Open Access Article
Open Access ArticleSimultaneously controlling conformational and operational stability of single-chain polymeric nanoparticles in complex media†
Stefan
Wijker
 ,
Rico
Monnink
,
Luc
Rijnders
,
Linlin
Deng
and
Anja R.A.
Palmans
,
Rico
Monnink
,
Luc
Rijnders
,
Linlin
Deng
and
Anja R.A.
Palmans
 *
*
Institute for Complex Molecular Systems, Laboratory of Macromolecular and Organic Chemistry, Eindhoven University of Technology, P.O. Box 513, 5600 MB, Eindhoven, The Netherlands. E-mail: a.palmans@tue.nl
First published on 4th April 2023
Abstract
Single-chain polymeric nanoparticles (SCPNs) comprising a solvatochromic pyrazoline adduct show conformational and operational stability in complex media and in cellular compartments; the connectivity of the adduct is crucial in modulating interactions with the surrounding media.
Folding single chains of amphiphilic heterograft copolymers into single-chain polymeric nanoparticles (SCPNs) of defined size and shape is a promising approach towards utilizing these synthetic polymers in bio-applications such as (cell) imaging,1–6 bio-orthogonal catalysis,7–12 and preservation of protein function.13 Two important factors regulate the successful application of SCPNs in complex media. First, there is a need for high conformational stability, wherein the SCPNs have the ability to retain size and compartmentalized shape. Second, SCPNs need good functional stability, whereby good performance over time is retained. Achieving this is not trivial, as interactions with hydrophobic proteins and peptides present in complex media affect stability and reduce performance.1 This has been highlighted by significant reductions in the activity of bio-orthogonal catalysts embedded in SCPNs when used in complex media.12,14
Recently, we showed that SCPNs with high conformational stability in complex media can be prepared by the introduction of covalent cross-links via coumarin dimerization after folding of the polymer chains via hydrophobic and hydrogen-bonding interactions.15 Since the fluorescence of the coumarin was lost after dimerization, the solvatochromic dye Nile Red was separately introduced to track the SCPNs in living cells and report on the presence of dye–cell interactions through solvatochromic shifts.1 We concluded that a covalent connectivity of the Nile Red to the polymer was crucial to reduce interactions with hydrophobic proteins in the cell culturing media. However, the need to covalently attach a fluorescent dye makes the synthesis of SCPNs more demanding.
Here, we set out to develop SCPNs that have the crosslinking and reporter function unified in one motif. We selected the nitrile imine-mediated tetrazole-ene cycloaddition, NITEC, which is a light-triggered, irreversible “click” reaction that proceeds at high reaction rates and without the need of a catalyst.16 The NITEC reaction was elegantly used by Barner-Kowollik and co-workers to intramolecularly crosslink polymers to SCPNs both in organic as well as in aqueous media.17–19 Interestingly, the NITEC reaction affords a fluorescent pyrazoline adduct.20,21 This adduct has been studied as a promising fluorophore in protic solutions,22 and for its solvatochromic properties.23 We show in this work that the solvatochromic properties of pyrazoline adduct can be transferred to provide a reporter function for SCPNs in complex media and living cells. Measuring the changes in the emission spectra resulting from interactions between the adduct and the surrounding media will permit to understand both the conformational as well as functional stability of SCPNs in complex media and living cells.
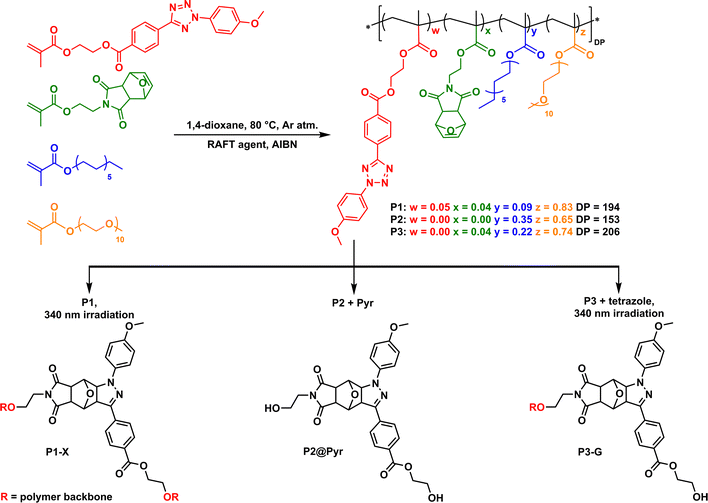
Three different polymers P1–P3 were prepared via RAFT copolymerization of hydrophobic and hydrophilic methacrylate derivatives (Scheme 1): oligo(ethylene glycol) (oEG) modified methacrylate to ensure water solubility, n-dodecyl (Dod) methacrylate to drive the hydrophobic collapse of the polymer in water, and methacrylates functionalized with either a furan-protected maleimide (pMal) or a tetrazole (Tet) moiety that act as the two precursors for the pyrazoline formation via the NITEC reaction. As the adduct connectivity is an important factor when considering SCPN interactions with surrounding media, we designed polymer P1–P3 to possess different microstructures. Apart from oEG and Dod grafts, P1 comprised both pMal and Tet grafts, each about 4%. P2 had no additional grafts, and P3 comprised only pMal grafts (4%). The RAFT synthesis resulted in polymers with incorporation ratios in good agreement with the feed ratios, a degree of polymerization (DP) between 150 and 200, and a relatively narrow molar mass dispersity (Đ around 1.3). The synthesis and characterization are described in detail in the ESI† (Section 2.2). All polymers readily dissolve in water and form nanoparticles with a small, defined size, with hydrodynamic radii RH between 6 and 8 nm as determined by dynamic light scattering (DLS) (see ESI,† Section 2.3).
P1, including both pMal and Tet, was crosslinked in water at dilute conditions (cpol = 1 mg mL−1) using irradiation with UV-light (λ = 340 nm, irradiance = 4 mW cm−2). This affords predominantly intramolecularly formed pyrazoline adducts (P1-X) as verified by DLS measurements (Scheme 1, bottom left, see ESI,† Section 2.3). The polymer concentration was kept low to suppress intermolecular cross-linking. P2 is used for the physical encapsulation of free pyrazoline adduct (Pyr) resulting in P2@Pyr. Pyr was synthesized separately, see Scheme 1 bottom middle, and we determined a fluorescence quantum yield Φpyr of 0.128 (see ESI† Section 2.4–2.6 for details). P3 contains pMal but no Tet grafts. Addition of free tetrazole and subsequent UV-light irradiation in water resulted in the formation of pyrazoline grafts connected to the polymer backbone via the maleimide moiety as dangling chains (P3-G) (Scheme 1, bottom right).
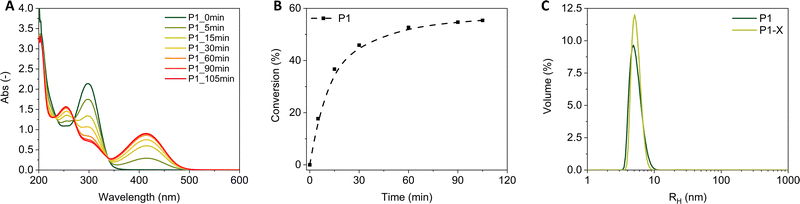
We first evaluated the solvatochromic behaviour of free Pyr (Scheme 1 and Fig. S26, ESI†) in solvents that differ in polarity. A bathochromic shift in the emission maxima from 500 nm in apolar p-xylene to 550 nm in polar acetic acid is observed (see ESI,† Section 2.5). This large shift indicates that Pyr can be used as a reporter to probe the polarity of its microenvironment when incorporated in SCPNs. Next, we probed the formation of the pyrazoline adduct in water for P1 and P3 using absorbance and fluorescence spectroscopy. Fig. 1A shows the absorbance spectrum over time during the NITEC reaction on P1. The decrease in absorbance around 300 nm corresponds to the disappearance of the tetrazole and the new bands appearing around 250 and 410 nm correspond to successful pyrazoline formation (see ESI,† Section 2.6). The conversion of the precursors into pyrazoline as a function of time was calculated via the feed ratios and the pyrazoline extinction coefficient (see ESI,† section 2.7) and is quantified in Fig. 1B, reaching 56% conversion after 105 minutes. For P1, 105 minutes was the optimum irradiation time, resulting in P1-X with, on average, 4.3 crosslinks per chain. Longer irradiation times lead to a net decrease in the amount of pyrazoline as the pyrazoline absorbance peak starts to decrease again, possibly due to photooxidation of the adduct.24 Formation of pyrazoline adducts in P3 proceeded similarly to P1, reaching a maximum pyrazoline concentration for P3-G after five hours irradiation at 66% conversion before a decrease of the pyrazoline absorption sets in. This corresponds to 5.4 grafts per chain. The absorbance and fluorescence spectra showed no significant changes after storing the polymer solutions for three months at ambient conditions, indicating that there is no dye degradation over time (see ESI,† Section 2.8). This highlights the excellent long-term stability of the pyrazoline adducts embedded in the amphiphilic polymers in water.
With P1-X and P3-G successfully prepared, we set out to investigate the interactions of the different polymer systems at high concentrations (cpol = 3 mg mL−1) with complex media. To this end, the fluorescence spectra in water—without competing interactions—were compared to those in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol% mixture of fetal bovine serum (FBS) and Dulbecco‘s modified eagle medium (DMEM). Fig. 2 shows an identical shape of the fluorescence spectra and no shift in the fluorescence maxima (543 nm for P1-X, and 537 nm for P2@Pyr and P3-G) when going from water to cell culture medium. It follows from these observations that the pyrazoline remains trapped inside the SCPNs and does not interact with the FBS proteins. This is likely caused by the insolubility of the pyrazoline adduct in water, see ESI,† Section 2.5 for details. As the systems are stable in cell culture medium, P1-G, P2@Pyr and P3-G were incubated with HeLa cells for 24 h with 10
90 vol% mixture of fetal bovine serum (FBS) and Dulbecco‘s modified eagle medium (DMEM). Fig. 2 shows an identical shape of the fluorescence spectra and no shift in the fluorescence maxima (543 nm for P1-X, and 537 nm for P2@Pyr and P3-G) when going from water to cell culture medium. It follows from these observations that the pyrazoline remains trapped inside the SCPNs and does not interact with the FBS proteins. This is likely caused by the insolubility of the pyrazoline adduct in water, see ESI,† Section 2.5 for details. As the systems are stable in cell culture medium, P1-G, P2@Pyr and P3-G were incubated with HeLa cells for 24 h with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol% FBS:DMEM solutions (cpol = 3 mg mL−1) in order to probe polymer–cell interactions. After successful internalization of the 3 polymers in the HeLa cells, confocal microscopy was used to visualize the cells and determine the fluorescence spectrum of the pyrazoline adducts (Fig. 3). Polymers P1-X and P3-G are internalized by cells, giving a dots-like cluster, whereas the free pyrazoline adduct of P2@Pyr seems to have been distributed homogeneously throughout the cytoplasm. The fluorescence spectra reveal that P1-X, in which the pyrazoline adduct acts as a cross-link, shows near identical fluorescence spectra in FBS and HeLa cells, indicating the absence of interactions. For P3-G, a blue-shift is observed in HeLa cells, which means that pyrazoline as dangling unit instead of as cross-link interacts with the cellular constituents. Finally, the largest blue-shift in HeLa cells is observed for P2@Pyr, indicating that the free pyrazoline adduct likely escapes from the SCPN and interacts with hydrophobic constituents, hereby showing the highest amount of interaction with the environment. The results for P1-X highlight that solvatochromic pyrazoline adducts act as stabilizing crosslinks for SCPN formation, and replaces the need for attaching fluorescent tracking moieties such as Nile Red. Analogous to what was observed for Nile Red,1 covalently linking the pyrazoline to the polymer backbone reduces interactions between the probe and its surroundings. But, the type of connectivity between the polymer and the pyrazoline matters, since the cross-linked adduct shows less interaction.
90 vol% FBS:DMEM solutions (cpol = 3 mg mL−1) in order to probe polymer–cell interactions. After successful internalization of the 3 polymers in the HeLa cells, confocal microscopy was used to visualize the cells and determine the fluorescence spectrum of the pyrazoline adducts (Fig. 3). Polymers P1-X and P3-G are internalized by cells, giving a dots-like cluster, whereas the free pyrazoline adduct of P2@Pyr seems to have been distributed homogeneously throughout the cytoplasm. The fluorescence spectra reveal that P1-X, in which the pyrazoline adduct acts as a cross-link, shows near identical fluorescence spectra in FBS and HeLa cells, indicating the absence of interactions. For P3-G, a blue-shift is observed in HeLa cells, which means that pyrazoline as dangling unit instead of as cross-link interacts with the cellular constituents. Finally, the largest blue-shift in HeLa cells is observed for P2@Pyr, indicating that the free pyrazoline adduct likely escapes from the SCPN and interacts with hydrophobic constituents, hereby showing the highest amount of interaction with the environment. The results for P1-X highlight that solvatochromic pyrazoline adducts act as stabilizing crosslinks for SCPN formation, and replaces the need for attaching fluorescent tracking moieties such as Nile Red. Analogous to what was observed for Nile Red,1 covalently linking the pyrazoline to the polymer backbone reduces interactions between the probe and its surroundings. But, the type of connectivity between the polymer and the pyrazoline matters, since the cross-linked adduct shows less interaction.
In summary, the NITEC reaction is a promising approach to include conformationally and functionally stabilizing fluorescent covalent cross-links in SCPNs. This method allows particle tracking via fluorescence without the need to incorporate additional fluorescent probes. Additionally, the fluorescence of the pyrazoline adduct is stable for a long time and no shift in the fluorescence maxima was observed in FBS:DMEM, corroborating both operational and conformational stability. In the presence of HeLa cells, the connectivity of the pyrazoline probe and the polymer is important as pyrazoline cross-links introduce additional stability to the polymer particles compared to dangling chains. Both approaches outperform free pyrazoline incorporated into cells via hydrophobic interactions. As such, the pyrazoline adduct reveals the influence of polymer architecture on cell interactions and gives guidelines for the future design of conformationally and functionally stable SCPNs for biological applications with excellent long-term stability. This novel approach to SCPN formation permits to design stable and traceable SCPNs that act as carriers for bio-orthogonal catalysts in complex media, which is the topic of future investigations.
This research was financed by the Dutch Ministry of Education, Culture and Science (Gravity program 024.001.035), and the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant Agreement no. 765497 (THERACAT).
Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Deng, L. Albertazzi and A. R. A. Palmans, Biomacromolecules, 2022, 23, 326–338 CrossRef CAS PubMed.
- A. P. P. Kröger and J. M. J. Paulusse, J. Controlled Release, 2018, 286, 326–347 CrossRef PubMed.
- I. Perez-Baena, I. Loinaz, D. Padro, I. García, H. J. Grande and I. Odriozola, J. Mater. Chem., 2010, 20, 6916–6922 RSC.
- R. Gracia, M. Marradi, U. Cossío, A. Benito, A. Pérez-San Vicente, V. Gómez-Vallejo, H. J. Grande, J. Llop and I. Loinaz, J. Mater. Chem. B, 2017, 5, 1143–1147 RSC.
- A. B. Benito, M. K. Aiertza, M. Marradi, L. Gil-Iceta, T. Shekhter Zahavi, B. Szczupak, M. Jiménez-González, T. Reese, E. Scanziani, L. Passoni, M. Matteoli, M. De Maglie, A. Orenstein, M. Oron-Herman, G. Kostenich, L. Buzhansky, E. Gazit, H.-J. J. Grande, V. Gómez-Vallejo, J. Llop and I. Loinaz, Biomacromolecules, 2016, 17, 3213–3221 CrossRef CAS PubMed.
- M. Collot, J. Schild, K. T. Fam, R. Bouchaala and A. S. Klymchenko, ACS Nano, 2020, 14, 13924–13937 CrossRef CAS PubMed.
- K. Liu, Y. Tian, R. Pitchimani, M. Huang, H. Lincoln and D. Pappas, Talanta, 2009, 79, 333–338 CrossRef CAS PubMed.
- J. Chen, K. Li, S. E. Bonson and S. C. Zimmerman, J. Am. Chem. Soc., 2020, 142, 13966–13973 CrossRef CAS PubMed.
- E. S. Garcia, T. M. Xiong, A. Lifschitz and S. C. Zimmerman, Polym. Chem., 2021, 12, 6755–6760 RSC.
- J. Chen, J. Wang, K. Li, Y. Wang, M. Gruebele, A. L. Ferguson and S. C. Zimmerman, J. Am. Chem. Soc., 2019, 141, 9693–9700 CrossRef CAS PubMed.
- Y. Deng, T. Wu, X. Chen, Y. Chen, Y. Fei, Y. Liu, Z. Chen, H. Xing and Y. Bai, J. Am. Chem. Soc., 2023, 145, 1262–1272 CrossRef CAS PubMed.
- A. Sathyan, S. Croke, A. M. Pérez-López, B. F. M. de Waal, A. Unciti-Broceta and A. R. A. Palmans, Mol. Syst. Des. Eng., 2022, 7, 1736–1748 RSC.
- B. Panganiban, B. Qiao, T. Jiang, C. DelRe, M. M. Obadia, T. D. Nguyen, A. A. A. Smith, A. Hall, I. Sit, M. G. Crosby, P. B. Dennis, E. Drockenmuller, M. Olvera de la Cruz and T. Xu, Science, 2018, 359, 1239–1243 CrossRef CAS PubMed.
- Y. Liu, S. Pujals, P. J. M. Stals, T. Paulöhrl, S. I. Presolski, E. W. Meijer, L. Albertazzi and A. R. A. Palmans, J. Am. Chem. Soc., 2018, 140, 3423–3433 CrossRef CAS PubMed.
- S. Wijker, L. Deng, F. Eisenreich, I. K. Voets and A. R. A. Palmans, Macromolecules, 2022, 55, 6220–6230 CrossRef CAS PubMed.
- J. S. Clovis, A. Eckell, R. Huisgen and R. Sustmann, Chem. Ber., 1967, 100, 60–70 CrossRef CAS.
- C. Heiler, J. T. Offenloch, E. Blasco and C. Barner-Kowollik, ACS Macro Lett., 2017, 6, 56–61 CrossRef CAS PubMed.
- C. Heiler, S. Bastian, P. Lederhose, J. P. Blinco, E. Blasco and C. Barner-Kowollik, Chem. Commun., 2018, 54, 3476–3479 RSC.
- J. T. Offenloch, J. Willenbacher, P. Tzvetkova, C. Heiler, H. Mutlu and C. Barner-Kowollik, Chem. Commun., 2017, 53, 775–778 RSC.
- R. Huisgen, M. Seidel, G. Wallbillich and H. Knupfer, Tetrahedron, 1962, 17, 3–29 CrossRef CAS.
- R. Huisgen, Angew. Chem., Int. Ed., 1963, 2, 565–598 CrossRef.
- Y.-K. Zhang, M. Li, L. Ruan and P. An, Chem. Commun., 2022, 58, 10404–10407 RSC.
- K. A. Haupa, A. Szukalski and J. Myśliwiec, J. Phys. Chem. A, 2018, 122, 7808–7818 CrossRef CAS PubMed.
- M. Soltani, H. R. Memarian and H. Sabzyan, J. Photochem. Photobiol., A, 2020, 389, 112285 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, experimental section, and additional characterization. See DOI: https://doi.org/10.1039/d3cc00763d |
| This journal is © The Royal Society of Chemistry 2023 |