Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cost-effective, high-performance Ni3Sn4 electrocatalysts for methanol oxidation reaction in acidic environments†
Danil W.
Boukhvalov
 ab,
Gianluca
D’Olimpio
ab,
Gianluca
D’Olimpio
 c,
Junzhe
Liu
d,
Corneliu
Ghica
e,
Marian Cosmin
Istrate
e,
Chia-Nung
Kuo
f,
Grazia Giuseppina
Politano
g,
Chin Shan
Lue
f,
Piero
Torelli
h,
Lixue
Zhang
c,
Junzhe
Liu
d,
Corneliu
Ghica
e,
Marian Cosmin
Istrate
e,
Chia-Nung
Kuo
f,
Grazia Giuseppina
Politano
g,
Chin Shan
Lue
f,
Piero
Torelli
h,
Lixue
Zhang
 *d and
Antonio
Politano
*d and
Antonio
Politano
 *c
*c
aCollege of Science, Institute of Materials Physics and Chemistry, Nanjing Forestry University, Nanjing 210037, P. R. China
bInstitute of Physics and Technology, Ural Federal University, Mira Str. 19, 620002 Yekaterinburg, Russia
cDepartment of Physical and Chemical Sciences, University of L’Aquila, via Vetoio, 67100 L’Aquila (AQ), Italy. E-mail: antonio.politano@univaq.it
dCollege of Chemistry and Chemical Engineering, Qingdao University, Qingdao 266071, P. R. China. E-mail: zhanglx@qdu.edu.cn
eNational Institute of Materials Physics, Atomistilor 405A, 077125 Magurele, Romania
fDepartment of Physics, National Cheng Kung University, 1 Ta-Hsueh Road, 70101 Tainan, Taiwan
gDepartment of Information Engineering, Infrastructures and Sustainable Energy (DIIES), University “Mediterranea” of Reggio Calabria, Loc. Feo di Vito, 89122 Reggio Calabria, Italy
hCNR-IOM, TASC Laboratory, Area Science Park-Basovizza, 34139 Trieste, Italy
First published on 17th April 2023
Abstract
Methanol (CH3OH) oxidation offers a promising avenue for transitioning to clean energy, particularly in the field of direct methanol fuel cells (DMFCs). However, the development of efficient and cost-effective catalysts for the methanol oxidation reaction (MOR) remains a critical challenge. Herein, we report the exceptional electrocatalytic activity and stability of Ni3Sn4 toward MOR in acidic media, achieving a performance comparable to that of commercial Pt/C catalysts. Our catalyst design incorporates Earth-abundant Ni and Sn elements, resulting in a material that is 1800 times more cost-effective than Pt/C. Density functional theory (DFT) modeling substantiates our experimental findings, shedding light on the favorable reaction mechanisms and kinetics on the Ni3Sn4 surface. Additionally, the as-synthesized Ni3Sn4 electrocatalyst demonstrates commendable durability, maintaining its electrocatalytic activity even after prolonged exposure to harsh acidic conditions.
DMFCs are attractive candidates for transportable power sources due to their superior energy density, safer storage and transportation, and ease of refuelling compared to traditional hydrogen-based fuel cells.1 However, the widespread commercialization of DMFCs has been limited due to the lack of efficient, stable, and cost-effective catalysts for MOR.2–4 Discovering cost-effective and stable catalysts in acidic environments is crucial for the advancement of DMFC technology.
The current state-of-the-art catalysts for MOR are platinum (Pt) and palladium (Pd), which often deliver onset potentials of about 450–550 mV and hold great potential application in DMFCs. However, the high cost of these precious metals is a major barrier to large-scale implementation of MOR catalysts for DMFCs. Many transition metal catalysts have been demonstrated to be active toward MOR in alkaline conditions, but MOR onset potentials of these catalysts are too high to be used in DMFCs.5–7 Furthermore, methanol decomposition on transition-metal surfaces generates poisoning adsorbates (especially CO8), which hinder their catalytic activity. Therefore, developing transition metal-based catalysts with high MOR activity, stability in acid conditions, and superior CO tolerance is of great significance and importance.
Recently, various Ni-based electrocatalysts, including Ni oxides, Fe–Ni nanoparticles, and Ni/Al2O3, have demonstrated impressive MOR performance under alkaline conditions.6,7,9,10 Nonetheless, due to Ni's tendency to dissolve in acidic environments, reports of Ni-based electrocatalysts' MOR activity in acidic media are scarce. While a Ni metal catalyst supported on partially sulfonated polyaniline has exhibited notable MOR activity and stability in acidic condition,11 pure nickel does not perform particularly well as an MOR catalyst. That being said, Ni-based alloys, characterized by their unique electronic structures and anti-corrosion properties,12 hold potential as catalyst candidates for MOR in acidic media.
Among the various Ni-based alloys, Ni–Sn compounds represent potential candidates, especially considering that the incorporation of Sn atoms makes CO adsorption energetically unfavorable, thus totally preventing the methanation reaction.13 Moreover, Ni–Sn compounds are abundant and widely available, making them an attractive alternative to Pt- and Pd-based catalysts from a cost perspective.14–16
Recently, Ni–Sn nanoparticles supported on carbon substrate have been tested for MOR in alkaline solution media, which showed reasonable catalytic performance.15 However, the MOR performance of Ni–Sn compounds in acidic media has not been reported yet. Considering the potential applications in DMFCs, it is highly worthwhile to explore the MOR performance of Ni–Sn alloys with appropriate Ni ratios in acidic conditions, given their commendable corrosion resistance.17,18
In this work, we synthetized Ni3Sn4 and employed a combination of experimental and theoretical techniques to investigate its electrocatalytic properties toward MOR in acid environments. Our findings reveal the exceptional performance of Ni3Sn4, including its high MOR activity, stability, and superior CO tolerance, making it a promising candidate for use in DMFCs.
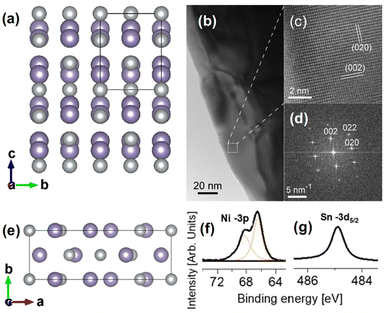
Single crystals of Ni3Sn4 were grown by the solution-growth method (see ESI,† Section S1). Ni3Sn4 (Fig. 1a) belongs to the C2/m [no. 12] (monoclinic unit cell) space group. The X-ray diffraction (XRD in ESI,† Fig. S1 and S2), transmission electron microscopy (TEM, Fig. 1b), HR-TEM (Fig. 1c), and small-area electron diffraction (SAED) experiments (Fig. 1d) confirmed the expected Ni3Sn4 structure (ICSD # 105363), with lattice parameters a = 12.236 Å, b = 4.063 Å, and c = 5.224 Å. The presence of a single doublet in Ni-3p and a single component in Sn-3d5/2 core levels further confirm the presence of a single Ni3Sn4 phase (see also X-ray absorption spectroscopy experiments reported in ESI†).
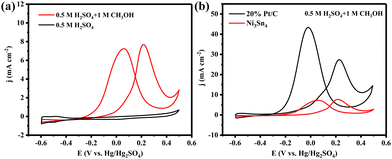
We measured the electrocatalytic activity of the obtained Ni3Sn4 toward MOR in a traditional three-electrode configuration with a scan rate of 50 mV s−1. In Fig. 2a, the oxidation peak at around −0.3 V vs. Hg/Hg2SO4 is well evident in the case of the 0.5 M H2SO4 + 1 M CH3OH mixture, while it is clearly absent in the case of 0.5 M H2SO4 only. This finding confirms the catalytic activity of Ni3Sn4 towards MOR. Fig. 2b shows the performance comparison between Ni3Sn4 and commercial 20% Pt/C in 0.5 M H2SO4 + 1 M CH3OH.
Evidently, Ni3Sn4 exhibits a comparable onset potential to Pt/C, which is also on par with some recently reported advanced Pt-based MOR catalysts.19 This suggests that MOR takes place more readily on Ni3Sn4 (Fig. 2b and Table S1, ESI†).
Taking into account the bulk structure of Ni3Sn4, it is not surprising that it exhibits a smaller current density (∼7.8 mA cm−2) compared to Pt/C (∼43.0 mA cm−2) (Fig. 2b). When normalizing the MOR current by the electrochemical active surface areas, the current density of Ni3Sn4 becomes much closer to that of Pt/C (Fig. S2, ESI†). Intriguingly, an examination of the CV curves in Fig. 2b and Fig. S2 (ESI†) reveals a larger If/Ib ratio (If and Ib are the peak currents of the forward and the backward curves, respectively) for Ni3Sn4 than for Pt/C, suggesting an enhanced CO tolerance for Ni3Sn4.19,20 Subsequently, we performed CO stripping tests on both Ni3Sn4 and commercial Pt/C samples (Fig. S3, ESI†). The onset potential of CO oxidation on the Pt/C is 0.0912 V vs. Hg/Hg2SO4, while the onset potential of CO oxidation on the Ni3Sn4 sample is 0.0178 V vs. Hg/Hg2SO4. This result indicates a superior capability of Ni3Sn4 to remove adsorbed CO compared to Pt/C.
Electrochemical impedance spectroscopy (EIS) was then used to gain some insight into the MOR activity (Fig. S4, ESI†). The fitted Nyquist data shows that the charge transfer resistances (Rct) of Ni3Sn4 and Pt/C are 6394 Ω and 3269 Ω in the mixture of 0.5 M H2SO4 + 1 M CH3OH, respectively. The larger Rct of Ni3Sn4 provides a kinetic explanation for its slightly lower MOR activity compared to Pt/C.
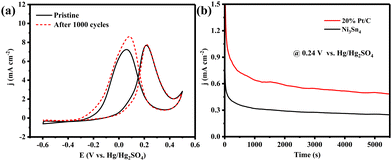
The durability of the Ni3Sn4 is also an indispensable parameter for potential applications. Firstly, the MOR process shows almost no decay after the accelerated electrochemical aging test of 1000 cycles (scan rate of 50 mV s−1, Fig. 3a). Then, we carried out the chronoamperometry at 0.24 V vs. Hg/Hg2SO4 to evaluate the long-time stability. In the chronoamperometric test, Ni3Sn4 displays a more gradual decay process compared to Pt/C (Fig. 3b), which signifies the commendable durability of the as-prepared Ni3Sn4 electrocatalyst. Consequently, it can be concluded that the non-noble metal-based Ni3Sn4 exhibits a Pt-like catalytic performance for MOR in acidic environments, while also offering cost reductions of up to 1800 times, thanks to the substitution of expensive Pt with an alloy composed of Earth-abundant Ni and Sn elements.
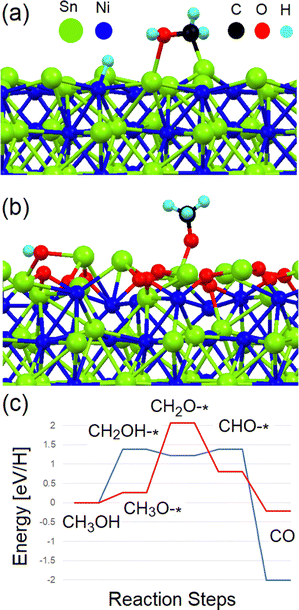
In order to reveal the pathway of MOR over the Ni3Sn4 surface, we performed first-principles simulations. For this purpose, we used density-functional based code Quantum Espresso.21 For the simulation of (010) surface of Ni3Sn4, we used a slab of 126 atoms (see Fig. 4a). Firstly, based on recent literature on transition-metal stannides,22,23 we checked the possible oxidation of the surface. For this purpose, physisorption and decomposition of molecular oxygen on the (010) surface of Ni3Sn4 were assessed. Both these processes are very energetically favourable (−1.4 and −1.1 eV per O2). Further oxidation of whole (010) surface (Fig. 4b) is also a very favourable exothermic process, as indicated by the corresponding energy of −3.65 eV per O2. Thus, modelling of MOR will be performed for two types of surfaces: reduced and oxidized.
In our simulations, we considered the step-by-step migration of hydrogen atoms from methanol to the substrate. The first step of the reaction displays a significant difference between reduced and oxidized Ni3Sn4 surfaces. On the reduced substrate, one hydrogen atom migrates from methyl group to surface Ni-atom, resulting into formation of –OHCH2 group attached to two Sn atoms on the Ni3Sn4 surface (see Fig. 4a). In the case of oxidized substrate, the first step is the formation of the –OCH3 group covalently bonded with Sn atom on substrate surface (Fig. 4b). On the other hand, on the reduced substrate, another hydrogen atom migrates from methyl group to oxygen, resulting into formation of –OHCH2– group to two Sn atoms on the substrate surface (see Fig. 4a). This difference in the reaction pathway determines the difference of the energies of the various steps of the reactions.
Calculations (Fig. 4c) indicate that, in the case of the reduced substrate, the energy cost of intermediate steps is about 1.1–1.3 eV, in qualitative agreement with the experimentally determined overpotential of about 0.9 eV (see Fig. 2 and 3). Note that the calculated energy cost is slightly larger that calculated for Pt-sites in tungsten carbide.24 Contrarily, one of the intermediate steps of MOR over the oxidized surface corresponds to an energy cost above 2 eV, while the first step (Fig. 4b) has a very low energy barrier (0.3 eV). These values are similar to obtained in previous calculations for other substrates.25–28
Additionally, the desorption of atomic hydrogen with the formation of H2 molecules is an exothermic process on the reduced surface (−0.3 eV), in contrast to the case of the oxidized surface. In the latter case, the removal of oxygen atoms is an endothermic process with the energy of about 0.9 eV. To merge results of the simulations with experiment, one can propose that MOR in sulphuric acid corresponds to the deoxygenation of oxidized Ni3Sn4, with following oxidation of methanol over reduced surface. The last step of our simulations is the evaluation of the stability of the Ni3Sn4 surface towards carbon monoxide poisoning. The desorption of CO from oxidized Ni3Sn4 surface is exothermic process with energy of −0.7 eV per CO, while an energy barrier of 0.6 eV per CO exists in the case of the reduced substrate, i.e., a value more than twice smaller than in the cases of the (111) and (100) surfaces of platinum (about 1.2 and 1.4 eV per CO, respectively).29 Thus, the desorption rate of CO is seven times larger for Ni3Sn4 than for platinum.
In conclusion, this study has shown that Ni3Sn4 exhibits a Pt-like catalytic performance for the MOR in acidic media, demonstrating comparable activity and stability to commercial 20% Pt/C. Theoretical simulations provide insights into the pathway of MOR on the Ni3Sn4 surface, highlighting significant differences between reduced and oxidized surfaces. The desorption of CO from the oxidized surface is found to be seven times faster than that of platinum, indicating Ni3Sn4 as a potential alternative to platinum-based catalysts for MOR. Using Ni3Sn4 as a catalyst for MOR can significantly reduce the cost of raw materials compared to platinum-based catalysts, making it a promising candidate for use in various applications, including methanol fuel cells. The findings of this study also suggest the potential of Ni3Sn4 in other catalytic processes and encourage further exploration of its diverse applications.
C. G. and M. C. I. acknowledge funding through contract POC 332/390008/29.12.2020-SMIS 109522. C.-N. K. and C. S. L. acknowledge funding through the National Science and Technology Council of Taiwan under Grant No. 109-2112-M-006-013 and No. 111-2124-M-006-007. J. L. and L. Z. acknowledge funds from the National Natural Science Foundation of China (No. 22075159) and Taishan Scholars Project of Shandong Province (No. tsqn202103058).
Conflicts of interest
There are no conflicts to declare.Notes and references
- Z. Xia, X. Zhang, H. Sun, S. Wang and G. Sun, Nano Energy, 2019, 65, 104048 CrossRef CAS
.
- J. Ding, Y. Liu, A. Li, Q. Chen, P. Dong, J. Mao and X. Wei, Chem. Commun., 2022, 58, 799–802 RSC
.
- X. Gao, J. Zhang, F. Song, Q. Zhang, Y. Han and Y. Tan, Chem. Commun., 2022, 58, 4687–4699 RSC
.
- J. Luo, P. Jiang, D. Wang, X. Yuan, H. Sun, L. Gan, C. Su and Q. Zhang, Chem. Commun., 2022, 58, 4755–4758 RSC
.
- J. Wang, B. Zhang, W. Guo, L. Wang, J. Chen, H. Pan and W. Sun, Adv. Mater., 2023, 2211099 CrossRef PubMed
.
- L. Yaqoob, T. Noor and N. Iqbal, Int. J. Energy Res., 2021, 45, 6550–6583 CrossRef CAS
.
- A. Yuda, A. Ashok and A. Kumar, Catal. Rev., 2022, 64, 126–228 CrossRef CAS
.
- R. Parsons and T. VanderNoot, J. Electroanal. Chem. Interfacial, 1988, 257, 9–45 CrossRef CAS
.
- S. L. Candelaria, N. M. Bedford, T. J. Woehl, N. S. Rentz, A. R. Showalter, S. Pylypenko, B. A. Bunker, S. Lee, B. Reinhart and Y. Ren, ACS Catal., 2017, 7, 365–379 CrossRef CAS
.
- Y. Wang, W. Chen, D. Pan, Q. Xu, J. Ma, J. Zheng and R. Li, Int. J. Electrochem. Sci., 2017, 12, 2194–2206 CrossRef CAS
.
- S. Das, K. Dutta and P. P. Kundu, J. Mater. Chem. A, 2015, 3, 11349–11357 RSC
.
- A. Mishra, Acta Metall. Sin. (Engl. Lett.), 2017, 30, 306–318 CrossRef CAS
.
- M. Fan, Y. Xu, J. Sakurai, M. Demura, T. Hirano, Y. Teraoka and A. Yoshigoe, Int. J. Hydrogen Energy, 2015, 40, 12663–12673 CrossRef CAS
.
- J. Li, Z. Luo, F. He, Y. Zuo, C. Zhang, J. Liu, X. Yu, R. Du, T. Zhang and M. F. Infante-Carrió, J. Mater. Chem. A, 2018, 6, 22915–22924 RSC
.
- J. Li, Z. Luo, Y. Zuo, J. Liu, T. Zhang, P. Tang, J. Arbiol, J. Llorca and A. Cabot, Appl. Catal., B, 2018, 234, 10–18 CrossRef CAS
.
- N. A. Barakat, F. S. Al-Mubaddel, M. R. Karim, M. Alrashed and H. Y. Kim, Int. J. Hydrogen Energy, 2018, 43, 21333–21344 CrossRef CAS
.
- E. W. Brooman, Met. Finish., 2001, 99, 100–102 CrossRef CAS
.
- C. Wan, X. Liu and J. Ye, Surf. Coat. Technol., 2019, 369, 244–251 CrossRef CAS
.
- Z. Li, X. Jiang, X. Wang, J. Hu, Y. Liu, G. Fu and Y. Tang, Appl. Catal., B, 2020, 277, 119135 CrossRef CAS
.
- H. Sun, L. Qi, X. Jiang, G. Fu, L. Xu, D. Sun, Z. Gu and Y. Tang, New J. Chem., 2017, 41, 8812–8817 RSC
.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, L. Michele, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed
.
- D. W. Boukhvalov, A. Marchionni, J. Filippi, C. N. Kuo, J. Fujii, R. Edla, S. Nappini, G. D'Olimpio, L. Ottaviano, C. S. Lue, P. Torelli, F. Vizza and A. Politano, J. Mater. Chem. A, 2020, 8, 2349–2355 RSC
.
- D. W. Boukhvalov, C.-N. Kuo, S. Nappini, A. Marchionni, G. D’Olimpio, J. Filippi, S. Mauri, P. Torelli, C. S. Lue, F. Vizza and A. Politano, ACS Catal., 2021, 11, 7311–7318 CrossRef CAS
.
- T. Sheng, X. Lin, Z.-Y. Chen, P. Hu, S.-G. Sun, Y.-Q. Chu, C.-A. Ma and W.-F. Lin, Phys. Chem. Chem. Phys., 2015, 17, 25235–25243 RSC
.
- J. R. B. Gomes and J. A. N. F. Gomes, Surf. Sci., 2001, 471, 59–70 CrossRef CAS
.
- S. Sakong and A. Gross, J. Phys. Chem. A, 2007, 111, 8814–8822 CrossRef CAS PubMed
.
- S. Liu, P. Jin, D. Zhang, C. Hao and X. Yang, Appl. Surf. Sci., 2013, 265, 443–451 CrossRef CAS
.
- C.-Q. Lv, C. Liu and G.-C. Wang, Catal. Commun., 2014, 45, 83–90 CrossRef CAS
.
- V. Pramhaas, M. Roiaz, N. Bosio, M. Corva, C. Rameshan, E. Vesselli, H. Grönbeck and G. Rupprechter, ACS Catal., 2021, 11, 208–214 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc01623d |
| This journal is © The Royal Society of Chemistry 2023 |