Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CaFeFeNbO6 – an iron-based double double perovskite†‡
K.
Ji
 a,
J. R.
Bedward
a,
J. R.
Bedward
 a,
Q.
Li
a,
P.
Manuel
b,
C.
Ritter
c and
J. Paul
Attfield
a,
Q.
Li
a,
P.
Manuel
b,
C.
Ritter
c and
J. Paul
Attfield
 *a
*a
aCentre for Science at Extreme Conditions (CSEC) and School of Chemistry, The University of Edinburgh, EH9 3FD, UK. E-mail: j.p.attfield@ed.ac.uk
bSTFC Rutherford Appleton Lab, ISIS Facility, Harwell Science and Innovation Campus, Didcot, OX11 0QX, UK
cInstitut Laue-Langevin, 38042 Grenoble Cedex, France
First published on 1st May 2023
Abstract
Ordering of cations is important for controlling properties of ABO3 perovskites, and CaFeFeNbO6 is the first example of an Fe-based AA’BB’O6 double double perovskite, with Ca2+/Fe2+ ordered on A-site columns, and Fe3+/Nb5+ at the octahedral B-sites. Substantial (37%) antisite disorder of the latter cations leads to spin glassy magnetism below a freezing transition at 12 K. The CaMnFeNbO6 analogue also shows substantial cation disorder and spin glassy behaviour. Comparison of synthesis pressures for ordered materials based on different A-site transition metals, suggests that pressures of at least 14–18 GPa will be required to discover the expected plethora of double double perovskites based on A’ cations smaller than Mn2+.
Cation ordering within ABO3 perovskite oxides generates new structures and emergent physical properties,1 most famously in B-cation ordered A2BB’O6 double perovskites where rock-salt ordering of B/B’ transition metal cations2–4 leads to ferrimagnetism and large magnetoresistance in Sr2FeMoO6 and related materials.5,6 A variety of AA’B2O6 double perovskites based on columnar A-cation ordering has also been discovered through high pressure synthesis. This was first reported in CaFeTi2O6, synthesised at 12–15 GPa,7 and several series based on A-site ordering of rare-earth (R) and Mn cations with the same P42/nmc double perovskite structure; e.g. RMn(Ga0.5Ti0.5)2O6 and RMn(Mn,Ti)2O6, have subsequently been reported from syntheses at lower pressure (6 GPa).8–11 CaMnTi2O6 and CaMn(Ti,V)2O6 derivatives have a symmetry-lowered P42mc double perovskite structure where loss of an inversion centre gives rise to ferroelectricity.12,13 Combining the columnar A-site order with 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 order of B-cations gives rise to AA’BB’O6 double double perovskites. RMn3O6 (NaNbO3-type, Pmmn) materials have columnar R/Mn A-site and layered Mn charge-ordering at the B-sites,14 but double perovskites based on orders of different metals have a tetragonal P42/n structure with columnar A/A’ and rocksalt type B/B’ orders.
1 order of B-cations gives rise to AA’BB’O6 double double perovskites. RMn3O6 (NaNbO3-type, Pmmn) materials have columnar R/Mn A-site and layered Mn charge-ordering at the B-sites,14 but double perovskites based on orders of different metals have a tetragonal P42/n structure with columnar A/A’ and rocksalt type B/B’ orders.
Many P42/n AA’BB’O6 double double perovskites with A’ = Mn have been synthesised at 10 GPa; RMnMnB’O6 (B’ = Sb15,16 and Ta17) and CaMnBB’O6 (B = Mn, Fe, Co, Ni for B’ = Re,18–20 and B/B’ = Fe/Ta,21 Cr/Sb, Fe/Sb22 and Mn/W23); and recently the first non-Mn analogue CaCuFeReO6 was made at 15.5 GPa.24 The CaA’FeReO6 (A’ = Mn, Mn0.5Cu0.5, Cu) materials are notable as ferrimagnets with Curie temperatures above 500 K and varying magnetoresistance properties.18,24 Recovery of CaCuFeReO6 from 15.5 GPa suggests that many further AA’BB’O6 double double perovskites based on other A’ metals may be accessible at pressures above 10 GPa. A’ = Fe is a likely possibility given the columnar cation ordering in double perovskite CaFeTi2O6. A-site Fe2+ perovskites are of interest given the predominance of (Mg, Fe)SiO3 perovskite in earth's lower mantle, and may have notable magnetic properties such as the unusual multiple-sublattice Fe2+ spin order recently reported in CaFe3Ti4O12,25 which has 1Ca2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3Fe2+ A-site order, but such recovered materials are rare. We report here high pressure exploration of CaFeFeNbO6 and the Mn-comparator CaMnFeNbO6, leading to the discovery that both form P42/n double double perovskites although with significant B-cation disorder leading to spin-glass ground states.
3Fe2+ A-site order, but such recovered materials are rare. We report here high pressure exploration of CaFeFeNbO6 and the Mn-comparator CaMnFeNbO6, leading to the discovery that both form P42/n double double perovskites although with significant B-cation disorder leading to spin-glass ground states.
Samples of ideal composition CaMFeNbO6 (M = Mn, Fe) were treated under high pressure and high temperature conditions in a Walker-type multianvil press. The precursor mixture comprised stoichiometric amounts of Ca2Fe2O5 (obtained by heating a pellet of CaCO3 and Fe2O3 at 1200 °C for 24 h under an N2 flow), MO (M = Mn, Fe) and Nb2O5. The mixture was packed into a Pt capsule, compressed to the synthesis pressure, heated over 10 minutes to the synthesis temperature, held at this temperature for 30 minutes, quenched to room temperature, and decompressed to ambient pressure. For M = Mn, reactions were performed at 10–12 GPa and 1000–1200 °C, and the optimum synthesis conditions were found to be 10 GPa and 1200 °C. Reactions were performed at 10–14 GPa and 1000–1400 °C for M = Fe, and the optimum synthesis conditions were found to be 14 GPa and 1400 °C. Laboratory powder X-ray diffraction (PXD) patterns were collected using a D2 Bruker diffractometer and powder neutron diffraction (PND) data were measured at the WISH beamline of the ISIS facility,26 and on diffractometer D20 at ILL. Magnetization was measured using an MPMS SQUID magnetometer.
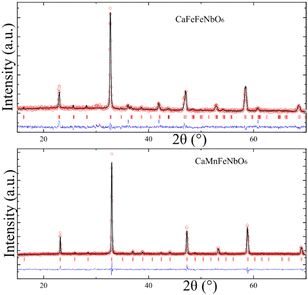
Powder X-ray diffraction showed that both CaMFeNbO6 (M = Mn, Fe) compositions form a majority perovskite-structured phase at the optimum conditions above. However, secondary phases were present and were not eliminated in repeated syntheses. Fits to the PXD data (Fig. 1) demonstrate that both products are consistent with the P42/n double double perovskite structure, although X-ray scattering does not discriminate well between different superstructure models and PND refinements were used to resolve these issues. Further results for CaMnFeNbO6 are shown in ESI‡ Fig. S1–S7.
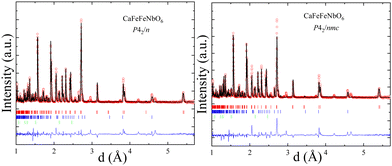
Fits to high resolution time-of-flight PND data for the CaFeFeNbO6 sample show that the main phase is a cation-ordered perovskite, but CaFeO3−x perovskite, FeO and unidentified impurity are also observed. A-cation occupancies were varied in initial refinements but no disorder or off-stoichiometry was found, in keeping with the very different respective coordination numbers of 10 and 4 at Ca and Fe sites. The PND data do not show a clear superstructure peak from Fe/Nb B-site ordering (expected at d = 4.4 Å), so refinements of P42/nmc double perovskite (CaFe(Fe0.5Nb0.5)2O6 where B-cations are fully disordered) and P42/n double double perovskite (CaFeFeNbO6 with partial or full Fe/Nb order) models were compared, making use of the good Fe/Nb neutron contrast (b = 9.54/7.05 fm), as shown in Fig. 2. Comparison of the fits by eye and through their residuals shows that the double double perovskite model gives a substantially better fit, so this is taken to be the best structural description. Refinement results are shown in Table 1 and Fig. 3.
| Atom, Wyckoff site | x | y | z | Biso (Å2) | Occ |
|---|---|---|---|---|---|
| Ca, 4e | 0.2500 | 0.7500 | 0.7791(1) | 1.5(2) | 1 |
| A1(Fe), 2a | 0.7500 | 0.7500 | 0.7500 | 1.6(1) | 1 |
| A2(Fe), 2b | 0.2500 | 0.2500 | 0.7500 | 1.6 | 1 |
| B1(Fe/Nb), 4c | 0.0000 | 0.5000 | 0.5000 | 1.6 | 0.37(3)/0.63 |
| B2(Fe/Nb), 4d | 0.0000 | 0.0000 | 0.5000 | 1.6 | 0.63/0.37 |
| O1, 8g | −0.065(1) | 0.548(1) | 0.243(1) | 1.4(1) | 1 |
| O2, 8g | −0.248(2) | −0.043(1) | 0.575(1) | 1.4 | 1 |
| O3, 8g | −0.227(1) | 0.059(1) | −0.039(1) | 1.4 | 1 |
| Ca–O1(x2) | 2.685(8) | B1–O1(x2) | 2.08(1) | ||
| Ca–O1(x2) | 2.877(6) | B1–O2(x2) | 2.03(1) | ||
| Ca–O2(x2) | 2.508(6) | B1–O3(x2) | 1.82(1) | ||
| Ca–O3(x2) | 2.486(8) | <B1–O> | 1.97(1) | ||
| Ca–O3(x2) | 2.359(8) | B2–O1(x2) | 1.97(1) | ||
| <Ca–O> | 2.583(8) | B2–O2(x2) | 2.02(1) | ||
| A1–O2(x4) | 2.084(5) | B2–O3(x2) | 2.17(1) | ||
| A2–O1(x4) | 2.104(8) | <B2–O> | 2.05(3) |
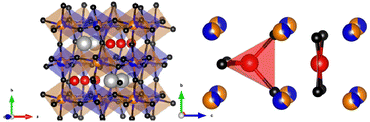
CaFeFeNbO6 is confirmed as having the P42/n double double perovskite structure, and so is the first reported Fe-based material in this family. Results in Table 1 show a substantial (37%) Fe/Nb antisite disorder at the B-sites. A similar (34%) antisite disorder was reported in the double perovskite Ca2FeNbO6,27 and no long range Fe/Nb cation order was found in a recent neutron diffraction study of Sr2FeNbO6.28 Disorder between Fe3+ and Nb5+ accounts for the lack of a clear superstructure peak at d = 4.4 Å for CaFeFeNbO6, and likely reflects their similar ionic radii (0.645 and 0.64 Å respectively) and a formal charge difference of only 2. Synthesis at higher pressure or lower temperature might reduce the disorder. Despite their similar radii, the average B1–O = 1.97 Å distance for the Nb-rich site is smaller than B2–O = 2.05 Å for the Fe-rich site. This may reflect disordered off-centre second-order Jahn–Teller distortions for d0 Nb5+ that reduce the crystallographic average distance. The average Ca–O distance of 2.58 Å is shorter than the expected value of 2.61 Å from ionic radii, whereas the A-site Fe–O distances of 2.08 and 2.10 Å are longer than expected values of 2.01 and 2.02 Å for tetrahedral and square planar coordinations, repectively. This reveals lattice strain from ordering large Ca2+ and small Fe2+ cations in alternate A-site columns of the perovskite framework.
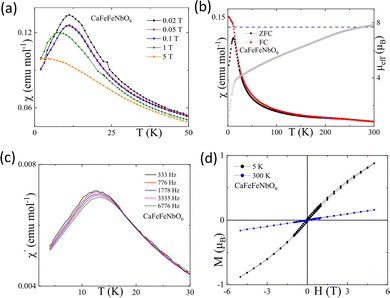
Magnetisation data for the CaFeFeNbO6 sample are shown in Fig. 4. The susceptibility χ in Fig. 4a reveals paramagnetic behaviour, and the effective magnetic moment per CaFeFeNbO6 unit (calculated as μeff = 2.83√(χT)) at high temperatures (>250 K) is close to the theoretical spin-only value of 7.7 μB for high spin Fe2+ and Fe3+ cations, hence confirming the mixed Fe-valency in CaFe2+Fe3+Nb5+O6. However, the inverse susceptibility plot (shown in ESI,‡ Fig. S8) does not show a linear (Curie–Weiss) variation over the paramagnetic regime, most likely due to impurity contributions. A broad susceptibility peak is observed at 12 K with divergence of zero-field cooled (ZFC) and field-cooled (FC) susceptibilities. This peak shifts to low temperature as applied field increases (Fig. 4b), and to higher temperature with increasing frequency in ac measurements (Fig. 4c). These susceptibility variations, and also the slightly non-linear and hysteretic magnetisation-field loop at 5 K (Fig. 4d), are all characteristic of a spin-glass, and further analysis of the ac susceptibility peak shift is shown in ESI,‡ Fig. S9.
Comparison of variable temperature powder neutron patterns for the CaFeFeNbO6 sample in Fig. 5 show two peaks that appear on cooling at long-d and so are most likely magnetic in origin. A prominent magnetic intensity appears on the peak at d = 4.59 Å from the CaFeO3−x impurity on cooling from 300 to 150 K. The magnetic transition is thus above that of stoichiometric CaFeO3−x (120 K)29 but below that of the O-vacancy ordered brownmillerite CaFeO2.5 (730 K),30 consistent with the presence of O-vacancies in this impurity. A weak second peak at d = 4.93 Å arises between 150 and 2 K. This could be associated with the 12 K magnetic transition in CaFeFeNbO6 but the peak does not index on the P42/n cell or simple supercells. As spin order in previous studied P42/n double double perovskites is usually ferrimagnetic and commensurate with the unit cell, and would give rise to strong magnetic diffraction peaks for high spin Fe2+ and Fe3+ cations, it is more likely that the d = 4.9 Å peak is due to spin ordering in an impurity phase.
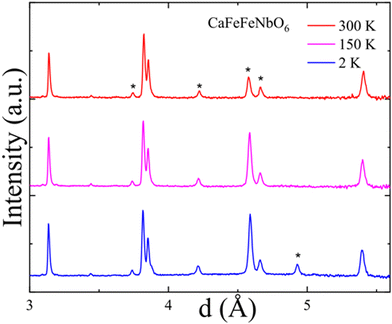 | ||
| Fig. 5 Comparison of long-d powder neutron patterns for the CaFeFeNbO6 sample at several temperatures with impurity peaks marked*. | ||
The magnetisation results discussed above plus the lack of clear magnetic diffraction peaks show that CaFeFeNbO6 is a spin-glass below freezing temperature Tf ≈ 12 K. This can be attributed to the high degree of disorder between magnetic Fe3+ and non-magnetic Nb5+ cations over the B-sites. Spin glass ground states were also reported for CaMnFeTaO6 which has substantial Fe/Ta disorder,21 and in Sr2FeNbO627 and many other cation-disordered double perovskites. The substantial Fe/Nb disorder in CaFeFeNbO6 also masks potentially interesting magnetic and conducting properties related to Fe2+/Fe3+ charge transfer which could lead to ferromagnetic double exchange as found in magnetite.
Results for the CaMFeNbO6 (M= Mn) analogue shown in ESI,‡ are very similar to those for M = Fe. CaMnFeNbO6 adopts the P42/n double double perovskite structure with a substantial (29%) Fe/Nb disorder at the B-sites, and also has 16% substitution of Fe for Mn at the A’ sites, so the phase has overall stoichiometry CaMn0.84Fe1.16NbO6. CaMnFeNbO6 shows paramagnetic behaviour at high temperatures and has a magnetic susceptibility peak at 27 K that shifts to higher temperature with increasing frequency in ac measurements. This and a lack of any magnetic diffraction peaks down to 2 K evidence a spin glass ground state.
CaFeFeNbO6 is the first reported AA’BB’O6P42/n double double perovskite based on A’ = Fe. As many A/A’ columnar-ordered perovskites are based on A’ = Mn but few with other metals, it is useful to compare synthesis pressures for analogue pairs; CaA’Ti2O6, A’ = Mn (7 GPa)12 and Fe (14–15 GPa);7 CaA’FeReO6, A’ = Mn (10 GPa)18 and Cu (15.5 GPa);24 and here CaMFeNbO6; A’ = Mn (10 GPa) and Fe (14 GPa). The extra synthesis pressures for other A’ cations relative to Mn2+ are in the range 4–8 GPa implying that further analogues of known AMnBB’O6 double double perovskites (synthesised at 10 GPa) may be accessible at pressure of 14 to 18 GPa for A’ = Fe, Cu and other divalent cations of a similar size. It is also notable that CaFeTi2O6 was initially synthesised at 14–15 GPa, but crystals were subsequently grown at 12 GPa using water as a mineraliser, so pressures for growth of new double double perovskites may be lowered by a few GPa in the same way.
In conclusion, CaFeFeNbO6 is the first reported example of a P42/n AA’BB’O6 double double perovskite based on A’ = Fe. The synthesised samples are stoichiometric with Fe2+/Fe3+ mixed valence, however, potentially interesting magnetic properties related to charge transfer are masked by antisite Fe/Nb disorder that leads to a spin glass ground state below a freezing transition at 12 K. The CaMnFeNbO6 analogue also shows substantial cation disorder and spin glassy behaviour. Comparison of optimum synthesis pressures for the latter and other pairs of ordered materials based on different A-site transition metals, suggests that pressures of at least 14–18 GPa will be required to discover the expected plethora of double double perovskites based on cations smaller than Mn2+.
We thank EPSRC for support, and STFC for provision of access to ISIS and ILL.
Conflicts of interest
There are no conflicts to declare.References
- G. King and P. M. Woodward, J. Mater. Chem., 2010, 20, 5785–5796 RSC; M. Shaikh, A. Fathima, M. J. Swamynadhan, H. Das and S. Ghosh, Chem. Mater., 2021, 33, 1594–1606 CrossRef CAS; A. Ghosh, G. Palanichamy, D. P. Trujillo, M. Shaikh and S. Ghosh, Chem. Mater, 2022, 34, 7563–7578 CrossRef.
- M. T. Anderson, K. B. Greenwood, G. A. Taylor and K. R. Poeppelmeier, Prog. Solid State Chem., 1993, 22, 197–233 CrossRef CAS.
- C. J. Howard, B. J. Kennedy and P. M. Woodward, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 463–471 CrossRef PubMed.
- S. Vasala and M. Karppinen, Prog. Solid State Chem., 2015, 43, 1–36 CrossRef CAS.
- K.-I. Kobayashi, T. Kimura, H. Sawada, K. Terakura and Y. Tokura, Nature, 1998, 395, 677–680 CrossRef CAS.
- D. Serrate, J. De Teresa and M. Ibarra, J. Phys.: Condens. Matter, 2006, 19, 023201 CrossRef.
- K. Lienenweber and J. Parise, J. Solid State Chem., 1995, 114, 277–281 CrossRef.
- G. Shimura, K. Niwa, Y. Shirako and M. Hasegawa, Eur. J. Inorg. Chem., 2017, 835–839 CrossRef CAS.
- A. A. Belik, L. Zhang, R. Liu, D. D. Khalyavin, Y. Katsuya, M. Tanaka and K. Yamaura, Inorg. Chem., 2019, 58, 3492–3501 CrossRef CAS PubMed.
- R. Liu, D. D. Khalyavin, N. Tsunoda, Y. Kumagai, F. Oba, Y. Katsuya, M. Tanaka, K. Yamaura and A. A. Belik, Inorg. Chem., 2019, 58, 14830–14841 CrossRef CAS PubMed.
- R. Liu, R. Scatena, D. D. Khalyavin, R. D. Johnson, Y. Inaguma, M. Tanaka, Y. Matsushita, K. Yamaura and A. A. Belik, Inorg. Chem., 2020, 59, 9065–9076 CrossRef CAS PubMed.
- A. Aimi, D. Mori, K.-I. Hiraki, T. Takahashi, Y. J. Shan, Y. Shirako, J. Zhou and Y. Inaguma, Chem. Mater., 2014, 26, 2601–2608 CrossRef CAS.
- M. Fukuda, T. Nishikubo, Z. Pan, Y. Sakai, M.-H. Zhang, S. Kawaguchi, H. Yu, Y. Okimoto, S.-Y. Koshihara, M. Itoh, J. Rödel and M. Azuma, Inorg. Chem., 2020, 59, 11749–11756 CrossRef CAS PubMed.
- L. Zhang, Y. Matsushita, K. Yamaura and A. A. Belik, Inorg. Chem., 2017, 56, 5210–5218 CrossRef CAS PubMed.
- E. Solana-Madruga, Á. M. Arévalo-López, A. J. Dos Santos-García, E. Urones-Garrote, D. Ávila-Brande, R. Sáez-Puche and J. P. Attfield, Angew. Chem., Int. Ed., 2016, 55, 9340–9344 CrossRef CAS PubMed.
- E. Solana-Madruga, Á. M. Arévalo-López, A. J. Dos santos-García, C. Ritter, C. Cascales, R. Sáez-Puche and J. P. Attfield, Phys. Rev. B, 2018, 97, 134408 CrossRef CAS.
- K. Ji, Y. Yuan, G. T. Moyo, C. Ritter and J. P. Attfield, J. Solid State Chem., 2022, 313, 123329 CrossRef CAS.
- G. M. McNally, Á. M. Arévalo-López, P. Kearins, F. Orlandi, P. Manuel and J. P. Attfield, Chem. Mater., 2017, 29, 8870–8874 CrossRef CAS.
- G. M. McNally, A. M. Arévalo-López, F. Guillou, P. Manuel and J. P. Attfield, Phys. Rev. Mater., 2020, 4, 064408 CrossRef CAS.
- E. Solana-Madruga, Y. Sun, Á. M. Arévalo-López and J. P. Attfield, Chem. Commun., 2019, 55, 2605–2608 RSC.
- P. Kearins, E. Solana-Madruga, K. Ji, C. Ritter and J. P. Attfield, J. Phys. Chem. C, 2021, 125, 9550–9555 CrossRef CAS.
- E. Solana-Madruga, P. S. Kearins, K. N. Alharbi, C. T. Lennon, C. Ritter and J. P. Attfield, Phys. Rev. Mater., 2021, 5, 054412 CrossRef CAS.
- K. Ji, K. N. Alharbi, E. Solana-Madruga, G. T. Moyo, C. Ritter and J. P. Attfield, Angew. Chem., Int. Ed., 2021, 133, 22422 CrossRef.
- E. Solana-Madruga, P. S. Kearins, C. Ritter, A. M. Arévalo-López and J. P. Attfield, Angew. Chem., Int. Ed., 2022, 61, e202209497 CrossRef CAS PubMed.
- M. A. Patino, F. D. Romero, M. Goto, T. Saito, F. Orlandi, P. Manuel, A. Szabó, P. Kayser, K. H. Hong, K. N. Alharbi, J. P. Attfield and Y. Shimakawa, Phys. Rev. Res., 2021, 3, 043208 CrossRef.
- L. C. Chapon, P. Manuel, P. G. Radaelli, C. Benson, L. Perrott, S. Ansell, N. J. Rhodes, D. Raspino, D. Duxbury, E. Spill and J. Norris, Neutron News, 2011, 22, 22–25 CrossRef.
- A. R. Chakhmouradian and R. H. Mitchell, J. Solid State Chem., 1998, 138, 272–277 CrossRef CAS.
- N. Kashima, K. Inoue, T. Wada and Y. Yamaguchi, Appl. Phys. A: Mater. Sci. Process., 2002, 74(Suppl.), S805–S807 CrossRef CAS.
- F. Kanamaru, H. Miyamoto, Y. Mimura, M. Koizumi, M. Shimada, S. Kume and S. Shin, Mater. Res. Bull., 1978, 13, 61–66 CrossRef.
- M. Ceretti, A. Piovano, A. Cousson, T. Berthier, M. Meven, G. Agostini, J. Schefer, O. Hernandez, C. Lamberti and W. Paulus, CrystEngComm, 2012, 14, 5771–5776 RSC.
Footnotes |
| † Data that support the findings of this study have been deposited at https://datashare.is.ed.ac.uk/handle/10283/838. |
| ‡ Electronic supplementary information (ESI) available: Supporting figures and tables. See DOI: https://doi.org/10.1039/d3cc01115a |
| This journal is © The Royal Society of Chemistry 2023 |