Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anomalous glucose-responsive rheological changes in a boronic acid-modified hyaluronan†
Ryotaro
Miki
 *,
Tsutomu
Yamaki
,
Masaki
Uchida
and
Hideshi
Natsume
*,
Tsutomu
Yamaki
,
Masaki
Uchida
and
Hideshi
Natsume
Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, 1-1 Keyakidai, Sakado, Saitama 350-0295, Japan. E-mail: rmiki@josai.ac.jp; Fax: +81-49-271-7052; Tel: +81-49-271-7052
First published on 11th April 2023
Abstract
Herein, we report anomalous glucose (Glc)-responsive gelation/solation in 3-aminophenylboronic acid-modified hyaluronic acid. With 5–20 mM Glc, gelation occurred, resulting in the formation of crosslinks via Glc, which could reversibly bind to the two boronic acid sites. Solation was induced at Glc concentrations of >80 mM.
In situ gelation materials that gelate in response to body conditions upon injection have the potential to be applied in drug delivery systems,1 regenerative medicine,2 and wound dressings.3 Reportedly, in situ gelation materials gelate in response to temperature, pH shifts, or ions.4 As hyaluronic acid (HA) has high biocompatibility and utility in biomaterial research,5,6 it can act as a base polymer in in situ gelation materials. Millimolar concentrations of glucose (Glc) exist in the subcutaneous interstitial fluid,7,8 and Glc is a prospective target stimulus for in situ gelation materials.
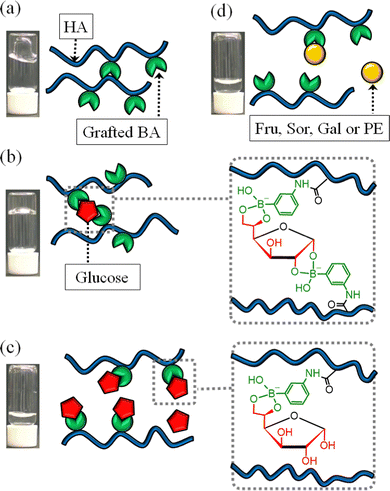
Phenylboronic acid (PBA) acts as a diol-sensor of polyols such as Glc by reversibly forming cyclic ester bonds with cis-diol (Fig. 1a).9,10 Therefore, PBA has been studied as a sugar sensor for smart insulin release systems11 and for various applications in analytical chemistry.12,13 Although various Glc-responsive rheological changes in PBA-modified polymers have been reported, these polymers show only unidirectional rheological shifts (e.g. solation with increasing Glc concentration).14–18 Glc was predominantly present as glucopyranose, with only small quantities of glucofuranose present in an equilibrium state.19 The glucofuranose form can bind two boronic acid moieties as it has two binding sites (Fig. 1b).19 Holz et al. reported that 3-aminophenylboronic acid (BA) modified HA (BA–HA) gelates in response to Glc in basic conditions (pH 10).20 However, the Glc concentration-dependent gelation/solation phenomenon has not been reported. Herein, we report unique Glc-responsive rheological changes, in which BA–HA turns into gel/sol with Glc using an injectable viscoelastic fluid.
BA–HA was synthesised using a slightly modified version of a previously reported method (Fig. 1c).20 BA was modified into sodium hyaluronate (50–110 kDa) via condensation with 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM). The degree of substitution was determined using 1H NMR21 based on the ratio of the integrated intensity of phenyl protons of BA (7.1–7.7 ppm, –C6H4) to that of HA methyl proton (at 1.85 and 1.40 ppm, –CH3). The degree of substitution of BA with HA was 21.8% per repeating unit (Fig. S1, ESI†). Samples of 12 mg mL−1 BA–HA were prepared using 0.2 M sodium carbonate buffer (pH 10.5) as a solvent. The polyols Glc, galactose (Gal), pentaerythritol (PE), fructose (Fru), and sorbitol (Sor) were added to the BA–HA solution (Fig. S2, ESI†). Gal22,23 and PE24–26 have the potential to gelate when added to BA–HA to form crosslinks, as these polyols can potentially bind two PBA moieties.
To study the gelation/solation behaviours, the visual appearance of the samples was observed. Native HA or BA–HA with/without polyols (1.02 mL of 12 mg mL−1) was added to glass vials, which were then inverted (Fig. 2a and b). The native HA dropped rapidly, whereas the BA–HA without polyols flowed downwards slowly. This indicates that modifying BA with HA increases its viscoelasticity, which derives from the self-crosslinking resulting from cyclic ester bonds between the PBA moieties and diol of HA.20,21 BA–HA without polyols could be injected through a 26G injection needle (outer diameter: 0.45 mm, inner diameter: 0.23 mm) (Fig. 2c). BA–HA without polyols could not be picked up using a pipette tip (Movie S1, ESI†). However, BA–HA with 5 mM Glc gelated and did not flow downward (Fig. 2b). Moreover, it could be picked up using a pipette tip, and it could be pulled from both sides with tweezers without breaking (Fig. 2d and e, Movie S2, ESI†). The BA–HA with 40 mM Glc flowed downward slowly, whereas the BA–HA with 80 mM Glc and 5 mM Gal, PE, Fru, and Sor dropped rapidly (Fig. 2b). Interestingly, the BA–HA became a gel or sol, depending on the Glc concentration. Gelation occurred selectively with Glc and not with the other polyols.
To study the polyol-responsive rheological properties of BA–HA, dynamic viscoelasticities were measured (Fig. 3a). The storage modulus (G′) of the BA–HA without polyols was higher than the loss modulus (G′′) above 2.6 rad s−1. The G′ of the BA–HA with 5 mM Glc was higher than the G′′ above 0.26 rad s−1, and higher than that of the BA–HA without polyols at all frequencies, indicating that the gel properties of the sample with 5 mM Glc were stronger than those of the samples without polyols. Although the G′ of BA–HA with 80 mM Glc was higher than the G′′ above 1.2 rad s−1, it was lower than that of BA–HA without polyols at all frequencies, indicating that the gel properties of the 80 mM Glc sample were weaker than those of the samples without polyols. To study the Glc concentration dependence of BA–HA, G′ and G′′ at 10 rad s−1 was plotted against the concentration (Fig. 3b). Interestingly, G′ increased in a Glc concentration-dependent manner up to 10 mM Glc and decreased thereafter. The G′ of the 40 mM Glc was lower than that of the sample without polyols (below the G′ line without polyols). To study the concentration dependence of the other polyols, G′ at 10 rad s−1 was plotted against the concentrations of Gal, PE, Fru, and Sor (Fig. 3c). Polyols other than Glc significantly decreased G′ in a concentration-dependent manner unlike in the case of Glc. The order of effect on decreasing G′ at 1.2 mM polyols was Sor > Fru > PE > Gal. This order almost corresponds to that of the binding constants of PBA with polyols at pH 7.4,9,10 suggesting that polyols other than Glc form a cyclic ester bond with one PBA moiety, which competitively inhibits cross-linking between the PBA moiety and the diol of HA.
We propose the mechanism of the rheological changes in BA–HA caused by the addition of polyols. Without polyols, the PBA moiety self-crosslinks20,21via a cyclic ester bond with the diol of HA because the viscoelasticity of BA–HA is clearly higher than that of native HA, and the addition of polyols such as Sor effectively decreased the viscoelasticity (Fig. 4a). In BA–HA with 5–20 mM Glc, gelation was induced to form crosslinks via Glc, which could reversibly bind to two PBA sites (Fig. 4b). The actual abundance ratio of α-D-glucofuranose and α-D-glucopyranose is unknown. Although only α-D-glucofuranose is depicted in Fig. 4b, a certain amount of α-D-glucopyranose was considered to be present. However, at Glc concentrations above 40 mM, Glc was predominantly bound to one PBA moiety, which competitively inhibited cross-linking, which led to solation and decreases in viscoelasticity (Fig. 4c). Such Glc-dependent changes in the binding mode have been reported in previous PBA-containing densely crosslinked-hydrogels that do not undergo a sol–gel transition.27–29 Those gels show a unique Glc-responsive shrinking and swelling. In these studies, the complex with boronic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 2
Glc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 predominantly formed at low Glc concentrations, whereas that with boronic acid
1 predominantly formed at low Glc concentrations, whereas that with boronic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 1
Glc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 predominantly formed at high Glc concentrations. Taking those studies into consideration, we infer that the results of this study are similar; boronic acid and Glc form a 2
1 predominantly formed at high Glc concentrations. Taking those studies into consideration, we infer that the results of this study are similar; boronic acid and Glc form a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex up to a certain Glc concentration. At higher Glc concentrations, boronic acid and Glc form a 1
1 complex up to a certain Glc concentration. At higher Glc concentrations, boronic acid and Glc form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. Polyols other than Glc (Gal, PE, Fru, and Sor) bind with one PBA moiety at lower concentrations than Glc, which inhibits cross-linking (Fig. 4d). Notably, even though Gal22,23 and PE24–26 have two potential binding sites for PBA moieties, Gal and PE function as monovalent ligands, and no cross-link is formed.
1 complex. Polyols other than Glc (Gal, PE, Fru, and Sor) bind with one PBA moiety at lower concentrations than Glc, which inhibits cross-linking (Fig. 4d). Notably, even though Gal22,23 and PE24–26 have two potential binding sites for PBA moieties, Gal and PE function as monovalent ligands, and no cross-link is formed.
In conclusion, we demonstrated that BA–HA viscoelastic fluid at pH 10.5 gelates at mild Glc concentrations (5 mM = 90 mg dL−1) corresponding to normal fasting blood Glc levels, and BA–HA solates in a Glc concentration-dependent manner at Glc concentrations above 80 mM (1440 mg dL−1). This is the first report describing a rheological shift from viscoelastic fluid to gel to sol in a Glc concentration-dependent manner by PBA-modified polymers. Such Glc-responsive rheological behaviour is useful for smart insulin release systems, where gelation in the subcutaneous interstitial fluid may allow a slow insulin release at normal blood Glc levels, hence enabling diabetes patients to autonomously avoid hypoglycaemia. At high blood Glc levels, the blood sugar level smoothly decreases via rapid insulin release in a Glc concentration-dependent manner. The development of this concept into an innovative diabetes treatment requires future research to address the preparation of viscoelastic HA exhibiting a gel/sol transition in a Glc concentration-dependent manner under physiological pH conditions using boronic acid derivatives with lower pKa.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Correa, A. K. Grosskopf, H. Lopez Hernandez, D. Chan, A. C. Yu, L. M. Stapleton and E. A. Appel, Chem. Rev., 2021, 121, 11385–11457 CrossRef CAS PubMed
.
- P. Bertsch, M. Diba, D. J. Mooney and S. C. G. Leeuwenburgh, Chem. Rev., 2023, 123, 834–873 CrossRef CAS PubMed
.
- H. Montazerian, E. Davoodi, A. Baidya, S. Baghdasarian, E. Sarikhani, C. E. Meyer, R. Haghniaz, M. Badv, N. Annabi, A. Khademhosseini and P. S. Weiss, Chem. Rev., 2022, 122, 12864–12903 CrossRef CAS PubMed
.
- M. Agrawal, S. Saraf, S. Saraf, S. K. Dubey, A. Puri, U. Gupta, P. Kesharwani, V. Ravichandiran, P. Kumar, V. G. M. Naidu, U. S. Murty, Ajazuddin and A. Alexander, J. Controlled Release, 2020, 327, 235–265 CrossRef CAS PubMed
.
- X. Zheng, B. Wang, X. Tang, B. Mao, Q. Zhang, T. Zhang, J. Zhao, S. Cui and W. Chen, Carbohydr. Polym., 2023, 299, 120153 CrossRef CAS PubMed
.
- X. Hou, D. Zhong, H. Chen, Z. Gu, Q. Gong, X. Ma, H. Zhang, H. Zhu and K. Luo, Carbohydr. Polym., 2022, 292, 119662 CrossRef CAS PubMed
.
- M. S. Boyne, D. M. Silver, J. Kaplan and C. D. Saudek, Diabetes, 2003, 52, 2790–2794 CrossRef CAS PubMed
.
- N. Elbarbary, O. Moser, S. Al Yaarubi, H. Alsaffar, A. Al Shaikh, R. A. Ajjan and A. Deeb, Diabetes Vasc. Dis. Res., 2021, 18, 1–12 Search PubMed
.
- G. Springsteen and B. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS
.
- J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769–774 CrossRef CAS
.
- J. Wang, Z. Wang, J. Yu, A. R. Kahkoska, J. B. Buse and Z. Gu, Adv. Mater., 2020, 32, 1902004 CrossRef CAS PubMed
.
- X. Sun and T. D. James, Chem. Rev., 2015, 115, 8001–8037 CrossRef CAS PubMed
.
- X. Sun, W. Zhai, J. S. Fossey and T. D. James, Chem. Commun., 2016, 52, 3456–3469 RSC
.
- S. H. Hong, S. Kim, J. P. Park, M. Shin, K. Kim, J. H. Ryu and H. Lee, Biomacromolecules, 2018, 19, 2053–2061 CrossRef CAS PubMed
.
- T. Figueiredo, Y. Ogawa, J. Jing, V. Cosenza, I. Jeacomine, J. D. M. Olsson, T. Gerfaud, J. G. Boiteau, C. Harris and R. Auzély-Velty, Polym. Chem., 2020, 11, 3800–3811 RSC
.
- Y. Xiang, S. Xian, R. C. Ollier, S. Yu, B. Su, I. Pramudya and M. J. Webber, J. Controlled Release, 2022, 348, 601–611 CrossRef CAS PubMed
.
- Y. Kotsuchibashi, R. V. C. Agustin, J. Y. Lu, D. G. Hall and R. Narain, ACS Macro Lett., 2013, 2, 260–264 CrossRef CAS PubMed
.
- M. Lin, P. Sun, G. Chen and M. Jiang, Chem. Commun., 2014, 50, 9779–9782 RSC
.
- X. Wu, Z. Li, X. X. Chen, J. S. Fossey, T. D. James and Y. B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048 RSC
.
- E. Holz and K. Rajagopal, Macromol. Chem. Phys., 2020, 221, 2000055 CrossRef CAS
.
- M. Li, X. Shi, B. Yang, J. Qin, X. Han, W. Peng, Y. He, H. Mao, D. Kong and Z. Gu, Carbohydr. Polym., 2022, 296, 119953 CrossRef CAS PubMed
.
- X. Wu, X. X. Chen, M. Zhang, Z. Li, P. A. Gale and Y. B. Jiang, Chem. Commun., 2016, 52, 6981–6984 RSC
.
- T. Hashimoto, M. Kumai, M. Maeda, K. Miyoshi, Y. Tsuchido, S. Fujiwara and T. Hayashita, Front. Chem. Sci. Eng., 2020, 14, 53–60 CrossRef CAS
.
- X. Qiu, Q. Cui, Q. Guo, T. Zhou, X. Zhang and M. Tian, Small, 2022, 18, 2107164 CrossRef CAS PubMed
.
- F. Zhao, A. Dong, L. Deng, R. Guo and J. Zhang, Polym. Chem., 2019, 10, 2436–2446 RSC
.
- A. Ozawa, A. Shimizu, R. Nishiyabu and Y. Kubo, Chem. Commun., 2015, 51, 118–121 RSC
.
- C. Zhang, M. D. Losego and P. V. Braun, Chem. Mater., 2013, 25, 3239–3250 CrossRef CAS
.
- J. Zhao, P. Liu and Y. Liu, Langmuir, 2018, 34, 7479–7487 CrossRef CAS PubMed
.
- V. L. Alexeev, A. C. Sharma, A. V. Goponenko, S. Das, I. K. Lednev, C. S. Wilcox, D. N. Finegold and S. A. Asher, Anal. Chem., 2003, 75, 2316–2323 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc01020a |
| This journal is © The Royal Society of Chemistry 2023 |