 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
POSSaxanes: active-template synthesis of organic–inorganic rotaxanes incorporating cubic silsesquioxane stoppers†
Rafał A.
Grzelczak
 ,
Anna
Władyczyn
,
Anna
Władyczyn
 ,
Agata
Białońska
,
Agata
Białońska
 ,
Łukasz
John
,
Łukasz
John
 * and
Bartosz
Szyszko
* and
Bartosz
Szyszko
 *
*
University of Wrocław, Faculty of Chemistry, 14 F. Joliot-Curie St., 50-383 Wrocław, Poland. E-mail: lukasz.john@uwr.edu.pl; bartosz.szyszko@uwr.edu.pl
First published on 1st June 2023
Abstract
The CuAAC active-template approach was exploited to construct rotaxanes incorporating cage-like silsesquioxane stoppers, namely, POSSaxanes. The compounds were characterized in the solution and solid state, providing the unprecedented molecular structures of POSS-incorporating rotaxanes.
The construction of hybrid systems merging organic and inorganic functionalities has emerged as an exciting approach allowing the formation of molecules and materials with novel functions.1 A unique group of such composites is mechanically interlocked materials that were demonstrated not only to present the features expected of the individual components, but to benefit from the novel, synergistic effects arising from the mechanical bond presence.2 In this context, rotaxanes comprise a group of particularly well-studied, mechanically interlocked molecules, which have demonstrated applications in areas spreading from molecular machines and motors, to stimuli-responsive polymers, and drug delivery systems.3
The alluring class of rotaxanes includes organic–inorganic hybrids, which, depending on the design, contain an inorganic macrocycle,1,4,5 a metal-based host,6 or an axle with the metal cation incorporated into the thread7 as its structural element,8 or as the motif organizing organic ligands into the coordination compound constituting the stopper(s).9,10 The emerging group of mechanically interlocked materials comprises MOFs embedding interlocked components,11 and systems with the thread attached to the metal, or metal oxide nanoparticles,12,13 or graphene.14
Polyhedral Oligomeric Silsesquioxanes (POSSs) are a group of hybrid building blocks of subnanometer and nanometer dimensions exploited for constructing novel materials with tailored functions.15 Within the vast group of cage-like silsesquioxanes, the cubic cages of general formula (RSiO1.5)8 are particularly attractive due to their intrinsic properties and facile functionalization. POSSs have found applications in catalysis, nanomedicine, and materials chemistry, where they were exploited as porous media, components of nanocomposites, and molecular encapsulants.16
Herein we report the facile synthesis of POSS-stoppered rotaxanes, i.e., POSSaxanes, based on the active template CuAAC approach. Active template (AT) synthesis is considered one of the most powerful tools for constructing MIMs. The critical element of the AT method relies upon the metal cation, which serves a dual purpose, functioning both as a template that organizes the components into the threaded architecture and as a catalyst taking part in the formation of the covalent bond resulting in the interlocked structure.17,18 Our interest in exploiting cubic silsesquioxanes for rotaxane synthesis stems from several reasons. The POSS cages are synthetically accessible, allowing for gram scale preparation in a relatively short time, and can be considered nanometer-sized stoppers, whose dimensions can be easily tuned with the appropriate substitution pattern of the silicon-based vertices. Notably, materials functionalized with cage-like silsesquioxanes have demonstrated improved resistance to thermal degradation and oxidative stability.19 Despite the evident benefits of the POSS motif, it was only incidentally exploited for synthesizing polypseudorotaxanes based on the cyclodextrin macrocycle.20,21
The POSSaxanes synthesis was envisaged to exploit the silsesquioxane precursors equipped with a single reactive arm terminated with either alkyne or azide functionality and seven chemically inert (iso-butyl or phenyl) substituents at the cube vertices, providing steric hindrance (Scheme 1). Once reacted in the presence of the macrocycle, these half-axle components were intended to introduce the POSS-stopper(s) into the rotaxane molecule. The essential starting materials for the half-axle syntheses, namely the open cages 1a/b, were either commercially available or could be easily obtained from the reaction of trimethoxysilane S14 with an aqueous solution of sodium hydroxide in THF. The azides 3a/b were synthesized by the corner capping of 1a/b with 3-halopropyl(trichlorosilane), followed by azidation of the initially formed 2a/b. The corner-capping of the commercially available triol 1b with 3-aminopropyl(trimethoxysilane) yielded amine 5b, which upon reaction with 4-propargyloxybenzoic acid chloride 4 in the presence of TEA/DMAP formed alkyne 6b in 74% yield. The alkyne 7 and azide 8 were additionally synthesized to target POSSaxanes stoppered with POSS at one end and a tetrahedral trityl-group at the other.
The crucial step in the rotaxane synthesis was realized through the active template copper(I)-catalyzed azide–alkyne cycloaddition between the half-axle components, namely the POSS-terminated alkyne or azides, in the presence of the macrocyclic compound (Scheme 2). In order to determine the versatility of the developed cage-like stoppers, the POSSaxane syntheses were evaluated by exploiting the bipyridine – A,22 and pyridine-incorporating B macrocycles23 reported by Goldup and Leigh, respectively. The two macrocyclic compounds differ in the incorporated coordinating motif but have cavities of comparable size, as defined by the smallest macrocyclic circuit composed of 26 (A) and 28 (B) atoms.
Three types of rotaxanes were targeted, incorporating the silsesquioxane stopper at the azide or alkyne side of the triazole moiety and those with the POSS groups terminating both ends of the axle (Scheme 2). The conditions developed for bipyridine macrocycle-based POSSaxanes required carrying out the reaction of A with a slight excess (1.2 equiv.) of azide 3a/b/8 and alkyne 6b/7 in THF or DCM in the presence of a catalytic amount (0.25 equiv.) of tetrakis(acetonitrile)copper(I) hexafluorophosphate and diisopropylethylamine (DIPEA, 1 equiv.) for ca. 20 hours. After the reaction workup and chromatographic separation, POSSaxanes 9b-A, 10b-A, and 11b-A, incorporating iso-Bu7-POSS stoppers, were isolated in 51–91% yield. The synthesis of POSSaxanes stoppered with Ph7-POSS groups required harsher conditions, namely carrying out the reaction at elevated temperature (40 °C) and for a longer time (ca. 40 hours). The POSSaxane 10a-A could be isolated following the modified synthetic protocol with a 59% yield.
The synthesis of POSSaxanes based on the pyridine-incorporating macrocycle B required reevaluating the previously developed conditions. In order to improve the yield of target compounds, THF solvent was replaced with poorly coordinating DCM, and the reaction time was elongated to 48–72 hours. Furthermore, a larger (2 equiv.) excess of half-axle components had to be used in some cases. Despite these modifications, the yields of 9b-B, 10b-B, and 11b-B were visibly lower than their bipyridine-incorporating analogs, reaching only 6–26%. In the extreme case of the Ph7-POSS-incorporating 10a-B, only traces of the target compound were detected in the reaction mixture. This result can be rationalized concerning the steric hindrance of the phenyl groups in the Ph7-POSS azide 3a hampering the effective formation of the intermediate copper(I)-azide–alkyne macrocyclic complex.
The formation of 10b-A, which can be considered a representative example of a POSSaxane group, is evident upon comparing its 1H NMR resonances with the spectra of the respective macrocycle A and independently synthesized thread 10b (Fig. 1; see ESI† for others). In particular, the resonances M2, M3, and M4 of the bipyridine unit were slightly up-field shifted from 7.61–7.55, and 7.09 ppm in the spectrum of A to 7.55, 7.35, and 7.03 ppm for 10b-A, respectively. The characteristic triazole CH singlet of the 10b thread underwent a downfield shift from 7.55 ppm to 8.30 ppm in 10b-A, resulting from the CH⋯N hydrogen bonding between the triazole linker of the axle and bipyridine of the macrocycle.24 The incorporation of the POSS stopper was also evident from the presence of the characteristic set of resonances corresponding to the protons of iso-butyl groups at the POSS vertices in the 1H NMR spectrum of 10b-A (Fig. S31, ESI†). In addition, the 29Si NMR spectrum demonstrated four overlapping resonances in the −66.0 to −67.0 ppm region, corresponding to the silicon vertices of the POSS stopper (inset in Fig. 1B).
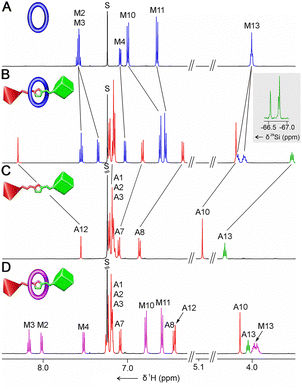 | ||
| Fig. 1 The 1H NMR spectra (CDCl3, 300 K, 500 MHz) of (A) A, (B) 10b-A, (C) 10b, and (D) 10b-A·(TFAH)n. The inset in B demonstrates the 29Si NMR spectrum. The aliphatic region was omitted for clarity. The resonance numbering is shown in Scheme 2. | ||
Remarkably, the molecular structures of two POSSaxanes 10a-A and 10b-A were determined in the solid state by means of XRD studies (Fig. 2). The monocrystals of 10a-A were grown by slow evaporation of THF/hexane solution, whereas those of 10b-A were obtained by diffusion of methanol into the ethyl acetate solution of the rotaxane.
 | ||
| Fig. 2 The X-ray molecular structure of (A) 10a-A and (B) 10b-A. Protons, except for the CH involved in HB, were omitted for clarity. (C) The packing diagram of 10b-A. | ||
The molecular structure of 10a-A demonstrated the rotaxane molecule composed of the macrocycle with the triazole-incorporating axle stoppered with the trityl and Ph7-POSS units (Fig. 2A). The triazole of the thread was located close to the bipyridine of A, resulting in CH⋯N hydrogen bonds of 2.58–2.60 Å length. Although the tetrahedral trityl group provided enough steric hindrance to play a stopper role for the A-based rotaxane, the POSS unit's dimensions are considerably larger. Despite the fact that the Si–O–Si distances within the cage-like silsesquioxane are 3.09–3.11 Å, the substitution of the silicon vertices with phenyl rings resulted in a stopper with impressive subnanometer-to-nanometer dimensions illustrated by the edge length ranging from 7.5–9.5 Å (Cpara-Ph⋯Cpara-Ph) to 8.4–10.8 Å (Hpara-Ph⋯Hpara-Ph). In comparison, the iso-Bu7-POSS stopper in 10b-A demonstrated slightly smaller dimensions, with the edge lengths being in the range of 5.7–9.0 Å (Cpara-Ph⋯Cpara-Ph)/7.5–10.6 Å (Hpara-Ph⋯Hpara-Ph), depending on the orientation of the alkyl groups at the vertices (Fig. 2B). Interestingly, as shown in the packing diagram of 10b-A, the molecules of the POSSaxane are arranged in the crystal network into trityl-macrocycle-POSS⋯POSS-macrocycle-trityl bilayers (Fig. 2C). This organization increased the contact surface area between the iso-butyl groups on one end of the rotaxane molecules and appropriately oriented phenyl rings on the other, enforcing the layer stabilization through intermolecular van der Waals and edge-to-face π–π stacking interactions.
Considering the potential applications of POSSaxanes for constructing new mechanically interlocked materials, their stability in the solution and solid-state was investigated. The prolonged heating of 10b-A in toluene at 100 °C did not result in degradation of the POSS-stopper nor rotaxane dethreading (Fig. S122, ESI†).
The acidification of 10b-A with trifluoroacetic acid yielded cationic 10b-A·(TFAH)n as the sole product, as monitored by 1H NMR titration (Fig. S52, ESI†). The 1H NMR spectrum of 10b-A·(TFAH)n demonstrated the features expected of a cationic rotaxane, with the characteristic up-field shift of the triazole proton resonance from 8.30 ppm (10b-A) to 6.43 ppm, being a result of disruption of the hydrogen bond network upon bipyridine protonation (Fig. 1D). In addition, the macrocycle's M2, M3, and M4 signals were markedly down-field shifted to 8.03, 8.16, and 7.51 ppm. Upon the addition of TEA to the sample of 10b-A·(TFAH)n, the rotaxane 10b-A was restored, with no dethreading or degradation products observed in the solution (Fig. S126, ESI†).
As the cubic silsesquioxane was previously demonstrated to undergo rearrangement reactions in the presence of fluoride anions, the stability of POSSaxanes in the presence of TBAF was investigated.25 Unexpectedly, the gradual increase of fluoride concentration in the solution of 10b-A in DCM-d2 resulted in new resonances (Fig. S123 and S124, ESI†). Upon the addition of 30 equiv. of TBAF, the resonances of the macrocycle A were detected, indicating that the rotaxane underwent dethreading. The MS analysis of the reaction mixture indicated the liberation of the macrocycle A and the formation of several silicon-containing degradation products that could not be unambiguously identified (Fig. S143, ESI†).
Eventually, the thermal stability of POSSaxanes was studied in the solid state employing thermogravimetric analysis (TGA) (Fig. 3, S144–S152, ESI†). All POSSaxanes incorporating the macrocycle A and the reference compound 12-A incorporating two trityl stoppers displayed similar thermal degradation profiles. The temperature values at 5% and 10% of weight loss were evaluated to determine the effect of the POSS moiety's presence. The installment of the POSS stoppers affected the degradation temperature of the rotaxanes. The T5% and T10% of 9b-A and 10b-A were increased to 350/362 °C (9b-A) and 356/368 °C (10b-A), in comparison to 313/347 °C detected for the reference 12-A. At the same time, the rotaxanes incorporating the 6b-originating stopper demonstrated decreased thermal stability, likely due to the presence of the amide functional group. In the case of B-based POSSaxanes, the modification of thermal stability was negligible (Fig. S149–S152, ESI†). Although the effect of the POSS stopper on the thermal stability of some rotaxanes is apparent, the inherent chemical structure of each MIM, in particular the presence of specific functional groups, must be taken into account to design a material with improved thermal stability characteristics.
Facile syntheses of cubic silsesquioxanes and their subnanometer-to-nanometer dimensions encouraged their exploitation to construct novel rotaxanes using the active template approach. The POSS-terminated rotaxanes were obtained with good-to-excellent yields indicating that the vertices-substituted silicon-containing cages can be considered a valuable group of stoppers, especially when the large macrocycles are to be exploited.26 POSSaxanes demonstrated stability in the solution at elevated temperature and under acidic conditions, yet the cleavage of POSS units in the presence of fluoride resulted in dethreading liberating the macrocyclic component. Incorporating the silsesquioxane cage into the rotaxane architecture was demonstrated to improve the thermal stability of the compounds in the solid state, encouraging their exploitation for the construction of novel functional, mechanically interlocked materials. Furthermore, POSSaxanes can be considered a unique group of Polyhedral Oligomeric Silsesquioxanes wherein a considerable part of the molecule is sterically protected by introducing MIM architecture. It is envisaged that such a modification of the POSS framework might influence the accessibility of silicon atoms at the cage corners and face, eventually affecting their reactivity.
The National Science Centre of Poland supported this work upon grant agreement no. 2020/38/E/ST4/00024.
Conflicts of interest
There are no conflicts to declare.Notes and references
- E. K. Brechin and L. Cronin, Angew. Chem., Int. Ed., 2009, 48, 6948–6949 CrossRef CAS PubMed.
- S. Mena-Hernando and E. M. Pérez, Chem. Soc. Rev., 2019, 48, 5016–5032 RSC.
- M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed.
- C.-F. Lee, D. A. Leigh, R. G. Pritchard, D. Schultz, S. J. Teat, G. A. Timco and R. E. P. Winpenny, Nature, 2009, 458, 314–318 CrossRef CAS PubMed.
- G. A. Timco, A. Fernandez, A. K. Kostopoulos, J. F. Charlton, S. J. Lockyer, T. R. Hailes, R. W. Adams, E. J. L. McInnes, F. Tuna, I. J. Vitorica-Yrezabal, G. F. S. Whitehead and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2018, 57, 10919–10922 CrossRef CAS PubMed.
- P. J. Altmann and A. Pöthig, Angew. Chem., Int. Ed., 2017, 56, 15733–15736 CrossRef CAS PubMed.
- Y. Suzaki, T. Taira, K. Osakada and M. Horie, Dalton Trans., 2008, 4823 RSC.
- B. A. Blight, X. Wei, J. A. Wisner and M. C. Jennings, Inorg. Chem., 2007, 46, 8445–8447 CrossRef CAS PubMed.
- S. J. Loeb and J. A. Wisner, Chem. Commun., 1998, 2757–2758 RSC.
- D. J. Hoffart and S. J. Loeb, Angew. Chem., Int. Ed., 2005, 44, 901–904 CrossRef CAS PubMed.
- V. N. Vukotic, K. J. Harris, K. Zhu, R. W. Schurko and S. J. Loeb, Nat. Chem., 2012, 4, 456–460 CrossRef CAS PubMed.
- A. Ulfkjær, F. W. Nielsen, H. Al-Kerdi, T. Ruβ, Z. K. Nielsen, J. Ulstrup, L. Sun, K. Moth-Poulsen, J. Zhang and M. Pittelkow, Nanoscale, 2018, 10, 9133–9140 RSC.
- B. Long, K. Nikitin and D. Fitzmaurice, J. Am. Chem. Soc., 2003, 125, 15490–15498 CrossRef CAS PubMed.
- C. Jia, H. Li, J. Jiang, J. Wang, H. Chen, D. Cao, J. F. Stoddart and X. Guo, Adv. Mater., 2013, 25, 6752–6759 CrossRef CAS PubMed.
- R. M. Laine and M. F. Roll, Macromolecules, 2011, 44, 1073–1109 CrossRef CAS.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
- V. Aucagne, K. D. Hänni, D. A. Leigh, P. J. Lusby and D. B. Walker, J. Am. Chem. Soc., 2006, 128, 2186–2187 CrossRef CAS PubMed.
- M. Denis and S. M. Goldup, Nat. Rev. Chem., 2017, 1, 0061 CrossRef CAS.
- D. Zhang, R. Yang, Z. Qin and W. Zhang, J. Therm. Anal. Calorim., 2023, 148, 2345–2355 CrossRef CAS.
- Y. Ni and S. Zheng, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1247–1259 CrossRef CAS.
- J. Huang, X. Li, T. Lin, C. He, K. Yi Mya, Y. Xiao and J. Li, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 1173–1180 CrossRef CAS.
- J. E. M. Lewis, R. J. Bordoli, M. Denis, C. J. Fletcher, M. Galli, E. A. Neal, E. M. Rochette and S. M. Goldup, Chem. Sci., 2016, 7, 3154–3161 RSC.
- V. Aucagne, J. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, D. A. Leigh, P. J. Lusby, V. E. Ronaldson, A. M. Z. Slawin, A. Viterisi and D. B. Walker, J. Am. Chem. Soc., 2007, 129, 11950–11963 CrossRef CAS PubMed.
- Y. Kawasaki, S. Rashid, K. Ikeyatsu, Y. Mutoh, Y. Yoshigoe, S. Kikkawa, I. Azumaya, S. Hosoya and S. Saito, J. Org. Chem., 2022, 87, 5744–5759 CrossRef CAS PubMed.
- A. R. Bassindale, Z. Liu, I. A. MacKinnon, P. G. Taylor, Y. Yang, M. E. Light, P. N. Horton and M. B. Hursthouse, Dalton Trans., 2003, 2945 RSC.
- H. Lahlali, K. Jobe, M. Watkinson and S. M. Goldup, Angew. Chem., Int. Ed., 2011, 50, 4151–4155 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2249743 and 2249744. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc01706k |
| This journal is © The Royal Society of Chemistry 2023 |



