Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bilateral π-extension of an open-[60]fullerene in a helical manner†
Yoshifumi
Hashikawa
 ,
Shumpei
Sadai
and
Yasujiro
Murata
,
Shumpei
Sadai
and
Yasujiro
Murata
 *
*
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011, Japan. E-mail: yasujiro@scl.kyoto-u.ac.jp
First published on 11th May 2023
Abstract
The conventional π-elongation of open-[60]fullerenes could only give unilaterally π-extended derivatives. Herein, we report the further π-elongation at another site to achieve bilateral π-elongation via a consecutive nucleophilic addition of 4,5-dimethyl-o-phenylenediamine. The thus-formed π-extended open-[60]fullerene bears two-fold diaza[n]helicene (n = 5 and 6) motifs in its skeleton. The crystallographic analysis revealed the characteristic helicene–fullerene interactions with close contacts of 3.09 and 3.14 Å.
Open-[60]fullerenes1 contain a geodesic polyarene with a formula of C50H10, which was firstly synthesized as the shortest end-cap (5,5) carbon nanotube (CNT) by Scott and co-workers in 2012.2 The (5,5) end-cap is a possible template molecule for CNT growth. The chemical vapor deposition in the presence of a mixed carbon source of CH4/C2H4 indeed produced single-walled CNTs with an average diameter of 0.82 nm.3 The similar preprogrammed bottom-up synthesis of CNTs has been demonstrated using unimolecular seeds such as cycloparaphenylene,4 truxene derivative,5 and open-[60]fullerene,6 in which ethanol is usually employed as a carbon source. These methods enable the production of CNTs with atomically-controlled diameters. However, the bay regions in the (5,5) end-cap resist Diels–Alder cycloadditions, thus being far less reactive toward nitroethylene and benzyne.7 Therefore, organic synthesis of molecular CNTs with an atomically precise π-conjugation length still remains a formidable challenge.
The consecutive chemical scission of [60]fullerene gives an open-form with a large orifice.1 This type of open-[60]fullerene could be regarded as a functional π-extended (5,5) end-cap (Fig. 1). Apart from the pristine (5,5) end-cap with a poor reactivity, open-[60]fullerenes are expected to be promising seeds for the growth of molecular CNTs.8 The unilateral π-extension of open-[60]fullerenes has been developed by Iwamatsu9 and us,10 independently, in which a fused quinoxaline was introduced to the geodesic π-conjugation (Fig. 1). The related conjugation motifs of such acceptor–acceptor (quinoxaline–open-[60]fullerene) hybrids were also prepared by Gan and co-workers.11 Recently, we synthesized π-extended open-[60]fullerenes with a fused imidazole (Fig. 1),10b showing a notable increase of the absorption coefficients in the visible region owing to donor–acceptor interactions.12 As exemplified above, open-[60]fullerenes allow us to precisely elongate their π-skeleton in a step-by-step manner while the unilateral π-elongation provides molecular platforms not ideal for the construction of tubular nanostructures. Thereby, multilateral and/or uniform π-elongation from the rim is highly demanded to chemically synthesize molecular CNTs. Herein, we report a bilateral π-elongation of an open-[60]fullerene, giving access to a π-extended (5,5) end-cap with two-fold embedded hetero[n]helicene motifs.
Recently, we have reported a unilateral π-elongation of an open-[60]fullerene giving tricarbonyl derivative 1 (Scheme 1).10a We had initially expected the further π-elongation from C(1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(1) and C(4)
O(1) and C(4)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(4) groups as footholds via two-fold dehydrative condensation with a suitable aromatic diamine. Thus, the reaction of 1 with 4,5-dimethyl-o-phenylenediamine was conducted in o-dichlorobenzene (ODCB) at 180 °C for 2 h. As a result, 2 was obtained in 49% isolated yield by a single dehydrative condensation, while the further reaction did not proceed at all (Scheme 1). The high selectivity at the C(1)
O(4) groups as footholds via two-fold dehydrative condensation with a suitable aromatic diamine. Thus, the reaction of 1 with 4,5-dimethyl-o-phenylenediamine was conducted in o-dichlorobenzene (ODCB) at 180 °C for 2 h. As a result, 2 was obtained in 49% isolated yield by a single dehydrative condensation, while the further reaction did not proceed at all (Scheme 1). The high selectivity at the C(1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(1) group is simply due to its higher accessibility (Fig. 2a). With the key precursor (2) in hand, we then tried the reaction in the presence of pyridine. The mass spectrometric analysis of the product (3) showed a molecular ion peak at m/z 1336.3477, which differs from the initially expected compound ([2–H2O]˙−) but was finally assignable to [2–2H]˙−. The absence of the amino group was confirmed by 1H NMR while 13C NMR spectrum of 3 (201 MHz, acetone-d6/CS2 (1
O(1) group is simply due to its higher accessibility (Fig. 2a). With the key precursor (2) in hand, we then tried the reaction in the presence of pyridine. The mass spectrometric analysis of the product (3) showed a molecular ion peak at m/z 1336.3477, which differs from the initially expected compound ([2–H2O]˙−) but was finally assignable to [2–2H]˙−. The absence of the amino group was confirmed by 1H NMR while 13C NMR spectrum of 3 (201 MHz, acetone-d6/CS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)) clearly indicated the presence of two carbonyl groups at δ 192.00 and 187.42 ppm. These results imply that the reaction occurred at the rim of the orifice undoubtedly with the amino group. The structure of 3 was unambiguously determined by X-ray crystallographic analysis (Fig. 3) and found to have an imidazole ring generated through a nitrogen-insertion between the C(1)–C(2) bond. Upon seeing the LUMO+1, the large orbital coefficient was found at the α,β-unsaturated carbonyl group where the C(2) atom (qNPA +0.016) is more positively charged rather than C(3) (qNPA −0.079) according to the natural population analysis (NPA) (Fig. 2b). Note that the LUMO has large coefficients on the entire [60]fullerene skeleton. As is the case with the addition of the diamine to 1, the amino group in 2 is not likely to attack the C(4)
5)) clearly indicated the presence of two carbonyl groups at δ 192.00 and 187.42 ppm. These results imply that the reaction occurred at the rim of the orifice undoubtedly with the amino group. The structure of 3 was unambiguously determined by X-ray crystallographic analysis (Fig. 3) and found to have an imidazole ring generated through a nitrogen-insertion between the C(1)–C(2) bond. Upon seeing the LUMO+1, the large orbital coefficient was found at the α,β-unsaturated carbonyl group where the C(2) atom (qNPA +0.016) is more positively charged rather than C(3) (qNPA −0.079) according to the natural population analysis (NPA) (Fig. 2b). Note that the LUMO has large coefficients on the entire [60]fullerene skeleton. As is the case with the addition of the diamine to 1, the amino group in 2 is not likely to attack the C(4)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(4) group due to the steric demand while it undergoes a nucleophilic addition to the C(2) atom, thus giving intermediate A which is transformed into Bvia aziridination (Fig. 2c). The subsequent ring-opening reaction allows for the nitrogen atom to be inserted into the rim of the orifice. The thus-formed dihydroimidazole derivative (C) is then oxidized to afford imidazole-fused 3.
O(4) group due to the steric demand while it undergoes a nucleophilic addition to the C(2) atom, thus giving intermediate A which is transformed into Bvia aziridination (Fig. 2c). The subsequent ring-opening reaction allows for the nitrogen atom to be inserted into the rim of the orifice. The thus-formed dihydroimidazole derivative (C) is then oxidized to afford imidazole-fused 3.
The single crystals of racemic 3 were obtained from a CS2/acetone solution. The solid-state structure for one of the two enantiomers is shown in Fig. 3. It is worth mentioning that the π-elongation was achieved on the geodesic [60]fullerene skeleton in a bilateral manner (Fig. 3a) where the two π-systems are embedded as diaza[n]helicenes (n = 5 and 6) with a single P-helicity (Fig. 3b), while another enantiomer consists of a single M-helicity. The torsion angles φ along the helical inner rims indicate the considerably larger distortion of the two helical motifs, when compared with non-substituted carbo[5] and [6]helicenes (16.4, 31.5, and 18.4° and 11.1, 30.1, 31.2, and 15.1°),13 reflecting the positive curvature of the [60]fullerene skeleton. Within the crystal, the molecules of 3 are arranged in close proximity with contact distances of 3.09 and 3.14 Å between the helically and spherically π-conjugated motifs (Fig. 3c).
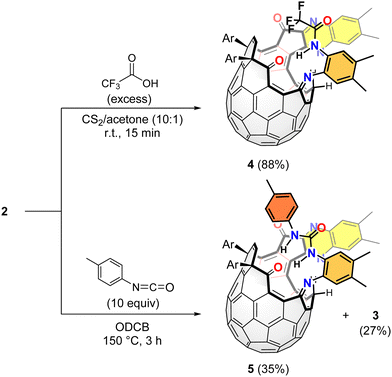
We also examined the chemical transformation of 2 at the amino group (Scheme 2). The reaction of 2 with trifluoroacetic acid smoothly gave the corresponding amide 4 in 88% yield under catalyst-free conditions. Such condensation is known to occur only if aniline is substituted with an electron-deficient group, which prevents it from generating a salt with the acid.14 In our case, the strong electron-accepting character of the [60]fullerene moiety is considered to make catalysts unnecessary. As another electrophile, we used p-tolyl isocyanate, which furnished the corresponding urea derivative 5 in 35% isolated yield together with 3 (27%).
To get insights into the electronic properties of 1–5, we measured absorption spectra in benzene (Fig. 4). Compound 6,15 which bears two carbonyl groups, was also measured as a reference molecule for evaluating the effect of the π-elongation in 1 and 3. As shown in Fig. 4a, the first π-elongation (6 → 1) drastically modulates the absorption properties with increased coefficients over the measured range. The embedded diaza[6]helicene moiety in 1 is well-conjugated with the [60]fullerene skeleton as found in the HOMO–1 and LUMO (B3LYP/6-31G(d)) (Fig. 4c). The second π-elongation (1 → 3) contributes to a slight increase in absorption at the visible region (Fig. 4a). According to theoretical calculations (Fig. 4c), the HOMO of 3 (−5.48 eV) is dominantly distributed on the aza[5]helicene moiety, which is less conjugated with the [60]fullerene core, while it overrides the level of the original HOMO (−5.69 eV) of 1 by the second π-elongation. The longest wavelength absorption of 3 originates from a charge transfer transition as opposed to 1 showing a π–π* character. Note that the HOMO–3 (−5.88 eV) and LUMO (−3.04 eV) of 3 bear a close resemblance with the HOMO–1 (−5.91 eV) and LUMO (−3.09 eV) of 1, respectively, without considerable perturbation to their energy levels by the second π-elongation.
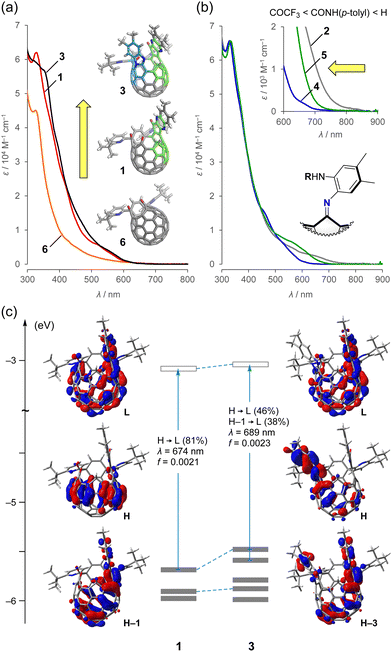 | ||
| Fig. 4 UV-vis-NIR absorption spectra of (a) 1, 3, and 6 and (b) 2, 4, and 5 (50 μM in benzene). (c) Kohn–Sham HOMO and LUMO levels of 1 and 3 with optical transitions (TD CAM-B3LYP/6-31G(d)//B3LYP/6-31G(d)). The transition energies were calibrated with a factor of 0.72.16 | ||
The aniline-substituted derivative (2) exhibited a near-infrared (NIR) absorption, which tails to 870 nm (Fig. 4b). The absorption edges were hypochromically shifted by varying the substituents: amide (4, 750 nm) < urea (5, 800 nm) < amine (2, 870 nm). The theoretical calculations suggested that the HOMO level is lowered in the order of 2 (−4.96 eV) > 5 (−5.06 eV) > 4 (−5.65 eV), while the LUMO levels are comparable (2, −2.97 eV; 5, −3.12 eV; 4, −3.08 eV) (B3LYP/6-31G(d)). Thus, the observed hypsochromic shift is ascribed to the magnitude of the donor character on the aniline moiety in 2, 4, and 5.
In summary, we achieved the second π-elongation of a unilaterally π-extended open-[60]fullerene (1) by the reaction with 4,5-dimethyl-o-phenylenediamine in the presence of pyridine. The structure of the product (3) was unambiguously determined by X-ray diffraction analysis, which revealed the bilateral π-elongation from the [60]fullerene core in a helical manner. The two embedded diaza[n]helicene (n = 5 and 6) moieties are severely distorted due to the positive curvature of the [60]fullerene skeleton. The close contacts between the helical and spherical π-motifs were found at distances of 3.09 and 3.14 Å. In this reaction, 2 is a key intermediate for the formation of 3 and its electronic structure was found to be modifiable by the reaction with electrophiles, thus observing the obvious hypochromic shift in the absorption spectra. Since 3 could be regarded as a π-extended (5,5) end-cap, this approach would open a way to create structurally well-defined molecular CNTs through stepwise chemical reactions.
Financial support was partially provided by the JSPS KAKENHI Grant Number JP17H06119 and JP22H04538, ISHIZUE 2022 of Kyoto University, and The Mazda Foundation. The NMR measurements were partly supported by the Joint Usage/Research Center (JURC) at the ICR, Kyoto University.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. Murata, Y. Murata and K. Komatsu, Chem. Commun., 2008, 6083–6094 RSC; (b) G. C. Vougioukalakis, M. M. Roubelakis and M. Orfanopoulos, Chem. Soc. Rev., 2010, 39, 817–844 RSC; (c) L. Shi and L. Gan, J. Phys. Org. Chem., 2013, 26, 766–772 CrossRef CAS.
- L. T. Scott, E. A. Jackson, Q. Zhang, B. D. Steinberg, M. Bancu and B. Li, J. Am. Chem. Soc., 2012, 134, 107–110 CrossRef CAS PubMed.
- B. Liu, J. Liu, H.-B. Li, R. Bhola, E. A. Jackson, L. T. Scott, A. Page, S. Irle, K. Morokuma and C. Zhou, Nano Lett., 2015, 15, 586–595 CrossRef CAS PubMed.
- H. Omachi, T. Nakayama, E. Takahashi, Y. Segawa and K. Itami, Nat. Chem., 2013, 5, 572–576 CrossRef CAS PubMed.
- (a) J. R. Sanchez-Valencia, T. Dienel, O. Gröning, I. Shorubalko, A. Mueller, M. Jansen, K. Amsharov, P. Ruffieux and R. Fasel, Nature, 2014, 512, 61–64 CrossRef CAS PubMed; (b) J. Tomada, T. Dienel, F. Hampel, R. Fasel and K. Amsharov, Nat. Commun., 2019, 10, 3278 CrossRef PubMed.
- X. Yu, J. Zhang, W. Choi, J.-Y. Choi, J. M. Kim, L. Gan and Z. Liu, Nano Lett., 2010, 10, 3343–3349 CrossRef CAS PubMed.
- L. T. Scott, Pure Appl. Chem., 2017, 89, 809–820 CrossRef CAS.
- C.-S. Chen and W.-Y. Yeh, Chem. – Eur. J., 2016, 22, 16425–16428 CrossRef CAS PubMed.
- (a) S.-i Iwamatsu, T. Uozaki, K. Kobayashi, S. Re, S. Nagase and S. Murata, J. Am. Chem. Soc., 2004, 126, 2668–2669 CrossRef CAS PubMed; (b) Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038–9041 CrossRef CAS PubMed.
- (a) Y. Hashikawa, S. Sadai and Y. Murata, Org. Lett., 2021, 23, 9586–9590 CrossRef CAS PubMed; (b) Y. Hashikawa, N. Fujikawa and Y. Murata, J. Am. Chem. Soc., 2022, 144, 23292–23296 CrossRef CAS PubMed; (c) S. Sadai, Y. Hashikawa and Y. Murata, Org. Lett., 2023, 25, 2815–2819 CrossRef CAS PubMed.
- (a) Y. Yu, L. Xu, X. Huang and L. Gan, J. Org. Chem., 2014, 79, 2156–2162 CrossRef CAS PubMed; (b) Z. Liu, Z. Liu, R. Gao, J. Su, Y. Qiu and L. Gan, Org. Chem. Front., 2022, 9, 320–328 RSC.
- (a) Y. Hashikawa, H. Yasui, K. Kurotobi and Y. Murata, Mater. Chem. Front., 2018, 2, 206–213 RSC; (b) Y. Hashikawa, S. Okamoto and Y. Murata, Commun. Chem., 2020, 3, 90 CrossRef CAS PubMed; (c) Y. Hashikawa and Y. Murata, Asian J. Org. Chem., 2022, 11, e202200357 CrossRef CAS; (d) Y. Hashikawa, N. Fujikawa, S. Okamoto and Y. Murata, Dalton Trans., 2022, 51, 17804–17808 RSC; (e) Y. Hashikawa, S. Sadai, S. Okamoto and Y. Murata, Angew. Chem., Int. Ed., 2023, 62, e202215380 CrossRef CAS PubMed.
- (a) A.-C. Bédard, A. Vlassova, A. C. Hernandez-Perez, A. Bes-sette, G. S. Hanan, M. A. Heuft and S. K. Collins, Chem. – Eur. J., 2013, 19, 16295–16302 CrossRef PubMed; (b) M. Dračínský, J. Storch, V. Církva, I. Císařová and J. Sýkora, Phys. Chem. Chem. Phys., 2017, 19, 2900–2907 RSC.
- J. Ohtaka, T. Sakamoto and Y. Kikugawa, Tetrahedron Lett., 2009, 50, 1681–1683 CrossRef CAS.
- (a) K. Kurotobi and Y. Murata, Science, 2011, 333, 613–616 CrossRef CAS PubMed; (b) Y. Hashikawa, M. Murata, A. Wakamiya and Y. Murata, J. Am. Chem. Soc., 2017, 139, 16350–16358 CrossRef CAS PubMed.
- (a) Y. Hashikawa and Y. Murata, Chem. Sci., 2020, 11, 12428–12435 RSC; (b) Y. Hashikawa, S. Okamoto and Y. Murata, Chem. – Eur. J., 2021, 27, 4864–4868 CrossRef CAS PubMed; (c) Y. Hashikawa, H. Kawasaki and Y. Murata, Organometallics, 2022, 41, 354–359 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures, spectra, and optimized geometries. CCDC 2236614. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00784g |
| This journal is © The Royal Society of Chemistry 2023 |