Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Long-term heat-storage materials based on λ-Ti3O5 for green transformation (GX)
Shin-ichi
Ohkoshi
 *a,
Marie
Yoshikiyo
*a,
Marie
Yoshikiyo
 *a,
Jessica
MacDougall
a,
Yusuke
Ikeda
a and
Hiroko
Tokoro
*ab
*a,
Jessica
MacDougall
a,
Yusuke
Ikeda
a and
Hiroko
Tokoro
*ab
aDepartment of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: ohkoshi@chem.s.u-tokyo.ac.jp; m-yoshikiyo@chem.s.u-tokyo.ac.jp
bDepartment of Materials Science, Faculty of Pure and Applied Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8573, Japan. E-mail: tokoro@ims.tsukuba.ac.jp
First published on 23rd May 2023
Abstract
Effective reuse of waste heat energy is an important energy savings issue for green transformation. In general, phase-change heat storage materials cannot store energy for a prolonged period. If a solid material could conserve the accumulated thermal energy and release it only on demand, then its heat-storage application potential is considerably widened. From this angle, in 2015, we proposed the concept of a long-term heat-storage material, in which latent heat is preserved until the material is triggered by an external stimulus. This feature article describes long-term heat-storage ceramics composed of lambda-trititanium-pentoxide (λ-Ti3O5) from their discovery to heat-storage properties and future applications.
1. Proposal of long-term heat-storage materials for a green transformation
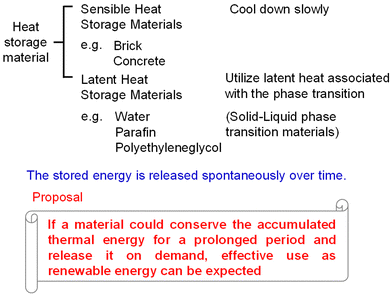
Climate change due to the rise of CO2 concentration is a global problem. To safeguard our planet and pass it to the next generation of humanity, it is imperative to develop technologies and green innovation along with the accompanying social transformation, green transformation (GX).1–14 Although producing nature-friendly energy (renewable energy) is vital, effective reuse of the produced energy and further energy savings are also important. From this point of view, we have developed eco-friendly materials (i.e., heat-storage materials).15,16 One material effectively reuses energy, while another is suitable for energy-conserving optical recordings.For GX, reuse of waste heat energy is instrumental. Currently, approximately 40% of the consumed energy from sources such as oil, gas, and coal is released into the atmosphere as waste heat. This causes several negative environmental effects.7,8 Approximately 80% of this wasted heat energy is below 200 °C (473 K) (Fig. 1).17 The development of high-performance heat-storage materials should help solve these problems. Known heat-storage materials include sensible ones such as bricks and concrete and solid–liquid latent heat-storage ones such as water, paraffin, and polyethylene glycol (Fig. 2).10–15 In general, the heat energy accumulated in a heat-storage material is slowly released over time. The lack of long-term heat storage is known as the “time-gap problem.” The ability to store heat long-term and release it on demand opens numerous practical applications.
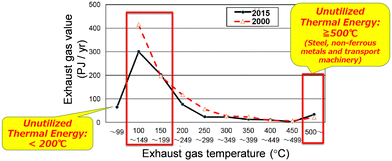 | ||
| Fig. 1 Temperature range vs. the total amount of heat energy for the 38% of input resource energy wasted annually as heat in Japan. Of the wasted heat, 78% of the total caloric value is below 200 °C. Modified from figure in ref. 17, “Toward future utilization of unused heat” of the New Energy and Industrial Technology Development Organization (NEDO), Japan. | ||
Recently, we reported a new concept called long-term heat-storage ceramic.15,16 A long-term heat-storage ceramic can conserve latent heat until the material is activated by an external stimulus. The material is lambda-trititanium-pentoxide (λ-Ti3O5), which shows a reversible phase transition to beta-trititanium-pentoxide (β-Ti3O5) by an external stimulus such as pressure. λ-Ti3O5 also exhibits a reversible photo-induced phase transition between β-Ti3O5 by light irradiation. Moreover, λ-Ti3O5 is a promising eco-friendly recording material composed of only common elements.
In this feature article, we describe λ-Ti3O5 and its metal-substituted series with potential as long-term heat-storage ceramics. This metal oxide can preserve the accumulated heat energy of the phase transition with β-Ti3O5. Applying external pressure releases the energy on demand. The rest of this article is organized as follows. Section 2 explains the discovery and basic physical properties of λ-Ti3O5. Section 3 describes the pressure-induced phase transition and heat-storage property. Section 4 demonstrates the control of the heat-storage property such as the necessary pressure for heat release and the heat-storage temperature. Section 5 discusses two other functionalities: light-induced and current-induced phase transitions. Finally, Section 6 provides the summary and future prospects.
2. Discovery of λ-Ti3O5 and its physical properties
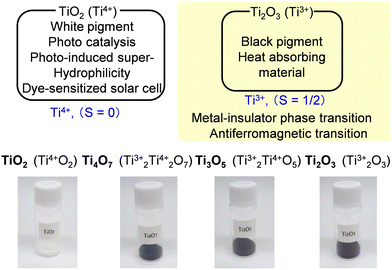
Titanium oxide, TiO2, is a common metal oxide. Tetravalent TiO2 is white. It is used as a white pigment for cosmetics or buildings and as a photocatalyst. By contrast, other titanium oxides, including trivalent titanium, show a black color. They are used in black-color cosmetics or as heat-absorbing materials (Fig. 3). The black color originates from the d-electron in the trivalent titanium. Combining the trivalent and tetravalent titanium ions generates various titanium oxides (TiO2, Ti4O7, Ti3O5, Ti2O3, etc.), which display a range of colors from white to pitch black.To discover new materials or functionalities, we investigated titanium oxide nanoparticles. Nanoparticles of white titanium oxide (TiO2) have been intensively studied for various applications such as photocatalysts. Despite their interesting features, nanoparticles of black titanium oxides are less studied. For example, single crystal of Ti3O5 exhibits a metal–insulator phase transition or an antiferromagnetic transition.18 Our research focuses on nanoparticle synthesis of Ti3O5 to elucidate the influence of particle size on the phase transition properties.15,16,19,20
Synthesis and morphology of λ-Ti3O5
We initially synthesized Ti3O5 as nanoparticles by the following process.15 First, nanoparticles were trapped in glass by a combination method of reverse-micelle and sol–gel processes. Then the precursor was sintered under a hydrogen flow to obtain titanium oxide nanoparticles in the glass matrix. Fig. 4(a) shows the transmission electron microscopy (TEM) image of the obtained nanoparticles in the glass matrix. Finally, the glass matrix was chemically etched to obtain the nanoparticles (Fig. 4(a), inset). The powder X-ray diffraction (PXRD) pattern indicated a new monoclinic structure (space group C2/m) (Fig. 4(b) and (c)). We discovered this new type of titanium oxide in 2010 and named it “lambda-trititanium-pentoxide (λ-Ti3O5)”.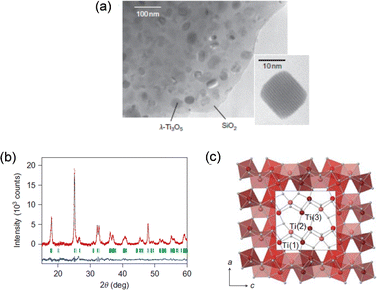 | ||
| Fig. 4 (a) TEM image of λ-Ti3O5 nanoparticles embedded in the SiO2 matrix. Inset shows a single nanocrystal. (b) PXRD pattern of λ-Ti3O5 in SiO2 with Rietveld analysis. Red dots, black lines, and blue lines show the observed pattern, calculated pattern, and their difference, respectively. Green bars show the calculated Bragg positions of λ-Ti3O5. (c) Crystal structure of monoclinic λ-Ti3O5. Reproduced from ref. 15, copyright 2010 Springer Nature. | ||
λ-Ti3O5 shows several types of morphologies, depending on the synthesis. The aforementioned initial synthesis of λ-Ti3O5 gave nanocrystals in a SiO2 matrix. The sol–gel method also produces λ-Ti3O5 nanocrystals.19 The sintering temperature can control the size of the nanocrystals: 8 ± 2 nm for 1123 °C, 9 ± 3 nm for 1133 °C, 9 ± 2 nm for 1143 °C, 10 ± 3 nm for 1153 °C, 11 ± 4 nm for 1163 °C, 13 ± 4 nm for 1173 °C, 25 ± 12 nm for 1200 °C, and 36 ± 15 nm for 1250 °C.
Another synthesis method to prepare large batches of λ-Ti3O5 in fewer steps is to simply sinter anatase-TiO2 nanoparticles (size = 7 nm) under a hydrogen flow.15Fig. 5(a) (upper) shows the scanning electron microscopy (SEM) image of the obtained sample, which has a coral-like morphology with a particle size of 2 μm. Fig. 5(a) (lower) shows the TEM image, indicating that the flake-form morphology is an assembly of primary nanocrystals with an average particle size of 25 nm. This approach can yield λ-Ti3O5 in large quantities.
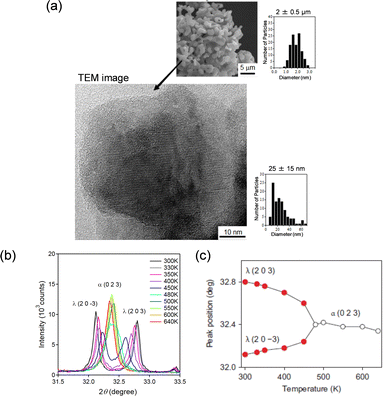 | ||
| Fig. 5 (a) HRTEM image of λ-Ti3O5 prepared from anatase-TiO2 and its nanocrystal size distribution. Inset shows the SEM image and the size distribution of flake-form λ-Ti3O5. (b) Temperature dependence of the PXRD pattern between 31.5° and 33.5°. (c) Temperature dependence of the (2 0 3) peak of λ-Ti3O5 and (0 2 3) peak of α-Ti3O5. Reproduced from ref. 15, copyright 2010 Springer Nature. | ||
PXRD measurements acquired as the temperature is increased show that the diffraction peaks of λ-Ti3O5 are converted to α-Ti3O5 peaks continuously, for example (2 0 −3) and (2 0 3) of λ-Ti3O5 → (0 2 3) of α-Ti3O5 (Fig. 5(b) and (c)). Furthermore, heating the sample to 640 K and subsequently cooling it to 300 K causes α-Ti3O5 peaks to return to λ-Ti3O5 peaks.
Magnetic and electric properties
Fig. 6(a) shows the magnetic susceptibility (χ) versus temperature (T) curve of the flake-form λ-Ti3O5 and that of a conventional single-crystal β-Ti3O5. The χ value of λ-Ti3O5 across the measured temperature range remains at ca. 2 × 10−4 emu per Ti atom, suggesting that λ-Ti3O5 is a Pauli paramagnet due to metallic conduction. The estimated electrical conductivity (σ) value for λ-Ti3O5 is 30 S cm−1, indicating that it is a near metallic conductor. This is consistent with the magnetic data. Fig. 6(b) shows the ultraviolet-visible (UV-vis) and infrared (IR) reflectance spectra. λ-Ti3O5 exhibits metallic absorption over these wavelength ranges. By contrast, β-Ti3O5 is a semiconductor with a band gap of 0.14 eV and a calculated conductivity of 3 × 10−2 S cm−1.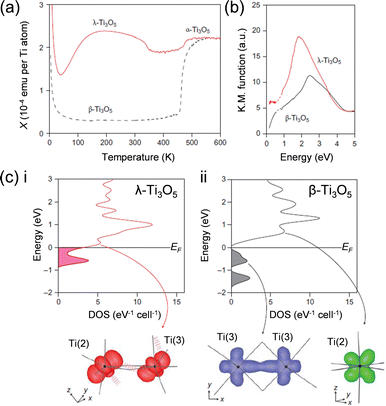 | ||
| Fig. 6 (a) χ vs. T at 0.5 T for flake-form λ-Ti3O5 (red) and single-crystal β-Ti3O5 (dashed black). (b) UV-vis and IR absorption spectra for flake-form λ-Ti3O5 (red) and single-crystal β-Ti3O5 (dashed black). (c) Band structures calculated by VASP for (i) λ-Ti3O5 and (ii) β-Ti3O5 with electron-density maps for the Fermi level displayed below. Reproduced from ref. 15, copyright 2010 Springer Nature. | ||
The valence states for the three Ti sites in λ-Ti3O5 were estimated using the link between the valence state and the bond length.21 The calculated valence states for Ti(1), Ti(2), and Ti(3) are +3.37, +3.20, and +3.53, respectively. These values are close to that of Ti(10/3)+, suggesting that the charge is delocalized over the structure. This further supports that λ-Ti3O5 is a metallic conductor. By contrast, the valence states of β-Ti3O5 suggest that it has a charge-localized system of Ti3+–Ti(11/3)+–Ti(10/3)+ as the valence states of Ti(1), Ti(2), and Ti(3) in β-Ti3O5 are +3.00, +3.79, and +3.32, respectively.
First-principles calculations for λ-Ti3O5 indicate that the bands residing close to the Fermi level are the t2g orbitals from the Ti ion octahedral 3d orbitals. These are split by coupling with adjacent Ti ions. The dxy orbital on Ti(2) forms slipped π stacking (akin to a zig-zag chain) with the dxy orbital on Ti(3), which is located at the Fermi level (Fig. 6(c), left), making λ-Ti3O5 a metallic conductor. In semi-conducting β-Ti3O5, the valence band at −0.60 eV consists of a bipolaron of Ti(3)–Ti(3) formed by σ-bonding with the dxy orbitals on Ti(3). The conduction band at +0.71 eV mainly consists of a vacant dxz orbital on Ti(2) (Fig. 6(c), right).
3. Pressure-induced phase transition and heat-storage properties of λ-Ti3O5
Material and morphology
The heat-storage properties and the pressure-induced phase transition were investigated using λ-Ti3O5 samples synthesized using rutile-TiO2 particles as the starting material.16 Rutile-TiO2 has a particle size of ca. 500 nm, which is much larger than anatase-TiO2 (Fig. 7, upper left). The obtained sample shows a coral-like morphology composed of rectangular-shaped nanorods measuring 200 nm × 30 nm, which we refer to as stripe-type λ-Ti3O5.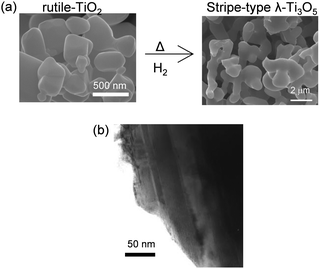 | ||
| Fig. 7 (a) SEM images of (i) rutile-TiO2 and (ii) stripe-type λ-Ti3O5. (b) TEM image of stripe-type λ-Ti3O5 showing 200 nm × 30 nm stripe-type crystal domains. Reproduced from ref. 16, copyright 2015 Springer Nature. | ||
Pressure-induced phase transition
The pressure (P) dependence of the crystal structure of stripe-type λ-Ti3O5 was measured by PXRD. Fig. 8(a) shows the PXRD pattern of the sample at 300 K under atmospheric pressure (P = 0.1 MPa). Rietveld analysis indicated that the sample is 80% λ-Ti3O5 and 20% β-Ti3O5. By applying P = 510 MPa, the PXRD pattern changes to that of β-Ti3O5 (Fig. 8(b)). Fig. 8(c) shows the pressure dependence of the phase fractions of λ-Ti3O5 and β-Ti3O5. The pressure where the fraction of λ-Ti3O5 becomes 50% (P1/2) is ∼60 MPa. Upon further heating, the pressure-formed β-Ti3O5 returns to λ-Ti3O5 at 470 K (Fig. 8(d)).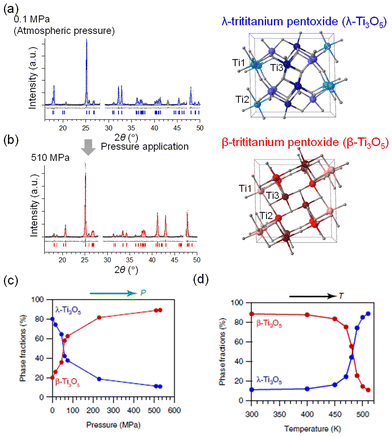 | ||
| Fig. 8 (a) PXRD pattern of stripe-type λ-Ti3O5 at ambient pressure. (b) PXRD of β-Ti3O5 after applying 510 MPa pressure. (c) Pressure dependence of the phase fractions of λ-Ti3O5 and β-Ti3O5. (d) Temperature dependence of the phase fractions of λ-Ti3O5 and β-Ti3O5. Reproduced from ref. 16, copyright 2015 Springer Nature. | ||
Accumulated heat energy and pressure-released energy
Differential scanning calorimetry (DSC) was performed for a detailed examination of the heat-storage process. The transition enthalpy (ΔH) for the first-order phase transition of β-Ti3O5 to λ-Ti3O5 is 230 ± 20 kJ L−1 (12 ± 1 kJ mol−1) (Fig. 9(a)). During the DSC measurement as the temperature decreased, a peak did not appear, indicating that the system conserves the accumulated heat energy of the phase transition from β-Ti3O5 to λ-Ti3O5.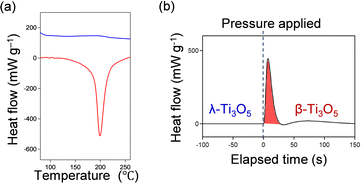 | ||
| Fig. 9 DSC measurements showing (a) the heat-induced transition of β-Ti3O5 to λ-Ti3O5 and (b) the pressure-induced phase transition of stripe-type λ-Ti3O5 to β-Ti3O5. Reproduced from ref. 16, copyright 2015 Springer Nature. | ||
To measure the amount of energy released, pressure was applied to the sample to induce a phase transition of stripe-type λ-Ti3O5 to β-Ti3O5. The released heat energy is 240 ± 40 kJ L−1, which is almost the same as the heat accumulated energy (Fig. 9(b)). The DSC curves show that this material conserves the heat energy of the first phase transition of β-Ti3O5 to λ-Ti3O5. However, this stored energy is released when a low pressure is applied to induce the reverse phase transition of λ-Ti3O5 to β-Ti3O5.
Mechanism
First-principles phonon-mode calculations were conducted for a deeper understanding of the pressure-induced phase transition. Fig. 10(a) shows the calculated phonon density of states (phonon DOS) for λ-Ti3O5 and β-Ti3O5. During the pressure-induced phase transition of λ-Ti3O5 to β-Ti3O5, the coordination geometry of Ti(3) changes. The Ti(3)–O(4) bond forms as the Ti(3)–O(5) bond breaks. The significant phonon modes of λ-Ti3O5 to this transition appear at 248.6 cm−1, 318.5 cm−1, and 445.8 cm−1. In the Bu phonon mode at 445.8 cm−1, Ti(3) vibrates away from O(5) and towards O(4) (Fig. 10(b), upper). During the reverse phase transition (thermally induced), where the Ti(3)–O(4) bond is broken and the Ti(3)–O(5) bond is reformed, the significant phonon modes are 226.7 cm−1 and 339.3 cm−1. In the Bu phonon mode at 226.7 cm−1, Ti(3) now vibrates away from O(4) towards O(5) (Fig. 10(b), lower).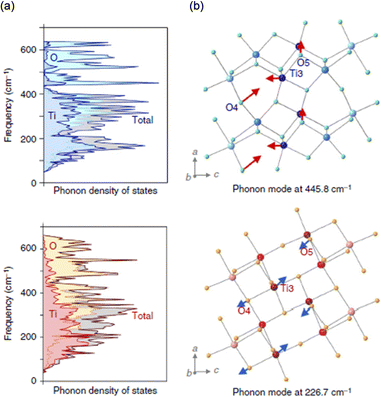 | ||
| Fig. 10 (a) Phonon DOS for stripe-type λ-Ti3O5 (upper) and β-Ti3O5 (lower). (b) Visualizations of the Bu phonon mode for stripe-type λ-Ti3O5 (upper) and β-Ti3O5 (lower). Reproduced from ref. 16, copyright 2015 Springer Nature. | ||
The Slichter and Drickamer mean field model22 was deployed to elucidate the thermodynamics of the pressure-induced phase transition from λ-Ti3O5 to β-Ti3O5. In this model, G is defined by the transition enthalpy (ΔH), the transition entropy (ΔS), and an interaction parameter between the λ-Ti3O5 and β-Ti3O5 phases. The change in Gibbs free energy (G) dictates the generation of stripe-type λ-Ti3O5vs. β-Ti3O5, and this change is thought to occur at the surface/interfacial energy of the nanoscale domain. Fig. 11(a) shows the G vs. λ-Ti3O5 fraction (x) curves from this calculation. Under atmospheric pressure (P = 0.1 MPa), the sample is stable as λ-Ti3O5 after it is formed at higher temperatures due to the energy barrier for the transition between λ-Ti3O5 and β-Ti3O5. When external pressure is applied, the G vs. x curves are affected. Because no energy barrier is present below 400 K for P = 60 MPa, there is a pressure-induced phase transformation from λ-Ti3O5 to β-Ti3O5. Fig. 11(b) plots the x vs. temperature curves for P = 0.1 MPa and P = 60 MPa, and Fig. 11(c) shows the x vs. pressure curve at 300 K, which represents the threshold of this pressure-induced transition.
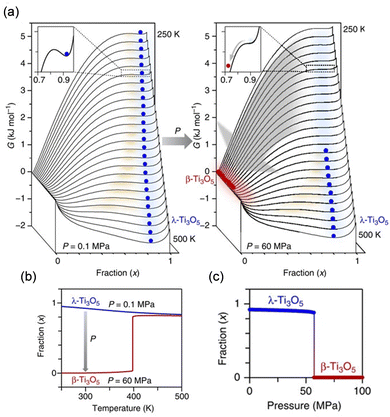 | ||
| Fig. 11 (a) Gibbs free energy (G) vs. fraction of λ-Ti3O5 (x) calculated by the Slichter–Drickamer mean-field model at (i) 0.1 MPa and (ii) 60 MPa external pressure. Blue and red circles denote λ-Ti3O5 and β-Ti3O5, respectively. (b) x vs. T curves at 0.1 MPa (blue) and 60 MPa (red) external pressures. (c) x vs. pressure curve at 300 K. Reproduced from ref. 16, copyright 2015 Springer Nature. | ||
In the SD model, this pressure-induced phase transition originates from the PΔV term of the enthalpy change (ΔH = ΔU + PΔV). From the phonon-mode calculations, the pressure-induced change of PΔV at 60 MPa is 0.19 kJ mol−1, which is two orders of magnitude larger than the change in internal energy (ΔU) of 1 × 10−3 kJ mol−1. The effect on ΔS is also negligible and does not significantly contribute to the phase transition.
In developing novel heat-storage materials, structural phase transition materials have potential as heat-storage materials. In addition to the sufficient magnitude of transition enthalpy, an important criterion for a heat-storage material is to possess a thermal hysteresis in the phase transition. The larger the hysteresis, the wider the possible temperature range is for heat-storage. In the case of λ-Ti3O5, the thermal hysteresis is so wide that this phase is maintained through all temperatures below the heat-storage temperature.15,16 This is the unique characteristic of λ-Ti3O5 realizing the “long-term” heat-storage ceramic that does not release the accumulated heat unless stimulated by pressure. λ-Ti3O5 and its metal-substituted series are the only “long-term” heat-storage materials up to date.
4. Tuning the heat-storage properties of λ-Ti3O5
Controlling the necessary pressure for heat release
Vehicles (e.g., cars, trucks, and buses) use heat energy from combusting fuel in an engine to gain power. The realization of technologies that can store and reuse waste heat while driving would drastically improve fuel consumption. For such applications, the necessary pressure for heat release must be low to compactly equip the heat-release system in an automobile. We investigated the effect of sintering conditions during the synthesis on the necessary pressure for heat release.23The sample was prepared by 1300 °C sintering of rutile-type TiO2 under a hydrogen gas flow. Rietveld analysis of the PXRD pattern determined that λ-Ti3O5 has a monoclinic structure with a C2/m space group. The sample is comprised of block-shaped crystals (Fig. 12). The crystal size is in the sub-micrometer range, which is notably larger than the previously reported samples (stripe-type λ-Ti3O5). This material is called block-type lambda-trititanium-pentoxide (block-type λ-Ti3O5), which reflects the primary particle morphology.
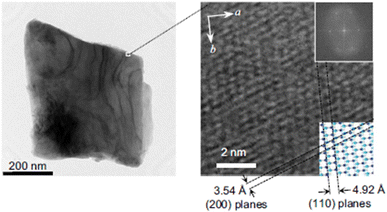 | ||
| Fig. 12 TEM image of block-type λ-Ti3O5 (left) with an enlarged image showing clear lattice fringes (right). Inset shows the FFT image (upper) and the corresponding lattices (lower). Reproduced from ref. 23, copyright 2019 Springer Nature. | ||
PXRD patterns were measured at different applied external pressures to investigate the pressure effect on block-type λ-Ti3O5 (Fig. 13(a) and (b)). As P increases, the phase fraction of λ-Ti3O5 decreases, whereas that of β-Ti3O5 increases until 30 MPa. Above 30 MPa, the phase fractions remain constant. The P1/2 value is 7 MPa.
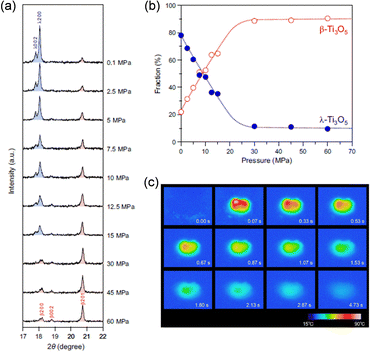 | ||
| Fig. 13 (a) Pressure-dependent PXRD patterns from 0.1 to 60 MPa external pressures. (b) Phase fraction vs. pressure curves of block type λ-Ti3O5 (blue) and β-Ti3O5 (red). (c) Thermographic images of block-type λ-Ti3O5 after applying external pressure at t = 0. Reproduced from ref. 23, copyright 2019 Springer Nature. | ||
Thermography was employed to visually measure the temperature change during a pressure-induced phase transition induced by striking the sample with a hammer. The initial thermal image is blue, and the starting temperature is 26.8 °C. After striking the sample, the thermal image changes to white, and then progressively turns red, orange, yellow, green, and finally returns to blue (Fig. 13(c)). The maximum temperature of the white area is 85.5 °C, which is reached less than 67 ms after applying pressure. These results demonstrate that the heat energy is released immediately upon applying pressure and the temperature exponentially decreases over time with a decay time of 1.7 s.
Materials for use in vehicles should have transition pressures below 10 MPa. Hence, this current heat-storage ceramic has potential for applications in automobile components near engines or mufflers, as the heat-storage ceramic can be employed to rewarm cooled internal systems when the vehicle is restarted. Additionally, changing the morphology can control the threshold pressure.
Controlling the heat-storage temperature
Another important property is the heat-storage temperature. λ-Ti3O5 stores heat near 200 °C and undergoes a phase transition from β-Ti3O5 to λ-Ti3O5. If the heat-storage temperature can be controlled and lowered, the potential for development as heat-storage materials will greatly expand. Thus, the effect of replacing titanium ions with other metal ions has been examined.The synthesis of magnesium-substituted λ-Ti3O5 was first reported by our group in 2017.24 Subsequently, in 2019, Wang et al. discovered that with Mg-substitution, the phase-transition temperature of λ-Ti3O5 to α-Ti3O5 to is decreased.25 We then investigated the effect on the pressure-induced phase transition of λ-Ti3O5 to β-Ti3O5 in 2022.26
Mg-substituted λ-Ti3O5 (λ-MgxTi3−xO5) was synthesized using the following procedure (Fig. 14(a)). First, an aqueous ammonia solution was added to a mixed dispersion of rutile TiO2 particles in aqueous magnesium acetate, forming the Mg ion-covered TiO2 precursor. This precursor was sintered at 1300 °C under a hydrogen gas flow to give λ-MgxTi3−xO5 (x = 0.015, 0.022, 0.043, and 0.053). SEM images showed that the samples have a coral-like shape. Increasing the Mg substitution gradually reduces the diameter of the coral-like structure (Fig. 14(b)). The diameter at x = 0.053 is about half that at x = 0.015.
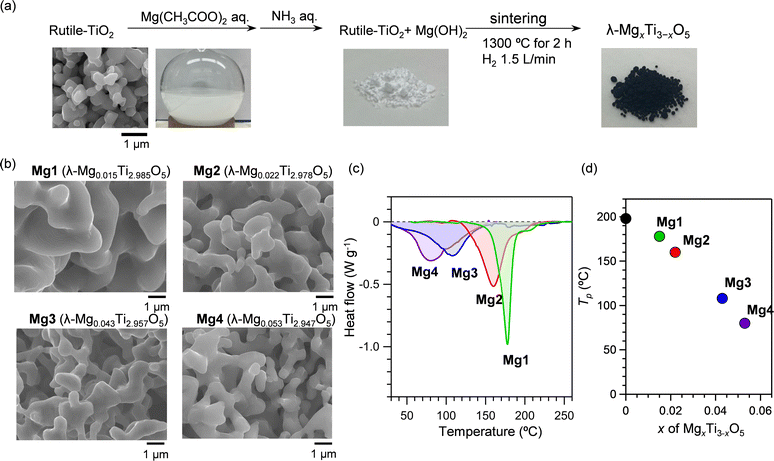 | ||
| Fig. 14 (a) Synthesis procedure of Mg-substituted λ-Ti3O5 (b) SEM images of λ-MgxTi3−xO5 with a coral-like morphology. (c) DSC measurements of Mg-substituted λ-Ti3O5 samples. (d) x vs. T dependence on the transition temperature. Reproduced from ref. 26, with permission from the Royal Society of Chemistry. | ||
The heat-storage properties were evaluated by DSC measurements. The DSC curves contain endothermic peaks at heat-storage temperatures of 178 °C (x = 0.015), 160 °C (x = 0.022), 108 °C (x = 0.043), and 80 °C (x = 0.053) (Fig. 14(c)). The transition enthalpy values are 227 kJ L−1 (x = 0.015), 223 kJ L−1 (x = 0.022), 215 kJ L−1 (x = 0.043), and 216 kJ L−1 (x = 0.053) (Fig. 14(d)). Mg cation substitution decreases the heat-storage temperature from 198 °C (x = 0) to 80 °C (x = 0.053), while maintaining the accumulated heat energy. This reduced heat-storage temperature can be understood by the narrowing of the thermal hysteresis loop caused by the decrease of the interaction parameter within the crystal due to defects, which is supported by mean-field thermodynamic simulations proposed by Slichter and Drickamer (the SD model).
Furthermore, an Sr-substituted λ-Ti3O5 (λ-SrxTi3−xO5) was synthesized.27 λ-SrxTi3−xO5 shows an endothermic peak at 67 °C (340 K) during the DSC measurement, which is below the boiling temperature of water (100 °C). Sc substitution can effectively reduce the heat-storage temperature of the heat-storage ceramics. First-principles calculations suggest that the decrease of the heat-storage temperature (= ΔH/ΔS) originates from the decrease in the ΔH value caused by Sc substitution.
As summarized in Fig. 15, the present long-term heat-storage property in λ-Ti3O5 provides insight into a new concept called heat-storage ceramics. Heat-storage ceramics can store heat energy for a prolonged period as latent heat and release this stored energy on demand by a pressure application. Furthermore, metal substitution on λ-Ti3O5 is an effective approach to broaden the heat-storage performance.
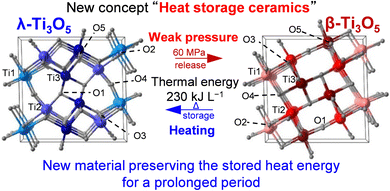 | ||
| Fig. 15 Concept of “heat-storage ceramics” showing an external stimulation-induced reversible phase transfer between λ-Ti3O5 (left) and β-Ti3O5 (right). | ||
As a future direction for the development of long-term heat-storage materials, controlling the thermal hysteresis is the key. Any first-order phase transition material with a thermal hysteresis can be a candidate for latent heat-storage materials, and the width of the thermal hysteresis decides the active temperature range and the energy storage period. A previous study indicates that the interaction parameter, which is affected by the crystalline size, defects, etc., influences the width of the thermal hysteresis.23,26,28 Exploring the hysteresis of various first-order phase transitions could lead to the discovery of new families of long-term heat-storage materials.
5. Other functions: light- and current-induced phase transitions
Light-induced phase transition
Herein we introduce a metal–semiconductor phase transition with λ-Ti3O5 at ambient temperature.15 This was the first demonstration of a photo-rewritable phenomenon for a metal oxide. This phenomenon is possible due to the specific state of λ-Ti3O5, which is trapped at a thermodynamic local energy minimum.Irradiating the flake form of λ-Ti3O5 with 532 nm nanosecond laser light (6 ns, five shots, 1.5 × 10−5 mJ μm−2 pulse−1) at room temperature causes the irradiated area to change from navy to brown. Further irradiating with 410 nm laser light (8 × 10−3 mW μm−2) restores the navy color at the irradiated spot (Fig. 16). The color change by alternating light irradiation with 532 nm and 410 nm light is repeatedly observed. The XRD pattern indicated that the brown area is β-Ti3O5. Irradiating with 532 nm light induces a phase transition from λ-Ti3O5 to β-Ti3O5, as demonstrated by the color change of navy to brown. By contrast, irradiating with 410 nm light induces the reverse transition. A similar phase-transition pattern appears when the sample is irradiated with 355 and 1064 nm nanosecond-pulsed laser light.
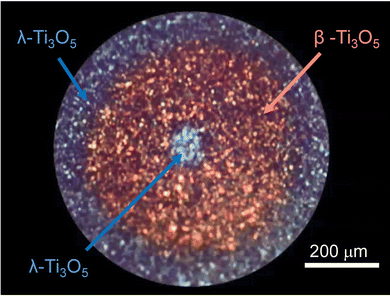 | ||
| Fig. 16 Photograph showing the result of irradiating flake-form λ-Ti3O5 with a 532 nm nanosecond laser pulse followed by a 410 nm nanosecond laser pulse. | ||
The SD model can explain the mechanism of the light-induced phase transition. As explained in Section 3, an energy barrier exists between the charge-localized β-Ti3O5 and charge-delocalized λ-Ti3O5 throughout the entire temperature range when an external pressure is not applied. The light-induced phase transition from λ-Ti3O5 (navy) to β-Ti3O5 (brown) is a transition from a thermodynamically trapped metastable phase at a local energy minimum state to the true stable phase by light. Since metallic absorption allows λ-Ti3O5 to effectively absorb light over a wide range of wavelengths from ultraviolet to near-infrared, the λ- to β-Ti3O5 transition appears by irradiating with 355, 532, or 1064 nm nanosecond-pulsed laser lights. The reverse photo-induced phase transition from β-Ti3O5 to λ-Ti3O5 is an excitation from the valence band to the conduction band on β-Ti3O5, where the excited state changes directly to λ-Ti3O5 in the pulsed laser irradiation or as a photothermal transition from β-Ti3O5 to λ-Ti3O5.
Recently, dynamic observations of the light-induced phase transition from β-Ti3O5 to λ-Ti3O5 were performed using ultrafast time-resolved PXRD measurements at the Swiss X-ray Free Electron Laser facility (Swiss-FEL).29 The experiments revealed that the crystal structure of the Ti3O5 crystal deforms within 500 fs after light irradiation and the phase transition is proceeded by strain waves propagating from the light-irradiated Ti3O5 surface through the crystal on the order of picoseconds (Fig. 17). This propagation speed is much faster than the phase transition caused by thermal diffusion (ca. 100 ns).
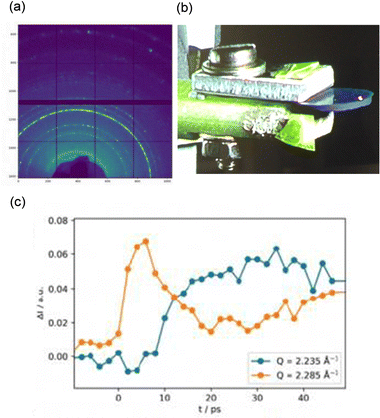 | ||
| Fig. 17 (a) Debye–Scherrer ring of a sample observed using 6.6 keV photons. (b) Photograph of sample and measurement setup. (c) Lattice distortion over time. Reproduced from ref. 29, copyright 2021 Springer Nature. | ||
Current-induced phase transition
As another stimulus, the electric current was investigated as a trigger for the phase transition.17 A pressure-formed sample of β-Ti3O5 was exposed to an electric current of 0.4 A mm−2, causing the brown sample to turn navy (Fig. 18(a)). The PXRD patterns prior to and following the applied electric current showed that β-Ti3O5 is converted into λ-Ti3O5 (Fig. 18(b)). The threshold current of the current-induced phase transition is 0.2 A mm−2, according to the electrical current dependence of the transformation of pressure-formed β-Ti3O5 to λ-Ti3O5 (Fig. 18(c)). This current-induced phase transition likely originates from (i) the breaking of charge ordering or (ii) the Joule heat.30–32 As shown in Fig. 6, β-Ti3O5 has a localized charge on Ti3+(3) and an empty orbital on Ti4+(2), while λ-Ti3O5 has a delocalized charge on Ti(2) and Ti(3). Applying an electric current to β-Ti3O5 impels the localized charge on Ti(3) to the vacant orbital of Ti(2), resulting in charge-delocalized λ-Ti3O5.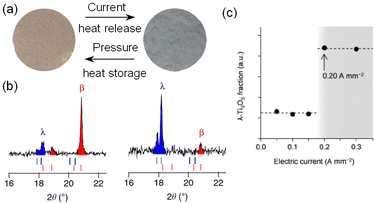 | ||
| Fig. 18 (a) Color change of the sample after exposure to an electric current of 0.4 A mm−2. (b) PXRD patterns before (left) and after (right) applying current. (c) Phase fraction of λ-Ti3O5vs. electric current. Reproduced from ref. 16, copyright 2015 Springer Nature. | ||
6. Summary and perspectives
This article details λ-Ti3O5 and a metal-substituted series called long-term heat-storage ceramics. This metal oxide can conserve the accumulated heat energy of the phase transition with β-Ti3O5 and release the stored energy on demand by applying external pressure. The first section provides the background that leads to the concept of long-term heat-storage ceramics. The second section explains how λ-Ti3O5 was first discovered and its physical properties with an emphasis on the phase transition between λ-Ti3O5 and β-Ti3O5. The third section introduces the pressure-induced phase transition from λ-Ti3O5 to β-Ti3O5 and the heat-storage property. The fourth section details approaches to control the heat-storage property. Increasing the particle size reduces the necessary pressure for heat release to 7 MPa, while substituting Ti with Mg or Sc decreases the heat-storage temperature below 100 °C. The fifth section illustrates two other features: a light-induced phase transition and a current-induced phase transition between λ-Ti3O5 and β-Ti3O5.Such a functional metal oxide with unique heat-storage properties has potential in diverse applications (Fig. 19). For example, λ-Ti3O5 may be used in automobile components near engines and mufflers to rewarm cooled internal systems using the conserved waste heat upon restarting.33,34 Additionally, this heat-storage ceramic has potential in solar power plants, which often have nitrate-based heat-storage tanks. As λ-Ti3O5 has both sensible and long-lasting latent heat storage, it should be a convenient alternative.5,6,35 Furthermore, Mg-substituted λ-Ti3O5 or Sc-substituted λ-Ti3O5 with low heat-storage temperatures should be useful to reuse heat waste at power plants or factories (Fig. 20), where 70% of the generated heat energy is wasted below 100 °C.3 Heat-storage materials functioning below the boiling point of water can recapture the thermal energy from cooling water in electric power plant turbines, which is vital to prevent rising sea-water temperatures. Moreover, the heat-storage temperature can easily be tuned by changing the Mg or Sc content in λ-Ti3O5 according to the target application. Such a tunable material series will expand opportunities to use thermal energy that has not been recycled.
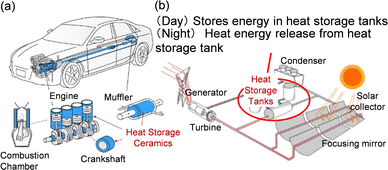 | ||
| Fig. 19 Suggested applications for heat-storage ceramic λ-Ti3O5 in (a) internal systems of vehicles for reheating engines and (b) energy-storage tanks in solar-power systems. Reproduced from ref. 23, copyright 2019 Springer Nature. | ||
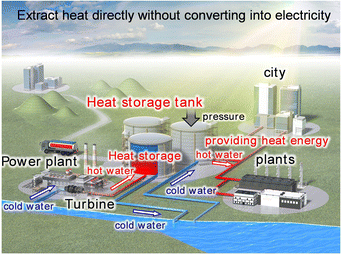 | ||
| Fig. 20 Suggested application for heat-storage ceramics, Mg- and Sc-substituted λ-Ti3O5 for the conservation of wasted heat energy in power plants or factories. Reproduced from ref. 27, reprinted with permission from AAAS. | ||
In addition to heat-storage applications, the present material has potential in pressure-sensitive conductivity or optical sensors because λ-Ti3O5 is a metallic conductor while β-Ti3O5 is a semiconductor. Furthermore, the light-induced and current-induced phase transitions between β-Ti3O5 and λ-Ti3O5 are enticing for developing advanced electronic devices. In particular, the light-induced phase transition opens possibilities for λ-Ti3O5 as a candidate for next-generation superhigh-density optical storage media. The future memory density is expected to achieve one terabit inch−2. The vast potential of λ-Ti3O5 has attracted many researchers: various synthesis methods have been developed,36–49 studies of phase transition mechanism have been reported,50–66 and novel functionalities are being explored.67–76 Since λ-Ti3O5 is composed of readily available elements of titanium and oxygen, it offers harmless, sustainable, and economic features, which are advantageous in industrial applications.
One of the most important tasks for the research of λ-Ti3O5 material family would be the investigation of the strategy for increasing the amount of stored energy, the ΔH value between λ-Ti3O5 and β-Ti3O5. This task is of high interest not only for practical applications but also for basic science unveiling the details of the structural transformation during the phase transition. Such studies will involve theoretical calculations of the phase transition dynamics as well as highly precise time-resolved measurements and novel synthetic approaches, i.e., various aspects of basic research. The present material holds numerous directions for future research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by a JSPS Grant-in-Aid for Scientific Research (A) (grant number 20H00369), a Japan Science and Technology FOREST Program (JPMJFR213Q), Yazaki Memorial Foundation for Science and Technology. We recognize the Cryogenic Research Center at The University of Tokyo, the Center for Nano Lithography & Analysis at The University of Tokyo, and Quantum Leap Flagship Program (Q-LEAP, grant number JPMXS0118068681) supported by MEXT.Notes and references
- D. Butler, Nature, 2007, 445, 768–769 CrossRef PubMed.
- D. Lindley, Nature, 2009, 458, 138–141 CrossRef CAS PubMed.
- S. A. Rattner and S. Garimella, Energy, 2011, 36, 6172–6183 CrossRef.
- D. B. Gingerich and M. S. Mauter, Environ. Sci. Technol., 2015, 49, 8297–8306 CrossRef CAS PubMed.
- G. W. Crabtree and N. S. Lewis, Phys. Today, 2007, 60, 37–42 CrossRef CAS.
- E. Cartlidge, Science, 2011, 334, 922–924 CrossRef PubMed.
- C. W. King, A. S. Holman and M. E. Webber, Nat. Geosci., 2008, 1, 283–286 CrossRef CAS.
- M. D. Bartos and M. V. Chester, Nat. Clim. Change, 2015, 5, 748–752 CrossRef.
- M. M. Farid, A. M. Khudhair, S. A.-K. Razack and S. Al-Hallaj, Energy Convers. Manage., 2004, 45, 1597–1615 CrossRef CAS.
- A. Sharma, V. V. Tyagi, C. R. Chen and D. Buddhi, Renewable Sustainable Energy Rev., 2009, 13, 318–345 CrossRef CAS.
- M. Barrio, D. O. López, J. L. Tamarit, P. Negrier and Y. Haget, J. Solid State Chem., 1996, 124, 29–38 CrossRef CAS.
- I. Gur, K. Sawyer and R. Prasher, Science, 2012, 335, 1454–1455 CrossRef PubMed.
- M. K. Nahas and F. H. Constable, Nature, 1938, 142, 837 CrossRef.
- K. S. Al-Jabri, A. W. Hago, A. S. Al-Nuaimi and A. H. Al-Saidy, Cem. Concr. Res., 2005, 35, 1472–1479 CrossRef CAS.
- S. Ohkoshi, Y. Tsunobuchi, T. Matsuda, K. Hashimoto, A. Namai, F. Hakoe and H. Tokoro, Nat. Chem., 2010, 2, 539–545 CrossRef CAS PubMed.
- H. Tokoro, M. Yoshikiyo, K. Imoto, A. Namai, T. Nasu, K. Nakagawa, N. Ozaki, F. Hakoe, K. Tanaka, K. Chiba, R. Makiura, K. Prassides and S. Ohkoshi, Nat. Commun., 2015, 6, 7037 CrossRef CAS PubMed.
- A. Kondo, NEDO, presented in part the 10th German-Japanese Environment and Energy Dialogue Forum, https://www.nedo.go.jp/content/100899759.pdf, Tokyo, October, 2019.
- M. Onoda, J. Solid State Chem., 1998, 136, 67–73 CrossRef CAS.
- T. Nasu, H. Tokoro, K. Tanaka, F. Hakoe, A. Namai and S. Ohkoshi, Mater. Sci. Eng., 2014, 54, 012008 CAS.
- Y. Araki, S. Ohkoshi and H. Tokoro, Mater. Today Energy, 2020, 18, 100525 CrossRef CAS.
- W. H. Zachariasen, J. Less-Common Met., 1978, 62, 1–7 CrossRef CAS.
- C. P. Slichter and H. G. Drickamer, J. Chem. Phys., 1972, 56, 2142–2160 CrossRef CAS.
- S. Ohkoshi, H. Tokoro, K. Nakagawa, M. Yoshikiyo, F. Jia and A. Namai, Sci. Rep., 2019, 9, 13203 CrossRef PubMed.
- S. Ohkoshi, Y. Maeno and T. Nasu, International Pat., WO2017/164083, 2017 Search PubMed.
- M. Wang, W. Huang, Z. Shen, J. Gao, Y. Shi, T. Lu and Q. Shi, J. Alloys Compd., 2019, 774, 1189–1194 CrossRef CAS.
- S. Ohkoshi, F. Jia, M. Yoshikiyo, K. Imoto, H. Tokoro, K. Nakagawa, Y. Maeno, A. Namai, R. Harada, K. Hattori, K. Kojima, K. Sugiura and T. Suganuma, Mater. Adv., 2022, 3, 4824–4830 RSC.
- Y. Nakamura, Y. Sakai, M. Azuma and S. Ohkoshi, Sci. Adv., 2020, 6, 5264 CrossRef PubMed.
- H. Tokoro, A. Namai, M. Yoshikiyo, K. Chiba, R. Fujiwara and S. Ohkoshi, Sci. Rep., 2018, 8, 63 CrossRef PubMed.
- C. Mariette, M. Lorenc, H. Cailleau, E. Collet, L. Guérin, A. Volte, E. Trzop, R. Bertoni, X. Dong, B. Lépine, O. Hernandez, E. Janod, L. Cario, V. Ta Phuoc, S. Ohkoshi, H. Tokoro, L. Patthey, A. Babic, I. Usov, D. Ozerov, L. Sala, S. Ebner, P. Böhler, A. Keller, A. Oggenfuss, T. Zmofing, S. Redford, S. Vetter, R. Follath, P. Juranic, A. Schreiber, P. Beaud, V. Esposito, Y. Deng, G. Ingold, M. Chergui, G. F. Mancini, R. Mankowsky, C. Svetina, S. Zerdane, A. Mozzanica, A. Bosak, M. Wulff, M. Levantino, H. Lemke and M. Cammarata, Nat. Commun., 2021, 12, 1239 CrossRef CAS PubMed.
- S. Ikeda, K. Miura, H. Yamamoto, K. Mizunuma, H. D. Gan, M. Endo, S. Kanai, J. Hayakawa, F. Matsukura and H. Ohno, Nat. Mater., 2010, 9, 721–724 CrossRef CAS PubMed.
- A. Asamitsu, Y. Tomioka, H. Kuwahara and Y. Tokura, Nature, 1997, 388, 50–52 CrossRef CAS.
- M. Yamanouchi, D. Chiba, F. Matsukura and H. Ohno, Nature, 2004, 428, 539–542 CrossRef CAS PubMed.
- A. Honda and S. Ohkoshi, JP Pat., JP6426658, 2018 Search PubMed.
- A. Honda and S. Ohkoshi, DE Pat., DE102017109005, 2018 Search PubMed.
- S. Garimella, K. Lockyear, D. Pharis, O. El Chawa, M. T. Hughes and G. Kini, Joule, 2022, 6, 956–971 CrossRef CAS.
- G. Liu, W. X. Huang and Y. Yi, J. Inorg. Mater., 2013, 28, 425–430 CrossRef CAS.
- Y. Chen and J. Mao, J. Mater. Sci.: Mater. Electron., 2014, 25, 1284–1288 CrossRef.
- N. Stem, M. L. de Souza, D. L. A. de Faria and S. G. dos Santos Filho, Thin Solid Films, 2014, 558, 67–74 CrossRef CAS.
- G. Chai, W. Huang, Q. Shi, S. Zheng and D. Wei, J. Alloys Compd., 2015, 621, 404–410 CrossRef CAS.
- D. Wei, W. Huang, Q. Shi, T. Lu and B. Huang, J. Mater. Sci.: Mater. Electron., 2016, 27, 4216–4222 CrossRef CAS.
- Z. Shen, Q. Shi, W. Huang, B. Huang, M. Wang, J. Gao, Y. Shi and T. Lu, Appl. Phys. Lett., 2017, 111, 191902 CrossRef.
- L. Wang, X. Zhang, W. Liu, W. Xu, A. Singh and Y. Lin, J. Mater. Sci.: Mater. Electron., 2017, 28, 6421–6425 CrossRef CAS.
- W. Qi, J. Du, Y. Peng, Y. Wang, Y. Xu, X. Li, K. Zhang, C. Gong, M. Luo and H. Peng, Vacuum, 2017, 143, 380–385 CrossRef CAS.
- Z. Ertekin, N. Ö. Pekmez and K. Pekmez, J. Solid State Electrochem., 2020, 24, 975–986 CrossRef CAS.
- H. Chen, Y. Hirose, K. Nakagawa, K. Imoto, S. Ohkoshi and T. Hasegawa, Appl. Phys. Lett., 2020, 116, 201904 CrossRef CAS.
- Y. Cai, Q. Shi, M. Wang, X. Lv, Y. Cheng and W. Huang, J. Alloys Compd., 2020, 848, 156585 CrossRef CAS.
- P. Sun, X. Hu, G. Wei, R. Wang, Q. Wang, H. Wang and X. Wang, Appl. Surf. Sci., 2021, 548, 149269 CrossRef CAS.
- P. Zhao, G. Li, W. Li, P. Cheng, Z. Pang, X. Xiong, X. Zou, Q. Xu and X. Lu, Trans. Nonferrous Met. Soc. China, 2021, 31, 3310–3327 CrossRef CAS.
- K. Yoshimatsu and H. Kumigashira, Cryst. Growth Des., 2022, 22, 703–710 CrossRef CAS.
- R. Liu and J.-X. Shang, Modell. Simul. Mater. Sci. Eng., 2012, 20, 035020 CrossRef.
- R. Liu, J.-X. Shang and F.-H. Wang, Comput. Mater. Sci., 2014, 81, 158–162 CrossRef CAS.
- A. Asahara, H. Watanabe, H. Tokoro, S. Ohkoshi and T. Suemoto, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 014303 CrossRef CAS.
- A. Ould-Hamouda, H. Tokoro, S. Ohkoshi and E. Freysz, Chem. Phys. Lett., 2014, 608, 106–112 CrossRef CAS.
- D. Olguín, E. Vallej and A. Rubio-Ponce, Phys. Status Solidi B, 2015, 252, 659–662 CrossRef.
- E. Vallejo and D. Olguín, Mater. Res. Express, 2015, 2, 126101 CrossRef.
- F. Hakoe, H. Tokoro and S. Ohkoshi, Mater. Lett., 2017, 188, 8–12 CrossRef CAS.
- K. Kobayashi, M. Taguchi, M. Kobata, K. Tanaka, H. Tokoro, H. Daimon, T. Okane, H. Yamagami, E. Ikenaga and S. Ohkoshi, Phys. Rev. B, 2017, 95, 085133 CrossRef.
- K. R. Tasca, V. Esposito, G. Lantz, P. Beaud, M. Kubli, M. Savoini, C. Giles and S. L. Johnson, ChemPhysChem, 2017, 18, 1385–1392 CrossRef CAS PubMed.
- X.-K. Fu, W.-Q. Chen, Z.-S. Jiang, B. Yang, X. Zhao and L. Zuo, Acta Phys. Sin., 2019, 68, 207301 CrossRef.
- X.-K. Fu, B. Yang, W.-Q. Chen, Z.-S. Jiang, Z.-B. Li, H.-L. Yan, X. Zhao and L. Zuo, Comput. Mater. Sci., 2020, 173, 109435 CrossRef CAS.
- X. Fu, X. Hao, W. Chen, B. Yang, X. Zhao and L. Zuo, J. Phys.: Condens. Matter, 2020, 32, 46LT01 CrossRef CAS PubMed.
- S. V. Hosseini, M. Abbasnejad and M. R. Mohammadizadeh, Phys. Rev. B, 2021, 104, 224101 CrossRef CAS.
- T. Takeda and S. Ohkoshi, Eur. J. Inorg. Chem., 2022, e202101037 CAS.
- T. Saiki, T. Yoshida, K. Akimoto, D. Indo, M. Arizono, T. Okuda and T. Katsufuji, Phys. Rev. B, 2022, 105, 075134 CrossRef CAS.
- D. Peng, N. Jin, Y. Liu, T. Yuan, P. Xiao and J. Ye, Mater. Today Commun., 2022, 31, 103332 CrossRef CAS.
- S. Jütten and T. Bredow, J. Phys. Chem. C, 2022, 126, 7809–7817 CrossRef.
- A. Kitada, G. Hasegawa, Y. Kobayashi, K. Kanamori, K. Nakanishi and H. Kageyama, J. Am. Chem. Soc., 2012, 134, 10894–10898 CrossRef CAS PubMed.
- G. Hasegawa, T. Sato, K. Kanamori, K. Nakano, T. Yajima, Y. Kobayashi, H. Kageyama, T. Abe and K. Nakanishi, Chem. Mater., 2013, 25, 3504–3512 CrossRef CAS.
- Z. Ertekin, U. Tamer and K. Pekmez, Electrochim. Acta, 2015, 163, 77–81 CrossRef CAS.
- Q. Shi, G. Chai, W. Huang, Y. Shi, B. Huang, D. Wei, J. Qi, F. Su, W. Xu and T. Lu, J. Mater. Chem. C, 2016, 4, 10279–10285 RSC.
- X. Zhang, W. Liu, H. Yu, X. Zhong, L. Wang, A. Singh and Y. Lin, Micro Nano Lett., 2016, 11, 811–813 CrossRef CAS.
- R. Alipour Moghadam Esfahani, S. K. Vankova, A. H. A. Monteverde Videla and S. Specchia, Appl. Catal., B, 2017, 201, 419–429 CrossRef CAS.
- R. Takahama, T. Ishii, D. Indo, M. Arizono, C. Terakura, Y. Tokura, N. Takeshita, M. Noda, H. Kuwahara, T. Saiki, T. Katsufuji, R. Kajimoto and T. Okuda, Phys. Rev. Mater., 2020, 4, 074401 CrossRef CAS.
- X. Fu, W. Chen, X. Hao, Z. Zhang, R. Tang, B. Yang, X. Zhao and L. Zuo, J. Mater. Chem. C, 2021, 9, 7976–7981 RSC.
- E. Widyastuti, F.-Y. Xu, C.-T. Chiu, J.-H. Jan, J.-L. Hsu and Y.-C. Lee, Catalysts, 2021, 11, 1416 CrossRef CAS.
- S. Zhang, Y. Zhang, Y. Huang, B. Lin, S. Ling, C. Mei and M. Pan, Chem. Eng. J., 2022, 450, 137910 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |