Open Access Article
Open Access ArticleCombining ligand-enhanced backdonation and steric shielding to stabilize a mono-substituted Au(I) carbene†
David
Vesseur
a,
Karinne
Miqueu
 b and
Didier
Bourissou
b and
Didier
Bourissou
 *a
*a
aLaboratoire Hétérochimie Fondamentale et Appliquée, Université Paul Sabatier/CNRS UMR 5069, 118 Route de Narbonne, Toulouse Cedex 09 31062, France. E-mail: didier.bourissou@univ-tlse3.fr
bCNRS/Université de Pau et des Pays de l’Adour, E2S UPPA, Institut des Sciences Analytiques et de Physico-Chimie pour l’Environnement et les Matériaux (IPREM, UMR 5254), Hélioparc, 2 Avenue du Président Angot, Pau Cedex 09 64053, France
First published on 11th April 2023
Abstract
A mono-substituted Au(I) carbene was prepared by reacting HC(N2)(Dmp) (Dmp = 2,6-dimesitylphenyl) with an (o-carboranyl)-diphosphine AuNTf2 complex. It is stable up to −10 °C and was characterized by NMR spectrocopy. According to DFT calculations, the chelating P^P ligand enhances Au → Ccarb backdonation, while the Dmp substituent provides kinetic stabilization but does not bias the electronic structure of the carbene complex.
Many catalytic transformations involve gold(I) carbene complexes as key intermediates and these species attract considerable attention.1 Important synthetic efforts have been devoted to the preparation and characterization of [LnAu
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C<]+ complexes2 with various substitution patterns at the carbenic center (with and without heteroelements) and various ancillary ligands at gold (from phosphines, N-heterocyclic carbenes to diphosphines). See Chart 1 for representative examples of recently isolated and/or spectroscopically characterized compounds. The nature of the AuCcarb bonds also raises fundamental questions, with the cationic gold carbene Au+
C<]+ complexes2 with various substitution patterns at the carbenic center (with and without heteroelements) and various ancillary ligands at gold (from phosphines, N-heterocyclic carbenes to diphosphines). See Chart 1 for representative examples of recently isolated and/or spectroscopically characterized compounds. The nature of the AuCcarb bonds also raises fundamental questions, with the cationic gold carbene Au+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< and metala-carbocationic (carbenoid) Au–C<+ structures as limit canonical forms.3 Most of the gold(I) carbene complexes reported so far are disubstituted, but in 2019, Echavarren et al. were able to observe monosubstituted gold(I) carbenes by NMR at −90 °C.4 Here, gold bears JohnPhos-type ligands and the carbene center is substituted by a mesityl (Mes) group. These species are stable only until −70 °C, but display rich reactivity.5
C< and metala-carbocationic (carbenoid) Au–C<+ structures as limit canonical forms.3 Most of the gold(I) carbene complexes reported so far are disubstituted, but in 2019, Echavarren et al. were able to observe monosubstituted gold(I) carbenes by NMR at −90 °C.4 Here, gold bears JohnPhos-type ligands and the carbene center is substituted by a mesityl (Mes) group. These species are stable only until −70 °C, but display rich reactivity.5
 | ||
Chart 1 Representative examples of recently isolated and/or spectroscopically characterized [LnAu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C<]+ complexes. C<]+ complexes. | ||
Over the past few years, our group has demonstrated that (o-carboranyl)-diphosphines are very efficient in enhancing backdonation from gold (thanks to P^P chelation) and stabilizing disubstituted gold(I) carbenes.2c,6 In this work, we envisioned to combine this approach with kinetic stabilization by a sterically demanding substituent. Hereafter, we report our first results along this line. A monosubstituted gold(I) carbene stable up to −10 °C has been prepared and characterized by multi-nuclear NMR spectroscopy. Its bonding situation was analysed computationally.
As sterically demanding aryl substituent, we choose the 2,6-dimesitylphenyl (Dmp) moiety which has been successfully employed by Hillhouse et al. to prepare low coordinate Ni complexes, (NHC)Ni(Dmp) and (P^P)Ni = CH(Dmp) species in particular.7,8 The 2,6-dimesitylphenyl diazomethane HC(N2)Dmp was prepared in 4 steps from mesityl bromide and 1,3-dichlorobenzene in an overall yield of 32%.9 The reported synthesis8a was modified and improved by replacing the highly toxic hydrazine and mercuric oxide for tosylhydrazine and sodium hydride.
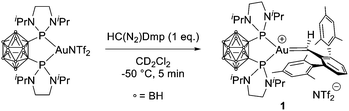
Adding the pseudo-cationic (P^P)AuNTf2 complex10 to HC(N2)Dmp at −50 °C in dichloromethane resulted in a quick colour change from pale yellow-orange to deep purple (Scheme 1).
The monosubstituted carbene 1 was characterized by low-temperature multi-nuclear NMR spectroscopy (at −80 °C).9 A single peak at δ 141.3 ppm was observed in the 31P NMR spectrum, indicating a clean and quantitative reaction. The 1H NMR spectrum shows a triplet at δ 13.36 ppm (JHP = 16.6 Hz) for the AuCH(Dmp) moiety (Fig. 1). This is approximately 1 ppm downfield shifted compared to the corresponding signal reported previously for the (JohnPhos)AuCH(Mes)+ complexes (δ 11.74–12.67 ppm).4 The 13C NMR resonance at δ 295.6 ppm (triplet, JCP = 85.6 Hz) is also very diagnostic for the carbene complex 1. It matches well with those found for the monosubstituted carbenes (δ 284.6–290.0 ppm).4 The correlation observed between the carbenic carbon and proton signals upon 1H–13C HSQC analysis further confirms the assignment. The 1H and 13C resonances associated with the Dmp moiety were assigned thanks to HSQC and HMBC experiments. The two mesityl groups were found to be inequivalent (two sets of signals were observed), indicating hindered rotation around the Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)(Dmp) bond.
C(H)(Dmp) bond.
The thermal stability of 1 was then investigated. No colour change was observed when the (P^P)AuNTf2 complex was added to HC(N2)Dmp at −80 °C. When the solution was warmed, the characteristic purple colour appeared at ca. −60 °C and then disappeared at ca. −10 °C. A variable temperature NMR monitoring was then carried out. A freshly prepared sample of 1 was analysed from −80 °C to room temperature by intervals of 10 °C.9 Accordingly, carbene 1 was found to survive up to −10 °C and to degrade completely within 5 minutes at 0 °C. The thermal stability of complex 1 substantiates the combined effect of Au → Ccarb backdonation (enhanced by the P^P ligand) and steric shielding (imparted by the Dmp substituent). The (JohnPhos)AuCH(Mes)+ complexes could be spectroscopically characterized at low temperature, but they were shown to decompose at temperatures between −90 and −70 °C.4
Despite our efforts, attempts to grow crystals suitable for XRD remained unsuccessful. To gain more insight into the structure and bonding situation of the (P^P)Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(Dmp)+ complex, Density Functional Theory (DFT) calculations were carried out. The actual compound was considered, without simplification of the substituents, but the counter-anion was omitted. It is referred hereafter as A-Dmp. The B3PW91/SDD+f(Au), 6-31G** (other atoms) level of theory was used to enable comparison with related complexes. The optimized structure (Fig. 2, left) shows a tricoordinate gold complex (the two P atoms chelate the metal with a bite angle close to 90°). The carbene center sits in a planar environment which substantially deviates from trigonal due to the dissymmetric substitution pattern. The AuCcarbH bond angle is contracted while the AuCcarbCipso bond angle is widened at ca. 108 and 144°, respectively (Table 1). The carbene plane is about perpendicular to the Au coordination plane, to minimize steric repulsions and maximize Au → Ccarb backdonation. Conversely, the phenyl ring of the Dmp moiety is coplanar with the carbene to accommodate the o,o′-Mes groups and enable π-donation to the vacant 2pπ(Ccarb) orbital. The Au
CH(Dmp)+ complex, Density Functional Theory (DFT) calculations were carried out. The actual compound was considered, without simplification of the substituents, but the counter-anion was omitted. It is referred hereafter as A-Dmp. The B3PW91/SDD+f(Au), 6-31G** (other atoms) level of theory was used to enable comparison with related complexes. The optimized structure (Fig. 2, left) shows a tricoordinate gold complex (the two P atoms chelate the metal with a bite angle close to 90°). The carbene center sits in a planar environment which substantially deviates from trigonal due to the dissymmetric substitution pattern. The AuCcarbH bond angle is contracted while the AuCcarbCipso bond angle is widened at ca. 108 and 144°, respectively (Table 1). The carbene plane is about perpendicular to the Au coordination plane, to minimize steric repulsions and maximize Au → Ccarb backdonation. Conversely, the phenyl ring of the Dmp moiety is coplanar with the carbene to accommodate the o,o′-Mes groups and enable π-donation to the vacant 2pπ(Ccarb) orbital. The Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ccarb bond length (1.975 Å) is comparable to those found in disubstituted (P^P)Au
Ccarb bond length (1.975 Å) is comparable to those found in disubstituted (P^P)Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)(R′)+ complexes.6 The Ccarb–Cipso bond length (1.428 Å) is similar to those found in (P^P)AuC(CF3)(Ar)+ complexes, suggesting some π-stabilization of the carbene center.6c The P^P ligand at gold and the Dmp substituent provide huge steric shielding. The central Au
C(R)(R′)+ complexes.6 The Ccarb–Cipso bond length (1.428 Å) is similar to those found in (P^P)AuC(CF3)(Ar)+ complexes, suggesting some π-stabilization of the carbene center.6c The P^P ligand at gold and the Dmp substituent provide huge steric shielding. The central Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH moiety is protected by the P substituents on one side and the flanking Mes groups of the Dmp substituent on the other side, as apparent from the space filling model (Fig. 2, right).
CH moiety is protected by the P substituents on one side and the flanking Mes groups of the Dmp substituent on the other side, as apparent from the space filling model (Fig. 2, right).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(Mes)+ complex B-Mes
CH(Mes)+ complex B-Mes
| A-Dmp | A-Mes | A-Ph | B-Mes | |
|---|---|---|---|---|
| Optimized structures | ||||
Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ccarb Ccarb |
1.975 | 1.979 | 1.959 | 2.007 |
| PAu | 2.458 | 2.429 | 2.422 | 2.378 |
| 2.456 | 2.441 | 2.427 | ||
| Ccarb–Cipso | 1.428 | 1.417 | 1.429 | 1.400 |
| PAuP | 88.94 | 89.44 | 89.14 | — |
| AuCH | 107.95 | 110.30 | 115.37 | 110.32 |
| AuCCipso | 143.76 | 138.55 | 133.04 | 138.51 |
| AuCCipsoCortho | 1.0 | −5.0 | 1.2 | −5.4 |
| NBO analyses | ||||
| WBI(AuCcarb) | 0.754 | 0.727 | 0.768 | 0.681 |
| WBI(CipsoCortho) | 1.294 | 1.344 | 1.128 | 1.438 |
| CT | −0.03 | −0.02 | −0.09 | 0.27 |
| CDA analyses | ||||
| Ccarb → Au donation | 0.482 | 0.447 | 0.365 | 0.391 |
| Au → Ccarb backdonation | 0.231 | 0.215 | 0.219 | 0.119 |
| d/b ratio | 2.09 | 2.08 | 1.67 | 3.29 |
Inspection of the frontier orbitals shows some Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ccarb π-bonding as the result of Au → Ccarb backdonation (Fig. 3). The HOMO/LUMO correspond mainly to bonding/anti-bonding combinations between an in-plane d(Au) orbital and the vacant 2pπ(carbene) orbital. The HOMO/LUMO energy gap (2.64 eV) is comparable to those found in disubstituted (P^P)Au
Ccarb π-bonding as the result of Au → Ccarb backdonation (Fig. 3). The HOMO/LUMO correspond mainly to bonding/anti-bonding combinations between an in-plane d(Au) orbital and the vacant 2pπ(carbene) orbital. The HOMO/LUMO energy gap (2.64 eV) is comparable to those found in disubstituted (P^P)Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)(R′)+ carbenes (2.34–2.58 eV).6 In addition, a Natural Localized Molecular Orbital (NLMO) associated to the Au → Ccarb backdonation evidences non-negligible mixing of the 2pπ(Ccarb) orbital with the d(Au)-centered orbital (9.1% Ccarb), in line with some Au
C(R)(R′)+ carbenes (2.34–2.58 eV).6 In addition, a Natural Localized Molecular Orbital (NLMO) associated to the Au → Ccarb backdonation evidences non-negligible mixing of the 2pπ(Ccarb) orbital with the d(Au)-centered orbital (9.1% Ccarb), in line with some Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ccarb π-bonding.9
Ccarb π-bonding.9
To further gauge the AuCcarb bonding, Natural Bond Orbital (NBO) analyses, Energy and Charge Decomposition Analyses (EDA and CDA) were performed (Table 1 and Table S4, ESI†).9 Accordingly, the interaction between (P^P)Au+ and CH(Dmp) (both in the singlet state) turned to be the best fragmentation to describe A-Dmp. The charge transfer (CT) from the carbene center to the metal fragment was found close to zero (−0.03 e), while the donation/backdonation ratio (2.09) indicates the predominance of Ccarb → Au donation, with significant Au → Ccarb backdonation however.
We then questioned whether the steric shielding of the Dmp substituent influences the bonding situation or not. We tried to prepare the related (P^P)Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(Mes)+ complex by the same route, i.e. reacting (P^P)Au(NTf2) with HC(N2)Mes. In this case, only diazo decomposition was observed and no gold carbene could be characterized. The carbenoid route employed by Echavarren et al.4 to access the (JohnPhos)AuCH(Ar)+ complexes was also tested but we could not find suitable conditions for the formal insertion of the carbene into the Au–Cl bond of the (P^P)AuCl complex. Mixing (P^P)AuCl with HC(N2)Mes only led to decomposition of the gold complex.11
CH(Mes)+ complex by the same route, i.e. reacting (P^P)Au(NTf2) with HC(N2)Mes. In this case, only diazo decomposition was observed and no gold carbene could be characterized. The carbenoid route employed by Echavarren et al.4 to access the (JohnPhos)AuCH(Ar)+ complexes was also tested but we could not find suitable conditions for the formal insertion of the carbene into the Au–Cl bond of the (P^P)AuCl complex. Mixing (P^P)AuCl with HC(N2)Mes only led to decomposition of the gold complex.11
We thus resorted to DFT and performed calculations on the (P^P)Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(Mes)+ and (P^P)Au
CH(Mes)+ and (P^P)Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(Ph)+ complexes, A-Mes and A-Ph, respectively (Table 1). No significant difference was noticed between the optimized geometries of A-Dmp, A-Mes and A-Ph. The MO, NBO and CDA analyses also reveal very similar bonding situations. Thus, the huge steric hindrance of the Dmp substituent provides kinetic stabilization but does not bias the electronic structure of the carbene complex. Comparison with the complex B-Mes bearing a JohnPhos-type ligand is also insightful. The key geometric features do not differ much, but it is worth noting that the Au
CH(Ph)+ complexes, A-Mes and A-Ph, respectively (Table 1). No significant difference was noticed between the optimized geometries of A-Dmp, A-Mes and A-Ph. The MO, NBO and CDA analyses also reveal very similar bonding situations. Thus, the huge steric hindrance of the Dmp substituent provides kinetic stabilization but does not bias the electronic structure of the carbene complex. Comparison with the complex B-Mes bearing a JohnPhos-type ligand is also insightful. The key geometric features do not differ much, but it is worth noting that the Au![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ccarb bond is longer (2.007 Å) while the Ccarb–Cipso bond length is slightly shorter (1.400 Å). This suggests weaker Au → Ccarb backdonation and stronger Ar → Ccarb π-donation in B-Mes than in A-Dmp, as further supported by the respective Wiberg Bond Indexes (WBI). The CT value (0.27 e) and d/b ratio (3.29) found for B-Mes by NBO and CDA confirm this view and highlight the Au → Ccarb backdonation enhancement induced by the chelating P^P ligand.
Ccarb bond is longer (2.007 Å) while the Ccarb–Cipso bond length is slightly shorter (1.400 Å). This suggests weaker Au → Ccarb backdonation and stronger Ar → Ccarb π-donation in B-Mes than in A-Dmp, as further supported by the respective Wiberg Bond Indexes (WBI). The CT value (0.27 e) and d/b ratio (3.29) found for B-Mes by NBO and CDA confirm this view and highlight the Au → Ccarb backdonation enhancement induced by the chelating P^P ligand.
In conclusion, a monosubstituted gold(I) carbene stable up to −10 °C has been prepared thanks to the combined use of a chelating (o-carboranyl)-diphosphine ligand, to enhance Au → Ccarb backdonation, and the sterically demanding Dmp substituent, to provide kinetic stabilization.
Future work will aim to extend this approach to other fleeting carbene complexes. Besides gold, it may be applicable to copper and silver, for which only few carbene complexes have been authenticated so far (Chart 2a).12 Other exciting targets are the low valent complexes LnMCR for which representatives of both the metalla carbene form and the carbyne complex have been recently reported (Chart 2b).13
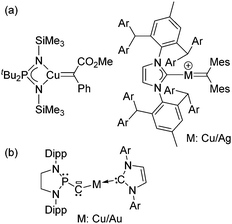 | ||
| Chart 2 Representative examples of (a) copper/silver carbene complexes and (b) metalla carbene/carbyne complex recently authenticated. | ||
Financial support from the Centre National de la Recherche Scientifique and the Université de Toulouse is gratefully acknowledged. The NMR service of ICT (Pierre Lavedan) is acknowledged for assistance with the variable-temperature NMR experiments. The “Direction du Numérique” of the Université de Pau et des Pays de l’Adour and Mésocentre de Calcul Intensif Aquitain (MCIA) are acknowledged for the support of computational facilities. This work was also granted access to the HPC resources of IDRIS under the allocation 2022-[AD010800045R1] made by GENCI. Laura Estévez is thanked for preliminary DFT calculations.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677–698 RSC; (b) L.-W. Ye, X.-Q. Zhu, R. L. Sahani, Y. Xu, P.-C. Qian and R.-S. Liu, Chem. Rev., 2021, 121, 9039–9112 CrossRef CAS PubMed; (c) T. Wang and A. S. K. Hashmi, Chem. Rev., 2021, 121, 8948–8978 CrossRef CAS PubMed.
- (a) R. J. Harris and R. A. Widenhoefer, Chem. Soc. Rev., 2016, 45, 4533–4551 RSC; (b) R. Peloso and E. Carmona, Coord. Chem. Rev., 2018, 355, 116–132 CrossRef CAS; (c) M. Navarro and D. Bourissou, Adv. Organomet. Chem., 2021, 76, 101–144 CrossRef.
- (a) D. Benitez, N. D. Shapiro, E. Tkatchouk, Y. Wang, W. A. Goddard III and F. D. Toste, Nat. Chem., 2009, 1, 482–486 CrossRef CAS PubMed; (b) Y. Wang, M. E. Muratore and A. M. Echavarren, Chem. – Eur. J., 2015, 21, 7332–7339 CrossRef CAS PubMed; (c) L. Nunes dos Santos Comprido, J. E. M. N. Klein, G. Knizia, J. Kästner and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2015, 54, 10336–10340 CrossRef CAS PubMed; (d) R. P. Herrera and M. C. Gimeno, Chem. Rev., 2021, 121, 8311–8363 CrossRef CAS PubMed.
- C. García-Morales, X.-L. Pei, J. M. Sarria Toro and A. M. Echavarren, Angew. Chem., Int. Ed., 2019, 58, 3957–3961 CrossRef PubMed.
- Gold(I) sulfonium benzylide complexes [(JohnPhos)AuCH(SR1R2)Ph]+ have also been reported and shown to undergo carbene transfer reactions: (a) R. G. Carden and R. A. Widenhoefer, Chem. – Eur. J., 2019, 47, 11026–11030 CrossRef PubMed; (b) M. Rivers, R. G. Carden and R. A. Widenhoefer, Organometallics, 2022, 41, 1106–1114 CrossRef CAS.
- (a) M. Joost, L. Estévez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune and D. Bourissou, Angew. Chem., Int. Ed., 2014, 53, 14512–14516 CrossRef CAS PubMed; (b) A. Zeineddine, F. Rekhroukh, E. D. Sosa Carrizo, S. Mallet-Ladeira, K. Miqueu, A. Amgoune and D. Bourissou, Angew. Chem., Int. Ed., 2018, 57, 1306–1310 CrossRef CAS PubMed; (c) M. Rigoulet, D. Vesseur, K. Miqueu and D. Bourissou, Angew. Chem., Int. Ed., 2022, 61, e202204781 CrossRef CAS PubMed.
- (a) C. A. Laskowski, D. J. Bungum, S. M. Baldwin, S. A. Del Ciello, V. M. Iluc and G. L. Hillhouse, J. Am. Chem. Soc., 2013, 135, 18272–18275 CrossRef CAS PubMed; (b) V. M. Iluc and G. L. Hillhouse, J. Am. Chem. Soc., 2014, 17, 6479–6488 CrossRef PubMed.
- Other examples using the bulky Dmp moiety for stabilization include α-diazoalkyl Cu(I) complexes and a terminally nitrogen coordinated Co(0) diazo complex: (a) V. M. Iluc, C. A. Laskowski and G. L. Hillhouse, Organometallics, 2009, 20, 6135–6138 CrossRef; (b) J. Du, W. Chen, Q. Chen, X. Leng, Y.-S. Meng, S. Gao and L. Deng, Organometallics, 2020, 39, 729–739 CrossRef CAS.
- See ESI† for details.
- M. Joost, A. Zeineddine, L. Estévez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune and D. Bourissou, J. Am. Chem. Soc., 2014, 42, 14654–14657 CrossRef PubMed.
- The reaction was tried in toluene and dichloromethane at room temperature and 40 °C.
- (a) B. F. Straub and P. Hofmann, Angew. Chem., Int. Ed., 2001, 40, 1288–1290 CrossRef CAS; (b) M. W. Hussong, W. T. Hoffmeister, F. Rominger and B. F. Straub, Angew. Chem., Int. Ed., 2015, 54, 10331–10335 CrossRef CAS PubMed; (c) M. Álvarez, M. Besora, F. Molina, F. Maseras, T. R. Belderrain and P. J. Pérez, J. Am. Chem. Soc., 2021, 143, 4837–4843 CrossRef PubMed.
- (a) C. Hu, X.-F. Wang, R. Wei, C. Hu, D. A. Ruiz, Y.-Y. Chang and L. L. Liu, Chemistry, 2022, 8, 2278–2289 CrossRef CAS; (b) R. Wei, X.-F. Wang, C. Hu and L. L. Liu, Nat. Synth., 2023, 2, 357–363 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization of complex 1, computational data (PDF). See DOI: https://doi.org/10.1039/d3cc01007d |
| This journal is © The Royal Society of Chemistry 2023 |