocluster Catalyst, J. Am. Chem. Soc., 2014, 136(31), 11034–11042 CrossRef CAS PubMed.
- W. Kim, E. Edri and H. Frei, Hierarchical Inorganic Assemblies for Artificial Photosynthesis, Acc. Chem. Res., 2016, 49, 1634–1645 CrossRef CAS PubMed.
- W. Zhong, R. Sa, L. Li, Y. He, L. Li, J. Bi, Z. Zhuang, Y. Yu and Z. Zou, A Covalent Organic Framework Bearing Single Ni Sites as a Synergistic Photocatalyst for Selective Photoreduction of CO2 to CO, J. Am. Chem. Soc., 2019, 141(18), 7615–7621 CrossRef CAS PubMed.
- Y.-S. Wei, M. Zhang, R. Zou and Q. Xu, Metal–Organic Framework-Based Catalysts with Single Metal Sites, Chem. Rev., 2020, 120(21), 12089–12174 CrossRef CAS PubMed.
- C.-C. Hou, H.-F. Wang, C. Li and Q. Xu, From metal-organic frameworks to single/dual-atom and cluster metal catalysts for energy applications, Energy Environ. Sci., 2020, 13(6), 1658–1693 RSC.
- L. Ran, Z. Li, B. Ran, J. Cao, Y. Zhao, T. Shao, Y. Song, M. K. H. Leung, L. Sun and J. Hou, Engineering Single-Atom Active Sites on Covalent Organic Frameworks for Boosting CO2 Photoreduction, J. Am. Chem. Soc., 2022, 144(37), 17097–17109 CrossRef CAS PubMed.
- A. Wagner, C. D. Sahm and E. Reisner, Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction, Nat. Catal., 2020, 3(10), 775–786 CrossRef CAS.
- X. Li, L. Liu, X. Ren, J. Gao, Y. Huang and B. Liu, Microenvironment modulation of single-atom catalysts and their roles in electrochemical energy conversion, Sci. Adv., 2020, 6(39), eabb6833 CrossRef CAS PubMed.
- T. L. Soucy, W. S. Dean, J. Zhou, K. E. Rivera Cruz and C. C. L. McCrory, Considering the Influence of Polymer–Catalyst Interactions on the Chemical Microenvironment of Electrocatalysts for the CO2 Reduction Reaction, Acc. Chem. Res., 2022, 55(3), 252–261 CrossRef CAS PubMed.
- Y. Pan, R. Lin, Y. Chen, S. Liu, W. Zhu, X. Cao, W. Chen, K. Wu, W.-C. Cheong, Y. Wang, L. Zheng, J. Luo, Y. Lin, Y. Liu, C. Liu, J. Li, Q. Lu, X. Chen, D. Wang, Q. Peng, C. Chen and Y. Li, Design of Single-Atom Co–N5 Catalytic Site: A Robust Electrocatalyst for CO2 Reduction with Nearly 100% CO Selectivity and Remarkable Stability, J. Am. Chem. Soc., 2018, 140(12), 4218–4221 CrossRef CAS PubMed.
- N. Mohd Adli, W. Shan, S. Hwang, W. Samarakoon, S. Karakalos, Y. Li, D. A. Cullen, D. Su, Z. Feng, G. Wang and G. Wu, Engineering Atomically Dispersed FeN4 Active Sites for CO2 Electroreduction, Angew. Chem., Int. Ed., 2021, 60(2), 1022–1032 CrossRef CAS PubMed.
- F. Pan, H. Zhang, K. Liu, D. Cullen, K. More, M. Wang, Z. Feng, G. Wang, G. Wu and Y. Li, Unveiling Active Sites of CO2 Reduction on Nitrogen-Coordinated and Atomically Dispersed Iron and Cobalt Catalysts, ACS Catal., 2018, 8(4), 3116–3122 CrossRef CAS.
- Y. Wang, J. Mao, X. Meng, L. Yu, D. Deng and X. Bao, Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications, Chem. Rev., 2019, 119(3), 1806–1854 CrossRef CAS PubMed.
- D. Qin, Y. Zhou, W. Wang, C. Zhang, G. Zeng, D. Huang, L. Wang, H. Wang, Y. Yang, L. Lei, S. Chen and D. He, Recent advances in two-dimensional nanomaterials for photocatalytic reduction of CO2: insights into performance, theories and perspective, J. Mater. Chem. A, 2020, 8(37), 19156–19195 RSC.
- X. Lin, S.-F. Ng and W.-J. Ong, Coordinating single-atom catalysts on two-dimensional nanomaterials: A paradigm towards bolstered photocatalytic energy conversion, Coord. Chem. Rev., 2022, 471, 214743 CrossRef CAS.
- Y. Li, T. Kong and S. Shen, Artificial Photosynthesis with Polymeric Carbon Nitride: When Meeting Metal Nanoparticles, Single Atoms, and Molecular Complexes, Small, 2019, 15(32), 1900772 CrossRef PubMed.
- J. Fu, S. Wang, Z. Wang, K. Liu, H. Li, H. Liu, J. Hu, X. Xu, H. Li and M. Liu, Graphitic carbon nitride based single-atom photocatalysts, Front. Phys., 2020, 15(3), 33201 CrossRef.
- M. Zhao, J. Feng, W. Yang, S. Song and H. Zhang, Recent Advances in Graphitic Carbon Nitride Supported Single-Atom Catalysts for Energy Conversion, ChemCatChem, 2021, 13(5), 1250–1270 CrossRef CAS.
- P. Huang, J. Huang, S. A. Pantovich, A. D. Carl, T. G. Fenton, C. A. Caputo, R. L. Grimm, A. I. Frenkel and G. Li, Selective CO2 Reduction Catalyzed by Single Cobalt Sites on Carbon Nitride under Visible-Light Irradiation, J. Am. Chem. Soc., 2018, 140(47), 16042–16047 CrossRef CAS PubMed.
- J. Wang, T. Heil, B. Zhu, C.-W. Tung, J. Yu, H. M. Chen, M. Antonietti and S. Cao, A Single Cu-Center Containing Enzyme-Mimic Enabling Full Photosynthesis under CO2 Reduction, ACS Nano, 2020, 14(7), 8584–8593 CrossRef CAS PubMed.
- Y. Yang, F. Li, J. Chen, J. Fan and Q. Xiang, Single Au Atoms Anchored on Amino-Group-Enriched Graphitic Carbon Nitride for Photocatalytic CO2 Reduction, ChemSusChem, 2020, 13(8), 1979–1985 CrossRef CAS PubMed.
- Y. Li, B. Li, D. Zhang, L. Cheng and Q. Xiang, Crystalline Carbon Nitride Supported Copper Single Atoms for Photocatalytic CO2 Reduction with Nearly 100% CO Selectivity, ACS Nano, 2020, 14(8), 10552–10561 CrossRef CAS PubMed.
- P. Chen, B. Lei, X. A. Dong, H. Wang, J. Sheng, W. Cui, J. Li, Y. Sun, Z. Wang and F. Dong, Rare-Earth Single-Atom La–N Charge-Transfer Bridge on Carbon Nitride for Highly Efficient and Selective Photocatalytic CO2 Reduction, ACS Nano, 2020, 14(11), 15841–15852 CrossRef CAS PubMed.
- S. Ji, Y. Qu, T. Wang, Y. Chen, G. Wang, X. Li, J. Dong, Q. Chen, W. Zhang, Z. Zhang, S. Liang, R. Yu, Y. Wang, D. Wang and Y. Li, Rare-Earth Single Erbium Atoms for Enhanced Photocatalytic CO2 Reduction, Angew. Chem., Int. Ed., 2020, 59(26), 10651–10657 CrossRef CAS PubMed.
- L. Cheng, H. Yin, C. Cai, J. Fan and Q. Xiang, Single Ni Atoms Anchored on Porous Few-Layer g-C3N4 for Photocatalytic CO2 Reduction: The Role of Edge Confinement, Small, 2020, 16(28), 2002411 CrossRef CAS PubMed.
- J. Fu, L. Zhu, K. Jiang, K. Liu, Z. Wang, X. Qiu, H. Li, J. Hu, H. Pan, Y.-R. Lu, T.-S. Chan and M. Liu, Activation of CO2 on graphitic carbon nitride supported single-atom cobalt sites, Chem. Eng. J., 2021, 415, 128982 CrossRef CAS.
- C. Cometto, A. Ugolotti, E. Grazietti, A. Moretto, G. Bottaro, L. Armelao, C. Di Valentin, L. Calvillo and G. Granozzi, Copper single-atoms embedded in 2D graphitic carbon nitride for the CO2 reduction, npj 2D Mater. Appl., 2021, 5(1), 63 CrossRef CAS.
- Z. Zhao, W. Liu, Y. Shi, H. Zhang, X. Song, W. Shang and C. Hao, An insight into the reaction mechanism of CO2 photoreduction catalyzed by atomically dispersed Fe atoms supported on graphitic carbon nitride, Phys. Chem. Chem. Phys., 2021, 23(8), 4690–4699 RSC.
- J.-H. Zhang, W. Yang, M. Zhang, H.-J. Wang, R. Si, D.-C. Zhong and T.-B. Lu, Metal-organic layers as a platform for developing single-atom catalysts for photochemical CO2 reduction, Nano Energy, 2021, 80, 105542 CrossRef CAS.
- P. Sharma, S. Kumar, O. Tomanec, M. Petr, J. Zhu Chen, J. T. Miller, R. S. Varma, M. B. Gawande and R. Zbořil, Carbon Nitride-Based Ruthenium Single Atom Photocatalyst for CO2 Reduction to Methanol, Small, 2021, 17(16), 2006478 CrossRef CAS PubMed.
- N. Pollak, P. Huang, H. Bell, G. Li and C. A. Caputo, Tunable Photocatalytic Production of Syngas Using Co@C3N4 and Black Phosphorus, ChemPhotoChem, 2021, 5(7), 674–679 CrossRef CAS.
- Y. Wang, Y. Qu, B. Qu, L. Bai, Y. Liu, Z.-D. Yang, W. Zhang, L. Jing and H. Fu, Construction of Six-Oxygen-Coordinated Single Ni Sites on g-C3N4 with Boron-Oxo Species for Photocatalytic Water-Activation-Induced CO2 Reduction, Adv. Mater., 2021, 33(48), 2105482 CrossRef CAS PubMed.
- X. Sun, L. Sun, G. Li, Y. Tuo, C. Ye, J. Yang, J. Low, X. Yu, J. H. Bitter, Y. Lei, D. Wang and Y. Li, Phosphorus Tailors the d-Band Center of Copper Atomic Sites for Efficient CO2 Photoreduction under Visible-Light Irradiation, Angew. Chem., Int. Ed., 2022, 61(38), e202207677 CAS.
- H. Ou, S. Ning, P. Zhu, S. Chen, A. Han, Q. Kang, Z. Hu, J. Ye, D. Wang and Y. Li, Carbon Nitride Photocatalysts with Integrated Oxidation and Reduction Atomic Active Centers for Improved CO2 Conversion, Angew. Chem., Int. Ed., 2022, 61(34), e202206579 CrossRef CAS PubMed.
- G. Gao, Y. Jiao, E. R. Waclawik and A. Du, Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide, J. Am. Chem. Soc., 2016, 138(19), 6292–6297 CrossRef CAS PubMed.
- X. Xiao, Y. Gao, L. Zhang, J. Zhang, Q. Zhang, Q. Li, H. Bao, J. Zhou, S. Miao, N. Chen, J. Wang, B. Jiang, C. Tian and H. Fu, A Promoted Charge Separation/Transfer System from Cu Single Atoms and C3N4 Layers for Efficient Photocatalysis, Adv. Mater., 2020, 32(33), 2003082 CrossRef CAS PubMed.
- Y. Cao, S. Chen, Q. Luo, H. Yan, Y. Lin, W. Liu, L. Cao, J. Lu, J. Yang, T. Yao and S. Wei, Atomic-Level Insight into Optimizing the Hydrogen Evolution Pathway over a Co1-N4 Single-Site Photocatalyst, Angew. Chem., Int. Ed., 2017, 56(40), 12191–12196 CrossRef CAS PubMed.
- Z. Chen, S. Mitchell, E. Vorobyeva, R. K. Leary, R. Hauert, T. Furnival, Q. M. Ramasse, J. M. Thomas, P. A. Midgley, D. Dontsova, M. Antonietti, S. Pogodin, N. López and J. Pérez-Ramírez, Stabilization of Single Metal Atoms on Graphitic Carbon Nitride, Adv. Funct. Mater., 2017, 27(8), 1605785 CrossRef.
- F. Yu, T. Huo, Q. Deng, G. Wang, Y. Xia, H. Li and W. Hou, Single-atom cobalt-hydroxyl modification of polymeric carbon nitride for highly enhanced photocatalytic water oxidation: ball milling increased single atom loading, Chem. Sci., 2022, 13(3), 754–762 RSC.
- P. Huang, J. Huang, J. Li, T. D. Pham, L. Zhang, J. He, G. W. Brudvig, N. A. Deskins, A. I. Frenkel and G. Li, Revealing the Structure of Single Cobalt Sites in Carbon Nitride for Photocatalytic CO2 Reduction, J. Phys. Chem. C, 2022, 126(20), 8596–8604 CrossRef CAS.
- J. Li, H. Zhang, W. Samarakoon, W. Shan, D. A. Cullen, S. Karakalos, M. Chen, D. Gu, K. L. More, G. Wang, Z. Feng, Z. Wang and G. Wu, Thermally Driven Structure and Performance Evolution of Atomically Dispersed FeN4 Sites for Oxygen Reduction, Angew. Chem., Int. Ed., 2019, 58(52), 18971–18980 CrossRef CAS PubMed.
- X. Qin, S. Zhu, F. Xiao, L. Zhang and M. Shao, Active Sites on Heterogeneous Single-Iron-Atom Electrocatalysts in CO2 Reduction Reaction, ACS Energy Lett., 2019, 4(7), 1778–1783 CrossRef CAS.
- P. Huang, J. Huang, J. Li, L. Zhang, J. He, C. A. Caputo, A. I. Frenkel and G. Li, Effect of Carbon Doping on CO2-Reduction Activity of Single Cobalt Sites in Graphitic Carbon Nitride, ChemNanoMat, 2021, 7(9), 1051–1056 CrossRef CAS.
- L. Jiang, X. Yuan, Y. Pan, J. Liang, G. Zeng, Z. Wu and H. Wang, Doping of graphitic carbon nitride for photocatalysis: A reveiw, Appl. Catal., B, 2017, 217, 388–406 CrossRef CAS.
- S. Vijay, W. Ju, S. Brückner, S.-C. Tsang, P. Strasser and K. Chan, Unified mechanistic understanding of CO2 reduction to CO on transition metal and single atom catalysts, Nat. Catal., 2021, 4, 1024–1031 CrossRef CAS.
- S. Sato, T. Arai and T. Morikawa, Toward Solar-Driven Photocatalytic CO2 Reduction Using Water as an Electron Donor, Inorg. Chem., 2015, 54(11), 5105–5113 CrossRef CAS PubMed.
- V. Kumaravel, J. Bartlett and S. C. Pillai, Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products, ACS Energy Lett., 2020, 5(2), 486–519 CrossRef CAS.
- H. Takeda, C. Cometto, O. Ishitani and M. Robert, Electrons, Photons, Protons and Earth-Abundant Metal Complexes for Molecular Catalysis of CO2 Reduction, ACS Catal., 2017, 7(1), 70–88 CrossRef CAS.
- C. L. Anfuso, D. Xiao, A. M. Ricks, C. F. A. Negre, V. S. Batista and T. Lian, Orientation of a Series of CO2 Reduction Catalysts on Single Crystal TiO2 Probed by Phase-Sensitive Vibrational Sum Frequency Generation Spectroscopy (PS-VSFG), J. Phys. Chem. C, 2012, 116, 24107–24114 CrossRef CAS.
- A. Ge, B. Rudshteyn, B. T. Psciuk, D. Xiao, J. Song, C. L. Anfuso, A. M. Ricks, V. S. Batista and T. Lian, Surface-Induced Anisotropic Binding of a Rhenium CO2-Reduction Catalyst on Rutile TiO2(110) Surfaces, J. Phys. Chem. C, 2016, 120, 20970–20977 CrossRef CAS.
- X. Li, X. Yang, J. Zhang, Y. Huang and B. Liu, In Situ/Operando Techniques for Characterization of Single-Atom Catalysts, ACS Catal., 2019, 9(3), 2521–2531 CrossRef CAS.
- X. Li, S. Wang, L. Li, Y. Sun and Y. Xie, Progress and Perspective for In Situ Studies of CO2 Reduction, J. Am. Chem. Soc., 2020, 142, 9567–9581 CAS.
- T. Zhang, Z. Chen, A. G. Walsh, Y. Li and P. Zhang, Single-Atom Catalysts Supported by Crystalline Porous Materials: Views from the Inside, Adv. Mater., 2020, 32(44), 2002910 CrossRef CAS PubMed.
- B. Hasa, Y. Zhao and F. Jiao, In Situ/Operando Characterization Techniques of Electrochemical CO2 Reduction, Annu. Rev. Chem. Biomol. Eng., 2023, 14(1), 165–185 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |