Open Access Article
Open Access ArticleThe emission efficiency of cationic solid state luminophores is directly proportional to the intermolecular charge transfer intensity†
Kaspars
Leduskrasts
 ,
Artis
Kinens
,
Artis
Kinens
 and
Edgars
Suna
and
Edgars
Suna
 *
*
Latvian Institute of Organic Synthesis, Aizkraukles 21, LV-1006, Riga, Latvia. E-mail: edgars@osi.lv
First published on 17th May 2023
Abstract
Cationic luminophores have recently emerged as a class of efficient emitters in both the solid state and solutions. However, the underlying processes that secure the emission in these luminophores are poorly understood. Here, we employ charge transfer integral (CTI) analysis in combination with X-ray single crystal data to uncover the emission mechanism in a series of pyridinium luminophores. We demonstrate that the solid state photoluminescence quantum yield (Φ) of cationic luminophores is directly proportional to the charge transfer (CT) intensity within a network of molecules in the crystal lattice. Electrostatic intermolecular interactions between π+-systems in the crystal lattice provide a disproportionately high contribution to the CT intensity and therefore are instrumental in achieving high Φ. In addition, the strength of electrostatic interactions can be increased by a through-space (TS) electron-donation strategy. Hence, electrostatic interactions can be utilized as a tool to achieve radiative CT, which is useful in the development of efficient luminophores, sensors and nonlinear optical materials.
Light emitting molecules (luminophores) are key components in various modern technological applications, such as organic light emitting diodes,1 light emitting electrochemical cells,2 organic photovoltaics,3 organic field effect transistors4 and sensors.5 Recent advances in luminophore design have rendered purely organic luminophores competitive or even superior to their inorganic or organometallic counterparts.6 Generally, purely organic luminophores comprise neutral π systems (e.g. phenyl or naphthyl rings)6b,7 and/or charged heteroaromatic subunits (π+-systems such as imidazolium or pyridinium (Py+) cations).8 Luminophores designed exclusively from π-systems are highly efficient in solution; however, they typically lose emission effectiveness in the solid state (SS).9 In contrast, the incorporation of a charged π+-system into a luminophore renders it highly emissive in the SS.8b,10 In particular, Py+-containing luminophores have demonstrated dual emission,11 aggregation induced emission (AIE)8,10,12 and thermally activated delayed fluorescence (TADF) behavior.13 Notably, charged π+-system-containing emitters display high emission efficiency also in solution14 and have found applications in sensing of alkylating reagents15 as well as reversible solid10c and solution state16 sensing of acids.
The high emission intensity of Py+-derived luminophores both in solution and the SS has been attributed to the formation of intermolecular electrostatic π+–π17 and π+–π+ interactions.8b,10a,b,15 However, the light emission mechanism associated with these electrostatic interactions is still poorly understood. We and others have demonstrated recently that the intermolecular π+–π interactions generate TS charge transfer (TSCT) bands in the crystal state.8b,11 In this study we offer quantitative means to evaluate CT intensity in a crystal lattice by calculating the total electronic coupling (Σ|V|) value between the luminophores as described below. We have also shown that that π+–π+ interactions significantly increase Σ|V| and enhance the SS photoluminescence quantum yields (Φ).
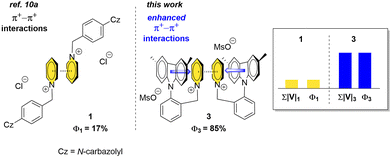
To unravel the role of the π+–π+ interactions in SS emission, we elected to modify a previously published Py+ luminophore 1.10a Specifically, a series of 1 analogues with the benzyl Py+ subunit ortho to the carbazole (2), 3,6-dimethylcarbazole (3) and 3,6-di-tert-butylcarbazole (4) substituents was prepared with mesylate (2a, 3), chloride (2b, 4a) and perchlorate (4b) as the counter ions (Fig. 1 and 2). We envisioned that 2–4 should be capable of forming key π+–π+ interactions in the SS similar to parent 1. We also hypothesized that placing the benzyl Py+ subunit in close spatial proximity18 to the carbazole ring could lead to a TS electron donation from the carbazole to the Py+ moiety and further enhance the strength of the intermolecular π+–π+ interactions in 2–4.
UV-Vis spectra for Py+ salts 2–4 were measured in MeCN solutions at room temperature and under ambient atmosphere at ca. 10−5 M concentration (see ESI,† pages S11–S16). The previously published Py+ salt 1 and the newly synthesized luminophores 2–4 featured four absorption bands at 238–241, 289–294, 321–333 and 334–345 nm (Table 1). Py+ salts 2–4 displayed negligible emission in MeCN solutions with Φ values below 0.1% and emission peaks at 344–357, 352–373 and 597–606 nm. The emission at 340–380 nm was attributed to the locally excited states, while the extremely broad and weak emission at around 600 nm was attributed to the intramolecular TSCT between the carbazole and Py+ moieties in solution. In the solid state, 2–4 displayed intense emission with maxima in the 437–490 nm range (see ESI,† pageS S17–S19). Notably, Py+ salts 2a, 2b, 3 and 4a showed considerably higher SS Φ (43.5, 35.0, 85.2, and 42.3%, respectively), as compared to the published analog 1 (17.3%). Obviously, placement of the benzyl Py+ moiety in close proximity to the carbazole subunit affected strongly the AIE properties of Py+ luminophores.
| Entry | Compound | λ abs, nm | Solution λem, nm | Solid λem, nm | Solution, Φ (%) | Solid, Φ (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 241, 290, 325, 337 | — | 505 | <0.1 | 17.3 |
| 2 | 2a | 238, 289, 321, 334 | 344, 360, 606 | 490 | <0.1 | 43.5 |
| 3 | 2b | 241, 289, 322, 334 | 352, 597 | 473 | <0.1 | 35.0 |
| 4 | 3 | 241, 296, 333, 345 | 357, 373, 602 | 488 | <0.1 | 85.2 |
| 5 | 4a | 240, 294, 328, 340 | 355, 370, 560 | 463, 480 | <0.1 | 42.3 |
| 6 | 4b | 241, 294, 328, 340 | 355, 370, 575 | 437 | <0.1 | 19.1 |
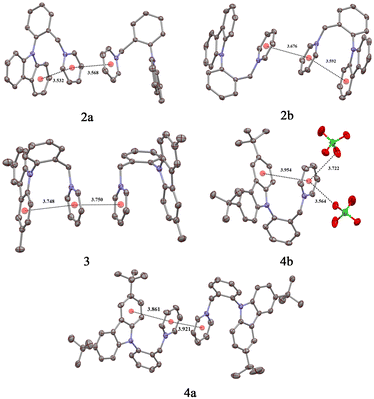
X-Ray crystallography was employed to analyze the mutual orientation of the proximate carbazole and Py+ moieties in the crystal lattices of emitters 2–4.19 Crystals of 2a, 2b, 3 and 4a suitable for X-ray analyses were obtained by Et2O vapor diffusion into their MeCN solutions, while crystallization from water was required for 4b. The emitters crystallized as monoclinic (2a, 2b, and 4a), triclinic (3), and orthorhombic (4b) colorless plates (2a, 4b) and prisms (2b, 3, and 4a) with P21/c (2a, 2b), P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (3), P21/n (4a) and Pbca (4b) space groups. As anticipated, relatively short distances between neighboring Py+ moieties in the crystal lattices of 2a (3.568 Å), 2b (3.676 Å), 3 (3.750 Å) and 4a (3.921 Å) were observed, indicating the presence of intermolecular π+–π+ interactions. However, 4b did not possess close inter-pyridinium contacts. Instead, the Py+ moiety in 4b was engaged in interactions with two perchlorate counter ions as evidenced by the relatively close 3.564 and 3.722 Å distances between the Py+ centroid and the respective oxygen atoms. The lack of SS π+–π+ interactions in 4b is obviously responsible for reduced SS Φ as compared to all other ortho-substituted Py+ salts (2a, 2b, 3, and 4a). This observation provides strong evidence for the importance of π+–π+ interactions in SS emission of 2–4. In the meantime, the large difference in Φ between ortho-substituted luminophores 2a, 2b, 3 and 4a, and parent emitter 1 suggested an involvement of additional favorable interactions in the crystal lattices of 2a, 2b, 3 and 4a. Indeed, a close distance between the carbazole and Py+ moieties was also measured for 2a (3.532 Å), 2b (3.592 Å), 3 (3.748 Å), and 4a (3.861 Å; see Fig. 3). Apparently, the superior Φ values for 2a, 2b, 3, and 4a as compared to 1 are to be attributed to enhancement of the key intramolecular π+–π+ interactions by the TS electron donation from the carbazole moiety to the Py+ ring.
(3), P21/n (4a) and Pbca (4b) space groups. As anticipated, relatively short distances between neighboring Py+ moieties in the crystal lattices of 2a (3.568 Å), 2b (3.676 Å), 3 (3.750 Å) and 4a (3.921 Å) were observed, indicating the presence of intermolecular π+–π+ interactions. However, 4b did not possess close inter-pyridinium contacts. Instead, the Py+ moiety in 4b was engaged in interactions with two perchlorate counter ions as evidenced by the relatively close 3.564 and 3.722 Å distances between the Py+ centroid and the respective oxygen atoms. The lack of SS π+–π+ interactions in 4b is obviously responsible for reduced SS Φ as compared to all other ortho-substituted Py+ salts (2a, 2b, 3, and 4a). This observation provides strong evidence for the importance of π+–π+ interactions in SS emission of 2–4. In the meantime, the large difference in Φ between ortho-substituted luminophores 2a, 2b, 3 and 4a, and parent emitter 1 suggested an involvement of additional favorable interactions in the crystal lattices of 2a, 2b, 3 and 4a. Indeed, a close distance between the carbazole and Py+ moieties was also measured for 2a (3.532 Å), 2b (3.592 Å), 3 (3.748 Å), and 4a (3.861 Å; see Fig. 3). Apparently, the superior Φ values for 2a, 2b, 3, and 4a as compared to 1 are to be attributed to enhancement of the key intramolecular π+–π+ interactions by the TS electron donation from the carbazole moiety to the Py+ ring.
The observed drop in SS Φ in the absence of intermolecular π+–π+ interactions for 4b indicated that an intermolecular process such as CT20 may play a key role in the mechanism of the SS emission for 1–4. The enhanced strength of the π+–π+ interactions in the crystalline framework should increase the electronic communication between luminophores, which can be approximated by the electronic coupling value V.21V is widely utilized for quantifying short range CT for holes and electrons22 by evaluating the HOMO–HOMO and LUMO–LUMO overlaps between neighboring luminophores. Accordingly, we performed a thorough CT integral (CTI) analysis for crystal state emitters 1–4 (see ESI,† pageS S25–S34). All possible CT pathways (CTPs) between a luminophore and all adjacent molecules in the crystal lattice were analyzed for both electron (blue vectors) and hole (red vectors) transfers (Fig. 4A) by Gaussian 09 and CATNIP23 software using geometries obtained from X-ray data.
Perchlorate, chloride and mesylate counter ions in 1–4 were disregarded in the CTI calculations. Thus, it is well known that perchlorates do not form a CT complex with Py+ ions.24 Furthermore, the formation of a CT complex between an anion and a Py+ ion would generate a new CT absorption band (CTAB) both in solution25 and the SS,26 resulting in colored solutions and solid materials. However, both mesylates 2a, 3 and chlorides 2b, 4a form a colorless solution in MeCN and their absorption spectra are similar to that of the perchlorate 4b. In contrast, the addition of TBAI as an iodide source to the colorless solution of perchlorate 4b in EtOAc afforded a yellow-colored solution and the appearance of a new CTAB red-shifted the absorption tail from 410 to 480 nm (see ESI,† Fig. S7). Obviously, the iodide anion engages in CT interactions with Py+ ions in solutions and this is a known phenomenon.24 Likewise, we have reported recently that green- or brown-colored Py+ iodide crystals feature an additional red-shifted CTAB that is absent in SS absorption spectra of the corresponding colorless crystalline perchlorates, mesylates and chlorides.8b,10a,b Overall, our observations conform to the literature24,25 showing the lack of CT between the Py+ ion and perchlorates, chlorides and mesylates. Consequently, the CTI calculations were performed using aromatic cations.
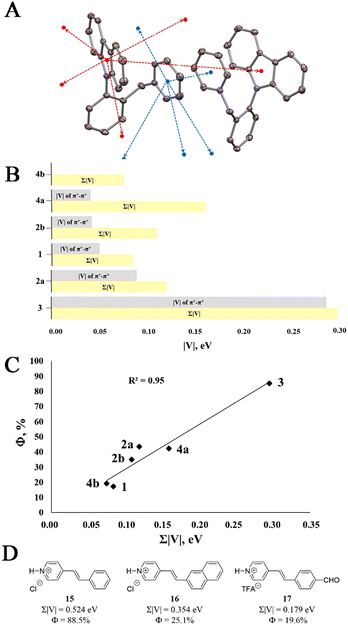
Cationic luminophore 2a features five electron and five hole transfer pathways, whereas 1, 2b, 3, 4a and 4b have five, seven, five, nine and three electron and five, seven, five, nine and three hole transfer pathways, respectively (see ESI,† pageS S25–27). As an illustration, a dimer in the crystal lattice of 2a is characterized by two (one red and one blue) inter-dimer pathways as well as by several other CTPs to adjacent molecules (the adjacent molecules have been omitted for clarity; see Fig. 4A). The effectiveness of each CTP was approximated by the intermolecular electronic coupling value V (see ESI,† page S28). Accordingly, higher absolute values of V (|V|) ensure a more plausible CT.
Having calculated |V| values for each of the CTPs under consideration, we selected the most plausible hole and electron CTPs for 1–4 in the crystal lattice. Specifically, three major electron and three major hole CTPs with the highest |V| values were selected for each luminophore. The sum of the electronic couplings Σ|V| for the six (three for hole and three for electron) major CTPs was calculated to be 0.084, 0.119, 0.109, 0.295, 0.159 and 0.075 eV for 1, 2a, 2b, 3, 4a and 4b, respectively (Fig. 4B and 4C). Notably, the disregarded CTPs contributed less than 3% to the Σ|V|. Among multiple hole and electron CTPs that contributed to Σ|V|, one pathway for each of the luminophores had a disproportionately high |V| value. These pathways were attributed to the intermolecular electrostatic π+–π+ interactions. Hence, the π+–π+ interactions contributed from 0% (in the absence of π+–π+ interactions for 4b) to 25% (in 4a), 38% (in 2b), 59% (in 1), 74% (in 2a) and 96% (in 3) of the total Σ|V| (Fig. 4B). The disproportionately high contribution of the π+–π+ interaction CTP to the overall electronic communications highlights its importance in the crystal state CT processes. Finally, the observed direct proportionality between calculated Σ|V| and Φ values provided additional evidence for the key role of CT in the crystal state emission of 1–4. Thus, the lowest Φ in the 17–20% range for 1 and 4b correlated well with the lowest calculated Σ|V| of 0.084 and 0.075 eV, respectively. The increased Φ for 2b (35.0%), 2a (43.5%) and 4a (42.3%) nicely correlated with the higher Σ|V| in the 0.100–0.160 eV range, while the highest Σ|V| of 0.295 eV corresponded to the most emissive luminophore 3 (85.2%, Fig. 4C). Notably, the observed good correlation (R2 = 0.95) between SS Φ and Σ|V| values also provided evidence that counter ions such as mesylates, chlorides and perchlorates do not directly participate in the SS emission of Py+ luminophores.
To verify the generality of our findings, we applied the SS CTI analysis for a series of highly planar Py+ luminophores 15–17 of completely distinct design10c (see Fig. 4D). Gratifyingly, good correlation between the calculated Σ|V| values and SS Φ was observed for 15–17. Thus, the highest Σ|V| value of 0.524 eV for 15 matched the highest solid state Φ of 88.5%. Likewise, the lowest Σ|V| value of 0.179 eV was calculated for 17, which featured poor Φ (19.6%; see ESI,† pages S35–S37 for details).
In conclusion, CTI analysis in combination with X-ray single crystal data has provided important insight into the emission mechanism of crystalline Py+ salts and revealed the key role of intermolecular π+–π+ interactions in the SS photoluminescence. Thus, SS Φ was shown to be proportional to the TSCT intensity that was quantified by calculating the total electronic coupling (Σ|V|) value between luminophores in the crystal lattice. The π+–π+ interactions provide a disproportionately high (up to 96%) contribution to Σ|V| between luminophore molecules. Hence, the π+–π+ interactions increase Φ for Py+ luminophores by enhancing CT intensity. The CT between Py+ moieties can be strengthened by TS electron donation from the electron-rich carbazole moiety to a Py+ ring. The stronger π+–π+ interactions translated into a significant rise in the SS Φ from 17% to 85%. We believe that our findings on the role of electrostatic (π+–π+) interactions in CT emission phenomena will be instrumental in the design of emitters with outstanding photoluminescence properties both in solution and in the SS.
This work was funded by ERDF project No. 1.1.1.1/18/A/063. We thank Dr S. Belyakov for X-ray crystallographic analysis and M. Baumane for the synthesis of compound 2a.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) Y. Shirota, J. Mater. Chem., 2000, 10, 1–25 RSC; (b) V. Anand, R. Mishra and Y. Barot, Dyes Pigm., 2021, 191, 109390 CrossRef CAS.
- (a) K. Matsuki, P. Jiang and T. Taishi, Adv. Funct. Mater., 2020, 30, 1908641 CrossRef CAS; (b) E. Fresta and R. D. Costa, J. Mater. Chem. C, 2017, 5, 5643–5675 RSC.
- (a) B. C. Thompson and M. J. F. Jean, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed; (b) R. O. Kesinro, A. O. Boyo, M. L. Akinyemi, M. E. Emetere and A. P. Aizebeokhai, IOP Conf. Ser.: Earth Environ. Sci., 2021, 665, 012036 CrossRef.
- (a) S. Kawata and Y. Kawata, Chem. Rev., 2000, 100, 1777–1788 CrossRef CAS PubMed; (b) W. Tang, Y. Huang, L. Han, R. Liu, Y. Su, X. Guo and F. Yan, J. Mater. Chem. C, 2019, 7, 790–808 RSC.
- H.-C. Gee, C.-H. Lee, Y.-H. Jeong and W.-D. Jang, Chem. Commun., 2011, 47, 11963–11965 RSC.
- (a) M. Y. Wong and E. Zysman-Colman, Adv. Mater., 2017, 29, 1605444 CrossRef PubMed; (b) S. Mukherjee and P. Thilagar, Chem. Commun., 2015, 51, 10988–11003 RSC.
- R. Katoh, K. Suzuki, A. Furube, M. Kotani and K. Tokumaru, J. Phys. Chem. C, 2009, 113, 2961–2965 CrossRef CAS.
- (a) P. Xie, Y. Zhou, X. Li, X. Liu, L. Liu, Z. Cao, J. Wang and X. Zheng, Chin. Chem. Lett., 2023, 34, 107582 CrossRef CAS; (b) K. Leduskrasts, A. Kinens and E. Suna, Chem. Commun., 2019, 55, 12663–12666 RSC; G. Zhang, X. Zhang, L. Kong, S. Wang, Y. Tian, X. Tao and J. Yang, Sci. Rep., 2016, 6, 37609 Search PubMed.
- (a) A. S. Klymchenko, Acc. Chem. Res., 2017, 50, 366 CrossRef CAS PubMed; (b) S. A. Jenekhe and J. A. Osaheni, Science, 1994, 265, 765 CrossRef CAS PubMed.
- (a) K. Leduskrasts and E. Suna, RSC Adv., 2019, 9, 460 RSC; (b) K. Leduskrasts and E. Suna, RSC Adv., 2020, 10, 38107 RSC; (c) X. Cui, Y. Hao, W. Guan, L. Liu, W. Shi and C. Lu, Adv. Opt. Mater., 2020, 8, 2000125 CrossRef CAS.
- X.-H. Jin, C. Chen, C.-X. Ren, L.-X. Cai and J. Zhang, Chem. Commun., 2014, 50, 15878–15881 RSC.
- C.-X. Yuan, X.-T. Tao, Y. Ren, Y. Li, J.-X. Yang, W.-T. Yu, L. Wang and M.-H. Jiang, J. Phys. Chem. C, 2007, 111, 12811–12816 CrossRef CAS.
- H.-L. Shen, P.-W. Hsiao, R.-H. Yi, Y.-H. Su, Y. Chen, C.-W. Lu and H.-C. Su, Dyes Pigm., 2022, 203, 110346 CrossRef CAS.
- E. Taipale, N. A. Durandin, J. K. Salunke, N. R. Candeias, T.-P. Ruoko, J. S. Ward, A. Priimagi and K. Rissanen, Mater. Adv., 2022, 3, 1703–1712 RSC.
- W. Chen, S. A. Elfeky, Y. Nonne, L. Male, K. Ahmed, C. Amiable, P. Axe, S. Yamada, T. D. James, S. D. Bull and J. S. Fossey, Chem. Commun., 2011, 47, 253–255 RSC.
- K. Leduskrasts and E. Suna, ChemistryOpen, 2021, 10, 1081–1086 CrossRef CAS PubMed.
- S. Yamada, Coord. Chem. Rev., 2020, 415, 213301 CrossRef CAS.
- The placement of two luminophore fragments in spatial proximity is frequently used to enhance photoluminescence properties: (a) H. Tsujimoto, D.-G. Ha, G. Markopoulos, H. S. Chae, M. A. Baldo and T. M. Swager, J. Am. Chem. Soc., 2017, 139, 4894–4900 CrossRef CAS PubMed; (b) C. Jiang, J. Miao, D. Zhang, Z. Wen, C. Yang and K. Li, Research, 2022, 2022, 1–12 CrossRef; (c) T. Huang, Q. Wang, G. Meng, L. Duan and D. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200059 CAS; (d) D.-R. Bai, X.-Y. Liu and S. Wang, Chem. – Eur. J., 2007, 13, 5713–5723 CrossRef CAS PubMed; (e) Y. Song, M. Tian, R. Yu and L. He, ACS Appl. Mater. Interfaces, 2021, 13, 60269–60278 CrossRef CAS PubMed; (f) H. Miranda-Salinas, Y.-T. Hung, Y.-S. Chen, D. Luo, H.-C. Kao, C.-H. Chang, K.-T. Wong and A. Monkman, J. Mater. Chem. C, 2021, 9, 8819–8833 RSC.
- CCDC 2216260, 2216261, and 2247114–2247116 contains the supplementary crystallographic data for luminophores 2–4.
- C. Schäfer, M. Ruggenthaler, H. Appel and A. Rubio, PNAS, 2019, 116, 4883–4892 CrossRef PubMed.
- (a) A. A. Voityuk, Phys. Chem. Chem. Phys., 2012, 14, 13789 RSC; (b) C.-P. Hsu, Acc. Chem. Res., 2009, 42, 509–518 CrossRef CAS PubMed.
- (a) A. Benny, R. Ramakrishnan and M. Hariharan, Chem. Sci., 2021, 12, 5064–5072 RSC; (b) A. A. Voityuk, N. Rösch, M. Bixon and J. Jortner, J. Phys. Chem. B, 2000, 104, 9740–9745 CrossRef CAS; (c) Z. Q. You and C. P. Hsu, Int. J. Quantum Chem., 2013, 114, 102–115 CrossRef.
- J. S. Brown, Catnip (version 1.9), 2018. Available from https://github.com/JoshuaSBrown/QC_Tools Search PubMed.
- E. M. Kosower and J. C. Burbach, J. Am. Chem. Soc., 1956, 78, 5838–5842 CrossRef CAS.
- Z.-X. Li, C.-H. Xu, W. Sun, Y.-C. Bai, C. Zhang, C.-J. Fang and C.-H. Yan, New J. Chem., 2009, 33, 853 RSC.
- L. Jacob, E. Rzeszotarska, M. Koyioni, R. Jakubowski, D. Pociecha, A. Pietrzak and P. Kaszyński, Chem. Mater., 2022, 34, 6476–6491 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2216260, 2216261, 2247114, 2247115, and 2247116. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc01674a |
| This journal is © The Royal Society of Chemistry 2023 |