 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ferrocene-driven single-chain polymer compaction†
Sebastian
Gillhuber
 abc,
Joshua O.
Holloway
abc,
Joshua O.
Holloway
 bc,
Hendrik
Frisch
bc,
Hendrik
Frisch
 bc,
Florian
Feist
bc,
Florian
Feist
 d,
Florian
Weigend
d,
Florian
Weigend
 e,
Christopher
Barner-Kowollik
e,
Christopher
Barner-Kowollik
 *bcd and
Peter W.
Roesky
*bcd and
Peter W.
Roesky
 *a
*a
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, Karlsruhe 76131, Germany. E-mail: roesky@kit.edu
bSchool of Chemistry and Physics, Queensland University of Technology (QUT), 2 George Street, Brisbane QLD 4000, Australia. E-mail: christopher.barnerkowollik@qut.edu.au
cCentre for Materials Science, Queensland University of Technology (QUT), 2 George Street, Brisbane QLD 4000, Australia
dInstitute of Nanotechnology (INT), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, Eggenstein-Leopoldshafen 76344, Germany. E-mail: christopher.barner-kowollik@kit.edu
eFachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, Marburg 35043, Germany
First published on 30th March 2023
Abstract
We introduce single-chain nanoparticles (SCNPs) exclusively folded by covalently bonded ferrocene units. Specifially, we demonstrate the ability of 2-ferrocenyl-1,10-phenanthroline to fuse single-chain collapse with the concomitant introduction of a donor functionality allowing the installation of a Pd-catalytic site, affording the first heterobimetallic ferrocene-functionalized SCNP.
Over generations, nature has optimized the constitution and shape of macromolecular structures for catalytic applications.1 One way of mimicking natural enzymes is the synthesis of so called single-chain nanoparticles (SCNPs), which are constructed from single polymer chains folded into compact nanoparticles via intramolecular linkages.2 While nature's perfection regarding sequence, conformation and configuration has never been reached for synthetic polymers,2a,3 controlled polymerization techniques4 allow for the synthesis of functional polymers with low dispersity. SCNP folding can principally be achieved by covalent or non-covalent interactions. Covalent folding can, for instance, be induced by (photo)cycloadditions,5 alkene metatheses6 or reactions of nucleophilic and electrophilic centers.7 Non-covalent crosslinking is often based on the formation of intramolecular hydrogen bonds8 or solvophobic interactions.9 The folding units can either be randomly distributed along the polymer chain (repeat unit folding) or be located at predefined positions (selective point folding).2c
In recent years, our teams have focused on the incorporation of rare-earth and transition metal ions into SCNPs, leading to artificial metalloenzymes.10 Thus, the adjustable characteristics of polymeric materials can be synergistically combined with the properties of metals.10,11 Another intriguing feature of metals is their potential function as a redox switch when more than one stable oxidation state is accessible, although this is as yet largely unexplored in SCNP chemistry. One of the most famous redox switches is the organometallic sandwich complex ferrocene.12 To the best of our knowledge, there are, up to now, only three examples of ferrocene-containing SCNPs reported in the literature.13 However, all of them make use of non-covalent folding approaches, and single-chain collapse was either induced by the formation of hydrogen bonds or β-cyclodextrin insertion complexes.13
Herein, we close a critical gap in the literature, introducing ferrocene as a covalently bonded SCNP crosslinker. The direct polymerization of a ferrocene-containing monomer is however challenging, typically leading to polymers featuring low molar masses or high dispersities and often poor solubility.14 Thus, we made use of a post-polymerization modification approach to incorporate ferrocene moieties into a polymer (Scheme 1). Specifically, a copolymer of styrene and chloromethyl styrene (Polymer P1) was synthesized using nitroxide-mediated polymerization (NMP).15 While styrene constitutes the inert polymer backbone, the chlorobenzyl groups allow for simple and efficient post-polymerization modification by nucleophilic substitution. Size exclusion chromatography (SEC) in N,N-dimethylacetamide (DMAc) gave an indication of the number-averaged molar mass (Mn) of 16,300 g mol−1 and dispersity (Đ) of 1.15 (Fig. 1b). Using 1H NMR spectroscopy, a chloromethyl styrene content of 14% was determined (see ESI† Chapter 3.1), and this copolymer was used as a platform for all reactions described in the current contribution. The described polymer is a random copolymer; therefore, all SCNP folding reactions reported here follow the repeat unit approach.2c As poly(styrene-co-chloromethyl styrene) was selected as open-chain precursor, a nucleophilic ferrocene derivative is necessary to induce single-chain collapse. The most simple one fulfilling this prerequisites is 1,1′-dilithioferrocene, which was therefore prepared in situ, and a solution of polymer P1 slowly added (Scheme 1).
1H NMR spectroscopy indicated the quantitative conversion of the initial polymer by disappearance of the resonance of the chlorobenzyl functionality at δ = 4.68–4.38 ppm (dashed box in Fig. 1a). All resonances of SCNP 1 are broadened compared to the corresponding ones of P1, which might result from a decrease in relaxation time caused by reduced chain-segment mobility upon single-chain collapse.10a,16
To ensure that only intramolecular crosslinking occurred, size sensitive techniques were employed. Fig. 1b shows the number-averaged size distributions of polymer P1 as well as 1, obtained via SEC in DMAc. The apparent peak molar mass (Mp) of 1 is about 5000 g mol−1 lower than that of P1, indicating a decrease in the hydrodynamic volume, characteristic for SCNP compaction (see ESI,† Fig. S7 for retention time plots).2a
To corroborate these findings, dynamic light scattering (DLS) measurements were conducted. Fig. 1c shows the number-averaged size distributions of polymer P1 as well as 1, recorded in tetrahydrofuran (THF). In line with the SEC results, a decrease in the hydrodynamic diameter Dh was observed for 1 (Dh = 4.68 nm) compared to P1 (Dh = 5.69 nm), also indicative of SCNP folding. It is worth noting here that DLS data of particles with such small hydrodynamic diameters needs to be treated with caution as they only possess weak scattering abilities.2d
Additionally, SCNP folding was evidenced by diffusion-ordered NMR spectroscopy (DOSY). Fig. 1d shows the superimposed diffusion domains of P1 and 1, revealing an increase in the diffusion coefficient of 1 (D = 1.99·10−10 m2 s−1) compared to P1 (D = 1.17·10−10 m2 s−1). According to the Stokes–Einstein equation, an increase in the diffusion coefficient goes in hand with a decrease in the hydrodynamic diameter, which again validates the assumed SCNP compaction.
For potential future applications of ferrocene-containing SCNPs, e.g. in redox-switchable catalysis, it is desirable to supplement them with an additional functionality. In an initial step, our interest focused on the combination of ferrocene with a second metal within an SCNP. To achieve this aim, two conceptually distinct approaches are conceivable. On the one hand, the synthesis of a terpolymer containing one co-monomer for folding and another one for metal complexation is possible. On the other hand, SCNP folding and the introduction of a functional group for metal complexation can be combined in an elegant all-in-one approach using a donor-functionalized ferrocene derivative. One candidate for the latter approach is 2-ferrocenyl-1,10-phenanthroline.17 Therefore, analogous to the reaction described above, 2-ferrocenyl-1,10-phenanthroline was lithiated (Scheme 2a). However, NMR studies show that the lithiation is not selective. Therefore, density functional theory (DFT) calculations were performed to deduce the most probable structure which is depicted in Scheme 2. The complexity of the reaction mixture obtained after lithiation of 2-ferrocenyl-1,10-phenanthroline and the determination of the most probable structure of the actual folding unit, based on DFT calculations, is discussed within the ESI† (Chapter 5.2).
 | ||
| Scheme 2 Synthesis of the 2-ferrocenyl-1,10-phenanthroline folded SCNP 2 (a) and Pd functionalization of SCNP 2 to obtain SCNP 2-Pd (b) (simplified scheme). | ||
The lithiated ferrocene derivative was subsequently reacted in situ with P1 to obtain 2. Again, 1H NMR spectroscopy indicated the quantitative conversion of the chlorobenzyl functionalities (δ = 4.68–4.38 ppm) in P1 (Fig. S5, ESI†).
SEC measurements in DMAc (Fig. 2) evidence that the apparent peak molar mass of 2 is decreased by approximately 4000 g mol−1 compared to P1, indicating successful SCNP folding. Compared to 1, the compaction is less pronounced.
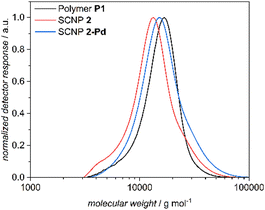 | ||
| Fig. 2 Number-averaged size distributions (SEC, DMAc, RI, PS cal.) of polymer P1 (black), SCNP 2 (red) and SCNP 2-Pd (blue). | ||
DLS measurements in THF corroborated the SEC results. A hydrodynamic diameter of Dh = 5.35 nm was observed for 2. Compared to P1 (Dh = 5.69 nm), the compaction is still evident, however, it is less pronounced than that of 1 (Dh = 4.68 nm). DOSY measurements gave a diffusion coefficient of D = 1.13·10−10 m2 s−1, similar to that of P1 (D = 1.17·10−10 m2 s−1).
SEC, DLS and DOSY clearly evidence SCNP compaction, however, it must be noted that 2 additionally contains an unidentified low molar mass impurity, as is evident from 1H NMR spectroscopy and SEC measurements (Fig. S5 and S9, ESI†). Purification leads to interchain crosslinking, evidenced by SEC (Fig. S9, ESI†) and a drastic decrease in solubility.
To perform catalytic transformations, SCNP 2 needed to be supplemented with an additional metal. As important prere-quisites, the metal must possess catalytic activity and should not undergo chemical reactions with either the SCNP crosslinker or the polymer backbone. To allow for future applications of the ferrocene redox switch, it is additionally necessary that the second metal does not interfere with oxidized ferrocenium moieties. One metal ion expected to fulfil these prerequisites is Pd(II). Therefore, SCNP 2 was reacted with [Pd(cod)Cl2] (cod = 1,5-cyclooctadiene) in a ligand substitution reaction to give SCNP 2-Pd (Scheme 2b). Contrary to 2, there is no evidence for the presence of a low molar mass impurity in the 1H NMR spectrum (Fig. S6, ESI†) and the SEC chromatogram of 2-Pd.
Relative to 2 and in agreement with the expectation that the Pd-functionalization leads to a less compact structure, and thus an increase in the hydrodynamic volume, an increase in the apparent molar mass is observed for 2-Pd (Fig. 2).
DLS measurements of 2-Pd in THF were conducted; however, only inconclusive data, with deviations larger than the actual estimated particle size, were obtained. Presumably, this is a consequence of absorption of the red laser light used in our DLS setup by the sample.
DOSY NMR corroborates the SEC findings as a slight increase in the diffusion coefficient of 2-Pd (D = 1.35·10−10 m2 s−1) compared to P1 (D = 1.17·10−10 m2 s−1) is observed.
Additionally, energy dispersive X-ray spectroscopy (EDX) measurements of 2-Pd were conducted and unambiguously evidence that Pd is indeed present (Fig. S15 and Table S6, ESI†). Successful Pd functionalization was corroborated by UV/Vis measurements combined with time-dependent DFT (TDDFT) calculations (Fig. S16–S18 and ESI† Chapter 5.3) showing that an additional absorption present in the UV/Vis spectrum of 2-Pd, which is absent in the corresponding spectrum of 2, originates from the PdCl2 moiety.
As a proof-of-principle illustrating that heterobimetallic ferrocene SCNPs are highly promising candidates for future applications, we assessed the ability of 2-Pd to function as homogeneous catalyst. To avoid undesirable interferences with the ferrocene moieties, no redox step should be present in the catalytic cycle. One reaction fulfilling this prerequisite is the intramolecular hydroamination of the aminoalkyne 2,2-diphenyl-4-heptyn-1-amine displayed in Fig. S1 (ESI†), as it most probably proceeds via a mechanistic pathway in which Pd remains in the +II oxidation state.18 The same reaction has already been used previously by our groups to demonstrate the catalytic activity of SCNPs.10f [HNMe2Ph][B(C6F5)4] is known to be an efficient co-catalyst for hydroamination reactions.19 The reaction progress was monitored by 1H NMR spectroscopy (Fig. S1, ESI†), and virtually quantitative conversion of the substrate was achieved within three hours at 60 °C, compared to zero conversion to the desired product when only the cocatalyst is present (Fig. S2, ESI†).
In summary, we exploit ferrocene as a simple and efficient crosslinker to collapse individual polymer chains into compact single-chain nanoparticles (SCNPs). For the first time, this was solely achieved by covalently bonded ferrocene. SCNP folding of poly(styrene-co-chloromethyl styrene) following the repeat unit approach was induced by in situ generated 1,1′-dilithioferrocene. Critically, we explored the ability of 2-ferrocenyl-1,10-phenanthroline to function as an elegant all-in-one SCNP crosslinker, combining single-chain collapse with the concomitant introduction of a donor functionality allowing for subsequent metal functionalization. Pd(II) functionalization gave access to the first heterobimetallic ferrocene SCNP, which proved to be an active catalyst for the intramolecular hydroamination of an aminoalkyne.
S.G. acknowledges the Fonds der Chemischen Industrie for financial support in form of a Kekulé fellowship (No. 110160). Helga Berberich is acknowledged for high temperature NMR and Dr Silke Wolf for EDX measurements. C.B.-K. and H.F. acknowledge an Australian Research Council (ARC) Laureate and DECRA Fellowship, respectively.
Conflicts of interest
There are no conflicts to declare.References
- (a) C. M. Dobson, Nature, 2003, 426, 884 CrossRef CAS PubMed; (b) C. B. Anfinsen, Science, 1973, 181, 223 CrossRef CAS PubMed.
- (a) C. K. Lyon, A. Prasher, A. M. Hanlon, B. T. Tuten, C. A. Tooley, P. G. Frank and E. B. Berda, Polym. Chem., 2015, 6, 181 RSC; (b) O. Altintas and C. Barner-Kowollik, Macromol. Rapid Commun., 2012, 33, 958 CrossRef CAS PubMed; (c) O. Altintas and C. Barner-Kowollik, Macromol. Rapid Commun., 2016, 37, 29 CrossRef CAS PubMed; (d) E. Blasco, B. T. Tuten, H. Frisch, A. Lederer and C. Barner-Kowollik, Polym. Chem., 2017, 8, 5845 RSC; (e) A. M. Hanlon, C. K. Lyon and E. B. Berda, Macromolecules, 2016, 49, 2 CrossRef CAS.
- M. A. R. Meier and C. Barner-Kowollik, Adv. Mater., 2019, 31, 1806027 CrossRef PubMed.
- (a) M. Szwarc, Living Polymers and Mechanisms of Anionic Polymerization, Springer-Verlag, Berlin, Heidelberg, Germany, 1983 Search PubMed; (b) M. Kato, M. Kamigaito, M. Sawamoto and T. Higashimura, Macromolecules, 1995, 28, 1721 CrossRef CAS; (c) J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559 CrossRef CAS; (d) P. Vana, T. P. Davis and C. Barner-Kowollik, Macromol. Theory Simul., 2002, 11, 823 CrossRef CAS; (e) R. B. Grubbs, Polym. Rev., 2011, 51, 104 CrossRef CAS; (f) K. Matyjaszewski, Macromolecules, 2012, 45, 4015 CrossRef CAS; (g) S. Perrier, Macromolecules, 2017, 50, 7433 CrossRef CAS.
- (a) E. Harth, B. V. Horn, V. Y. Lee, D. S. Germack, C. P. Gonzales, R. D. Miller and C. J. Hawker, J. Am. Chem. Soc., 2002, 124, 8653 CrossRef CAS PubMed; (b) A. Tuteja, M. E. Mackay, C. J. Hawker, B. Van Horn and D. L. Ho, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1930 CrossRef CAS; (c) J. N. Dobish, S. K. Hamilton and E. Harth, Polym. Chem., 2012, 3, 857 RSC; (d) T. E. Dukette, M. E. Mackay, B. Van Horn, K. L. Wooley, E. Drockenmuller, M. Malkoch and C. J. Hawker, Nano Lett., 2005, 5, 1704 CrossRef CAS PubMed; (e) O. Altintas, J. Willenbacher, K. N. R. Wuest, K. K. Oehlenschlaeger, P. Krolla-Sidenstein, H. Gliemann and C. Barner-Kowollik, Macromolecules, 2013, 46, 8092 CrossRef CAS; (f) I. Perez-Baena, I. Loinaz, D. Padro, I. García, H. J. Grande and I. Odriozola, J. Mater. Chem., 2010, 20, 6916 RSC; (g) A. R. de Luzuriaga, N. Ormategui, H. J. Grande, I. Odriozola, J. A. Pomposo and I. Loinaz, Macromol. Rapid Commun., 2008, 29, 1156 CrossRef; (h) N. Ormategui, I. García, D. Padro, G. Cabañero, H. J. Grande and I. Loinaz, Soft Matter, 2012, 8, 734 RSC; (i) A. R. de Luzuriaga, I. Perez-Baena, S. Montes, I. Loinaz, I. Odriozola, I. García and J. A. Pomposo, Macromol. Symp., 2010, 296, 303 CrossRef; (j) H. Frisch, J. P. Menzel, F. R. Bloesser, D. E. Marschner, K. Mundsinger and C. Barner-Kowollik, J. Am. Chem. Soc., 2018, 140, 9551 CrossRef CAS PubMed.
- A. E. Cherian, F. C. Sun, S. S. Sheiko and G. W. Coates, J. Am. Chem. Soc., 2007, 129, 11350 CrossRef CAS PubMed.
- (a) A. Sanchez-Sanchez, D. A. Fulton and J. A. Pomposo, Chem. Commun., 2014, 50, 1871 RSC; (b) L. Buruaga and J. A. Pomposo, Polymers, 2011, 3, 1673 CrossRef CAS; (c) J. B. Beck, K. L. Killops, T. Kang, K. Sivanandan, A. Bayles, M. E. Mackay, K. L. Wooley and C. J. Hawker, Macromolecules, 2009, 42, 5629 CrossRef CAS PubMed; (d) J. Wen, L. Yuan, Y. Yang, L. Liu and H. Zhao, ACS Macro Lett., 2013, 2, 100 CrossRef CAS PubMed.
- (a) O. Altintas, E. Lejeune, P. Gerstel and C. Barner-Kowollik, Polym. Chem., 2012, 3, 640 RSC; (b) O. Altintas, T. Rudolph and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2566 CrossRef CAS; (c) O. Altintas, P. Gerstel, N. Dingenouts and C. Barner-Kowollik, Chem. Commun., 2010, 46, 6291 RSC.
- (a) R. S. Lokey and B. L. Iverson, Nature, 1995, 375, 303 CrossRef CAS; (b) J. C. Nelson, J. G. Saven, J. S. Moore and P. G. Wolynes, Science, 1997, 277, 1793 CrossRef CAS PubMed; (c) D. D. Prabhu, K. Aratsu, Y. Kitamoto, H. Ouchi, T. Ohba, M. J. Hollamby, N. Shimizu, H. Takagi, R. Haruki, S. I. Adachi and S. Yagai, Sci. Adv., 2018, 4, eaat8466 CrossRef PubMed; (d) P. F. Wu, A. Pietropaolo, M. Fortino, S. Shimoda, K. Maeda, T. Nishimura, M. Bando, N. Naga and T. Nakano, Angew. Chem., Int. Ed., 2022, 61, e202210556 CAS.
- (a) J. Willenbacher, O. Altintas, V. Trouillet, N. Knöfel, M. J. Monteiro, P. W. Roesky and C. Barner-Kowollik, Polym. Chem., 2015, 6, 4358 RSC; (b) N. D. Knöfel, H. Rothfuss, J. Willenbacher, C. Barner-Kowollik and P. W. Roesky, Angew. Chem., Int. Ed., 2017, 56, 4950 CrossRef PubMed; (c) N. D. Knöfel, H. Rothfuss, P. Tzvetkova, B. Kulendran, C. Barner-Kowollik and P. W. Roesky, Chem. Sci., 2020, 11, 10331 RSC; (d) J. L. Bohlen, B. Kulendran, H. Rothfuss, C. Barner-Kowollik and P. W. Roesky, Polym. Chem., 2021, 12, 4016 RSC; (e) A. E. Izuagbe, V. X. Truong, B. T. Tuten, P. W. Roesky and C. Barner-Kowollik, Macromolecules, 2022, 55, 9242 CrossRef CAS; (f) P. H. Maag, F. Feist, H. Frisch, P. W. Roesky and C. Barner-Kowollik, Macromolecules, 2022, 55, 9918 CrossRef CAS.
- (a) J. Willenbacher, O. Altintas, P. W. Roesky and C. Barner-Kowollik, Macromol. Rapid Commun., 2014, 35, 45 CrossRef CAS PubMed; (b) H. Rothfuss, N. D. Knöfel, P. W. Roesky and C. Barner-Kowollik, J. Am. Chem. Soc., 2018, 140, 5875 CrossRef CAS PubMed; (c) H. Rothfuss, N. D. Knöfel, P. Tzvetkova, N. C. Michenfelder, S. Baraban, A.-N. Unterreiner, P. W. Roesky and C. Barner-Kowollik, Chem. – Eur. J., 2018, 24, 17369 CrossRef CAS; (d) N. D. Knöfel, H. Rothfuss, C. Barner-Kowollik and P. W. Roesky, Polym. Chem., 2019, 10, 86 RSC.
- G. B. Kauffman, J. Chem. Educ., 1983, 60, 185 CrossRef CAS.
- (a) T. H. Galow, F. Ilhan, G. Cooke and V. M. Rotello, J. Am. Chem. Soc., 2000, 122, 3595 CrossRef CAS; (b) F. Wang, H. Pu and X. Che, Chem. Commun., 2016, 52, 3516 RSC; (c) F. Wang, H. Pu, Y. Ding, R. Lin, H. Pan, Z. Chang and M. Jin, Polymer, 2018, 141, 86 CrossRef CAS.
- (a) R. Pietschnig, Chem. Soc. Rev., 2016, 45, 5216 RSC; (b) A. S. Gamble, J. T. Patton and J. M. Boncella, Makromol. Chem., Rapid Commun., 1992, 13, 109 CrossRef CAS; (c) C. Herfurth, D. Voll, J. Buller, J. Weiss, C. Barner-Kowollik and A. Laschewsky, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 108 CrossRef CAS; (d) M. Gallei, R. Klein and M. Rehahn, Macromolecules, 2010, 43, 1844 CrossRef CAS; (e) M. Mazurowski, M. Gallei, J. Y. Li, H. Didzoleit, B. Stuhn and M. Rehahn, Macromolecules, 2012, 45, 8970 CrossRef CAS; (f) C. G. Hardy, L. X. Ren, T. C. Tamboue and C. B. Tang, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1409 CrossRef CAS.
- J. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes and B. Charleux, Prog. Polym. Sci., 2013, 38, 63 CrossRef CAS.
- J. He, L. Tremblay, S. Lacelle and Y. Zhao, Soft Matter, 2011, 7, 2380 RSC.
- I. R. Butler and J.-L. Roustan, Can. J. Chem., 1990, 68, 2212 CrossRef CAS.
- T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795 CrossRef PubMed.
- J.-W. Pissarek, D. Schlesiger, P. W. Roesky and S. Blechert, Adv. Synth. Catal., 2009, 351, 2081 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc00736g |
| This journal is © The Royal Society of Chemistry 2023 |


