Open Access Article
Open Access ArticlePd-Catalyzed intermolecular asymmetric allylic dearomatization of 1-nitro-2-naphthols with MBH adducts†
Qing-Xia
Zhang
,
Jia-Hao
Xie
,
Qing
Gu
and
Shu-Li
You
 *
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032, China. E-mail: slyou@sioc.ac.cn
First published on 1st March 2023
Abstract
An asymmetric allylic dearomatization reaction of 1-nitro-2-naphthol derivatives with Morita–Baylis–Hillman (MBH) adducts has been developed. By utilizing Pd catalyst derived from Pd(OAc)2 and Trost ligand (R,R)-L1, the reaction proceeded smoothly in 1,4-dioxane at room temperature, affording substituted β-naphthalenones in good yields (up to 92%) and enantioselectivity (up to 90% ee). A range of substituted 1-nitro-2-naphthols and MBH adducts were found to be compatible under the optimized conditions. This reaction provides a convenient method for the synthesis of enantioenriched 1-nitro-β-naphthalenone derivatives.
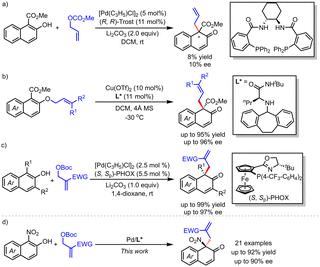
Dearomatization reactions can transform planar aromatic compounds into three-dimensional chiral molecules.1 β-Naphthalenone is a core skeleton widely found in natural products and biologically active molecules.2 Therefore, extensive efforts have been dedicated to the construction of β-naphthalenones through dearomatization of 2-naphthol derivatives. In this regard, asymmetric dearomatization of 1,3-disubstituted-2-naphthols has led to the formation of C–C,3 C–N,4 C–O5 and other C–X (X = F, Cl, S) bonds with high efficiency in the past decade.6 However, introducing a substituent at the C3 position of 2-naphthol is critical for regulating C/O selectivity and enantioselectivity of the reaction. Good chemoselectivity could be obtained only with 1,3-disubstituted 2-naphthols, wherein the steric hindrance originating from the 1,3-disubstituted groups would effectively prevent the competitive O-alkylation process. Correspondingly, C1-monosubstituted 2-naphthols usually suffer from poor regio- and enantio-selective control in the dearomatization reactions. Recently, dearomatization of C1-monosubstituted 2-naphthol derivatives has been accomplished through amination, halogenation, hydroxylation, azidation, and arylation reactions.7 However, there are limited examples for their asymmetric varients.7e,i–l,8 In 2014, our group realized an allylic dearomatization of methyl 2-hydroxy-1-naphthoate in excellent chemo- and regio-selectivities. Unfortunately, low enantioselective control (10% ee) was observed in the presence of the (R,R)-Trost ligand after systematically screening the chiral ligands (Scheme 1a).9 Later, Ishihara and coworkers10 reported an elegant Cu-catalyzed asymmetric [1,3] O-to-C rearrangement reaction, giving β-naphthalenones in excellent yields and enantioselectivity (Scheme 1b). Despite of this elegant work, the development of novel intermolecular asymmetric dearomatization reaction of 1-substituted 2-naphthols for the synthesis of β-naphthalenones is still in great demand.
In our recent work, palladium-catalyzed asymmetric allylic alkylation of 1,3-disubstituted-2-naphthols with MBH adducts was relized (Scheme 1c).11 Considering our continuous interests on asymmetric allylic dearomatization reactions, we recently investigated palladium-catalyzed allylic dearomatization reaction of 1-nitro-2-naphthol with MBH adducts. This reaction proceeded smoothly in the presence of Pd catalyst derived from the Trost ligand, resulting in 1-nitro-2-naphthalenones with moderate to excellent chemo- and enantio-selective control (≥19/1 C/O selectivity, up to 90% ee, Scheme 1d). Herein, we report the details of this study.
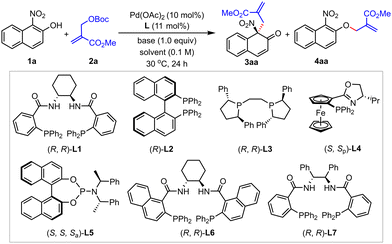
The initial exploration of the reaction was launched between 1-nitro-2-naphthol 1a and methyl-substituted MBH carbonate 2a (1.2 equiv.) in the presence of Pd(OAc)2 (10 mol%), Trost ligand (R,R)-L1 (11 mol%), and Li2CO3 (1.0 equiv.) in 1,4-dioxane under argon at 30 °C. To our delight, the reaction proceeded smoothly to deliver 3aa in 78% NMR yield and 84% ee. Meanwhile, the etherification product 4aa was obtained in 16% NMR yield (Table 1, entry 1). Encouraged by these preliminary results, various phosphine ligands were then investigated. The reaction did not occur when (R)-BINAP (L2), (R,R)-BPE (L3) or (S,Sp)-PHOX ligand (L4) was used. The Feringa ligand (S,S,Sa)-L5 delivered the dearomatization product in racemic form, but in excellent yield and regioselectivity (entry 5, 97% yield, 19/1 C/O selectivity). Further screening of Trost-type ligands revealed that both L6 and L7 were slugglish in this reaction (entry 6, <5% yield; entry 7, 11% yield, 2/5 C/O selectivity, 80% ee). Thus, (R,R)-L1 was proved to be the optimal ligand in terms of reactivity and selectivity (for complete screening of ligands, see the ESI† for details). Subsequently, the solvent effect was investigated with (R,R)-L1. The reaction in DCM, toluene or THF delivered 3aa in poor yield and C/O selectivity with moderate enantioselectivity (entries 8–10, 13–43% yields, 2/1–5/11 C/O selectivity, 74–87% ee). The examination of bases indicated that Et3N gave the best results (entry 13, 92% NMR yield, 84% ee). Besides, the reaction with Et3N in THF was examined. It could occur smoothly with good enantioselective control, albeit with slightly reduced yield and C/O selectivity (entry 14, 60% yield, 15/1 C/O selectivity, 87% ee). Therefore, 1,4-dioxane was still chosen as the optimal solvent. Lowering the reaction temperature slightly enhanced the enantioselectivity (entry 15, 86% ee). When the reaction was conducted on a 0.2 mmol scale, 3aa was obtained in 92% yield and 86% ee (entry 16). Notably, the reaction of methyl 2-hydroxy-1-naphthoate with 2a can occur smoothly under the optimized conditions (83% NMR yield), but with poor C/O selectivity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
| Entry | Ligand | Base | Solvent | Yield of 3aab (%) | C/Oc (3aa/4aa) | eed (%) |
|---|---|---|---|---|---|---|
| a General conditions: 1a (0.1 mmol), 2a (0.12 mmol), Pd(OAc)2 (10 mol%), ligand (11 mol%), base (1.0 equiv.) in solvent (1.0 mL) at 30 °C. b NMR yields using 1,3,5-trimethylbenzene as an internal standard. c Determined by 1H NMR analysis of the crude reaction mixture. d Determined by HPLC analysis with a chiral stationary phase. e n.d. = not detected. f 22 mol% (S,S,Sa)-L5 was used. g At 25 °C. h The reaction was carried out in 0.2 mmol scale. i Isolated yield. | ||||||
| 1 | L1 | Li2CO3 | 1,4-Dioxane | 78 | 5/1 | 84 |
| 2 | L2 | Li2CO3 | 1,4-Dioxane | n.d.e | — | — |
| 3 | L3 | Li2CO3 | 1,4-Dioxane | n.d. | — | — |
| 4 | L4 | Li2CO3 | 1,4-Dioxane | n.d. | — | — |
| 5f | L5 | Li2CO3 | 1,4-Dioxane | 97 | 19/1 | 0 |
| 6 | L6 | Li2CO3 | 1,4-Dioxane | <5 | — | — |
| 7 | L7 | Li2CO3 | 1,4-Dioxane | 11 | 2/5 | 80 |
| 8 | L1 | Li2CO3 | DCE | 43 | 2/1 | 87 |
| 9 | L1 | Li2CO3 | Toluene | 13 | 1/2 | 74 |
| 10 | L1 | Li2CO3 | THF | 30 | 5/11 | 87 |
| 11 | L1 | K2CO3 | 1,4-Dioxane | 65 | 33/10 | 84 |
| 12 | L1 | Cs2CO3 | 1,4-Dioxane | 66 | 33/10 | 84 |
| 13 | L1 | Et3N | 1,4-Dioxane | 92 | >19/1 | 84 |
| 14 | L1 | Et3N | THF | 60 | 15/1 | 87 |
| 15g | L1 | Et3N | 1,4-Dioxane | 90 | >19/1 | 86 |
| 16gh | L1 | Et3N | 1,4-Dioxane | 92i | >19/1 | 86 |
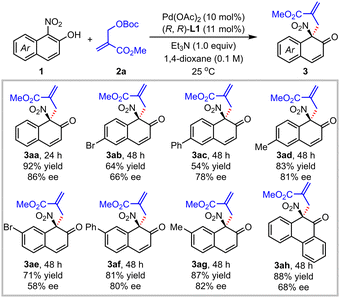
With the optimized conditions in hand (entry 16, Table 1), a series of 1-nitro-2-naphthols bearing different substituents were examined to test the generality of this asymmetric allylic dearomatization process (Table 2). When phenyl or methyl group was introduced at the C6 position of 2-naphthol, dearomatized products 3ac and 3ad could be delivered smoothly with excellent regioselectivity and reasonable enantioselectivity (3ac and 3ad, 54–83% yields, 78–81% ee, >19/1 C/O selectivity). However, the enantioselectivity and yield of the reaction decreased slightly when a Br group was introduced at either the C6 or C7 position (3ab and 3ae, 64–71% yields, 58–66% ee). Besides, different substituents at the C7 position of the naphthalene ring were investigated. The reactions occurred smoothly with excellent C/O selectivity and enantioselectivity for 7-phenyl and 7-methyl substituted 1-nitro-2-naphthols (3af, 81% yield, 80% ee; 3ag, 87% yield, 82% ee). For 10-nitro-9-phenanthrenol, 88% yield and 68% ee were obtained for the dearomatized product 3ah.
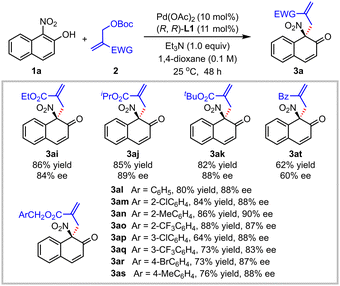
Subsequently, the reactions of 1-nitro-2-naphthol 1a with various MBH carbonates 2 were examined (Table 3). With the increase of steric hindrance of the ester group of the MBH adduct, the enantiomeric excess values of the dearomatized products varied slightly and comparable yields were obtained by prolonging the reaction time (3ai, 86% yield, 84% ee; 3aj, 85% yield, 89% ee; 3ak, 82% yield, 88% ee). Good yield and enantioselectivity were well maintained for the reaction of benzyl-substituted MBH carbonate (3al, 80% yield, 88% ee). Meanwhile, a variety of substituents on the benzene ring of the benzyl-substituted MBH carbonates were examined. As shown in Table 3, the optimized conditions were compatible with MBH carbonates bearing an electron-donating, electron-withdrawing or halogen group at the ortho position of the phenyl ring, such as methyl, trifluoromethyl or chloride group (3am–3ao, 84–88% yields, 87–90% ee). To be noted, the absolute configuration of product 3an (>99% ee after recrystallization) was assigned as R by X-ray diffraction analysis, and those of other products were assigned by analogy. Various meta or para-substituted benzyl-type MBH carbonates were tolerated well, affording the target products 3ap–3as in 64–76% yields and 83–88% ee. The benzoyl-substituted MBH carbonate could also be transformed to the desired product 3at in 62% yield and 60% ee. When the reactions with either tert-butyl (2-cyanoallyl) carbonate or methyl 2-(((tert-butoxycarbonyl)oxy)(phenyl)methyl)acrylate were attempted, no corresponding dearomatized products were observed.
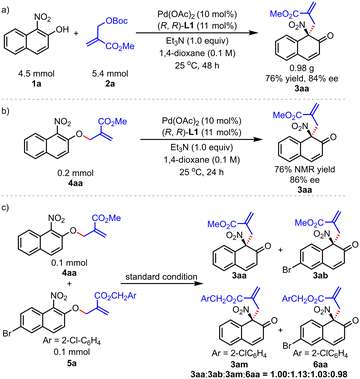
A gram-scale reaction was performed to examine the practicality of the current dearomatization reaction. To our delight, subjecting 1-nitro-2-naphthol 1a on a 4.5 mmol scale under the optimal conditions led to 3aa (0.98 g) in 76% yield without notable loss of enantioselectivity (84% ee, Scheme 2a). To gain insights into the reaction mechanism, the O-alkylation product 4aa was subjected to the standard conditions. Interestingly, dearomatized product 3aa was obtained in 76% NMR yield and the same enantioselectivity (86% ee) of the standard reaction (Scheme 2b). A crossover reaction was carried out using substrates 4aa and 5a. As shown in Scheme 2c, products 3aa, 3ab, 3am and 6aa were obtained in a ratio of 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.13
1.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.03
1.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.98. These results indicate that the process is an intermolecular reaction.
0.98. These results indicate that the process is an intermolecular reaction.
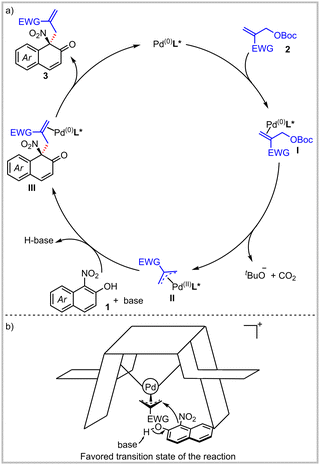
On the basis of the above experimental results and the reported literature,9,12 a plausible mechanism is proposed as depicted in Scheme 3a. The direct allylic alkylation of α-C of naphthol is feasible in the reaction. First, palladium(0) is coordinated with MBH adduct 2 to form species I. The π-allylpalladium species II is generated by the oxidative addition of species I with the release of tert-butoxy anion and carbon dioxide. The α-C of 2-naphthol directly attacks species II, resulting in the dearomatized product 3. Finally, the released palladium(0) complex enters into the next catalytic cycle. Meanwhile, the absolute configuration of the product is predicted to be R by the favored transition state model (Scheme 3b), which is consistent with the result confirmed by the single crystal structure.
In summary, we have developed an asymmetric allylic dearomatization of 1-nitro-2-naphthol derivatives with MBH adducts by using Pd catalyst derived from palladium acetate and Trost ligand. This strategy provides a direct and convenient access to a variety of β-naphthalenones bearing a nitro group in moderate to excellent yields and good enantioselectivity. Diverse functional groups were well tolerated for both 1-nitro-2-naphthols and MBH adducts.
This work is supported by NSFC (21821002, 22031012), and Science and Technology Commission of Shanghai Municipality (21520780100, 22JC1401103).
Conflicts of interest
The authors declare no competing interests.Notes and references
- For selected reviews: (a) L. Pouységu, D. Deffieux and S. Quideau, Tetrahedron, 2010, 66, 2235 CrossRef; (b) C. Zheng and S.-L. You, Nat. Prod. Rep., 2019, 36, 1589 RSC; (c) J. An and M. Bandini, Eur. J. Org. Chem., 2020, 4087 CrossRef CAS; (d) Y.-Z. Liu, H. Song, C. Zheng and S.-L. You, Nat. Synth., 2022, 1, 203 CrossRef; (e) X.-Y. Liu and Y. Qin, Green Synth. Catal., 2022, 3, 25 CrossRef ; For selected examples: ; (f) B. D. Horning and D. W. MacMillan, J. Am. Chem. Soc., 2013, 135, 6442 CrossRef CAS PubMed; (g) R. J. Phipps and F. D. Toste, J. Am. Chem. Soc., 2013, 135, 1268 CrossRef CAS PubMed; (h) L. Song, H. Yao and R. Tong, Org. Lett., 2014, 16, 3740 CrossRef CAS; (i) X. Feng, G. Jiang, Z. Xia, J. Hu, X. Wan, J.-M. Gao, Y. Lai and W. Xie, Org. Lett., 2015, 17, 4428 CrossRef CAS PubMed; (j) R. Coffinier, M. E. Assal, P. A. Peixoto, C. Bosset, K. Miqueu, J. M. Sotiropoulos, L. Pouységu and S. Quideau, Org. Lett., 2016, 18, 1120 CrossRef CAS PubMed; (k) C.-H. Weng, D.-S. Hsu and C.-C. Liao, J. Org. Chem., 2016, 81, 11421 CrossRef CAS PubMed; (l) X. Liang, T.-Y. Zhang, X.-Y. Zeng, Y. Zheng, K. Wei and Y.-R. Yang, J. Am. Chem. Soc., 2017, 139, 3364 CrossRef CAS PubMed; (m) H.-F. Tu, X. Zhang, C. Zheng, M. Zhu and S.-L. You, Nat. Catal., 2018, 1, 601 CrossRef CAS; (n) Y. Wang, W.-Y. Zhang, J.-H. Xie, Z.-L. Yu, J.-H. Tan, C. Zheng, X.-L. Hou and S.-L. You, J. Am. Chem. Soc., 2020, 142, 19354 CrossRef CAS PubMed; (o) B. Gao, F. Yao, Z. Zhang and H. Ding, Angew. Chem., Int. Ed., 2021, 60, 10603 CrossRef CAS PubMed; (p) X. Chang, C. Che, Z.-F. Wang and C.-J. Wang, CCS Chem., 2021, 3, 1484 Search PubMed; (q) S. Ma, Z. Li, P. Yu, H. Shi, H. Yang, J. Yi, Z. Zhang, X. Duan, X. Xie and X. She, Org. Lett., 2022, 24, 5541 CrossRef CAS PubMed.
- For selected reviews: (a) S. P. Roche and J. A. Porco Jr., Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed; (b) J.-H. Fan, Y.-J. Hu, L.-X. Li, J.-J. Wang, S.-P. Li, J. Zhao and C.-C. Li, Nat. Prod. Rep., 2021, 38, 1821 RSC ; For selected examples: ; (c) Y. Lin, G. B. Jones, G.-S. Hwang, L. Kappen and I. H. Goldberg, Org. Lett., 2005, 7, 71 CrossRef CAS PubMed; (d) T. Cao, L. Zhu, J. Huang and Z. Yang, Org. Lett., 2022, 24, 1491 CrossRef CAS PubMed.
- For selected examples: (a) T. Oguma and T. Katsuki, J. Am. Chem. Soc., 2012, 134, 20017 CrossRef CAS PubMed; (b) C.-X. Zhuo and S.-L. You, Angew. Chem., Int. Ed., 2013, 52, 10056 CrossRef CAS PubMed; (c) D. Yang, L. Wang, F. Han, D. Li, D. Zhao and R. Wang, Angew. Chem., Int. Ed., 2015, 54, 2185 CrossRef CAS PubMed; (d) X.-Q. Li, H. Yang, J.-J. Wang, B.-B. Gou, J. Chen and L. Zhou, Chem. – Eur. J., 2017, 23, 5381 CrossRef CAS PubMed; (e) D. Shen, Q. Chen, P. Yan, X. Zeng and G. Zhong, Angew. Chem., Int. Ed., 2017, 56, 3242 CrossRef CAS; (f) H.-F. Tu, C. Zheng, R.-Q. Xu, X.-J. Liu and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 3237 CrossRef CAS PubMed; (g) M. T. Peruzzi, S. J. Lee and M. R. Gagné, Org. Lett., 2017, 19, 6256 CrossRef CAS PubMed; (h) X. Liu, P. Wang, L. Bai, D. Li, L. Wang, D. Yang and R. Wang, ACS Catal., 2018, 8, 10888 CrossRef CAS; (i) S.-B. Tang, H.-F. Tu, X. Zhang and S.-L. You, Org. Lett., 2019, 21, 6130 CrossRef CAS PubMed; (j) J. Hu, S. Pan, S. Zhu, P. Yu, R. Xu, G. Zhong and X. Zeng, J. Org. Chem., 2020, 85, 7896 CrossRef CAS PubMed; (k) B. G. Das, S. Shah, A. Das and V. K. Singh, Org. Lett., 2021, 23, 6262 CrossRef CAS PubMed; (l) W. Wei, A. Scheremetjew and L. Ackermann, Chem. Sci., 2022, 13, 2783 RSC.
- (a) S.-G. Wang, Q. Yin, C.-X. Zhuo and S.-L. You, Angew. Chem., Int. Ed., 2015, 54, 647 CAS; (b) J. Nan, J. Liu, H. Zheng, Z. Zuo, L. Hou, H. Hu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2015, 54, 2356 CrossRef CAS; (c) X. Lian, L. Lin, G. Wang, X. Liu and X. Feng, Chem. – Eur. J., 2015, 21, 17453 CrossRef CAS PubMed; (d) A. Changotra, S. Das and R. B. Sunoj, Org. Lett., 2017, 19, 2354 CrossRef CAS PubMed.
- (a) Y. Zhang, Y. Liao, X. Liu, X. Xu, L. Lin and X. Feng, Chem. Sci., 2017, 8, 6645 RSC; (b) M. Uyanik, T. Kato, N. Sahara, O. Katade and K. Ishihara, ACS Catal., 2019, 9, 11619 CrossRef CAS; (c) L. Wang, H. Zhu, T. Peng, Y. Xu, Y. Hou, S. Li, S. Pang, H. Zhang and D. Yang, Chin. Chem. Lett., 2022, 33, 4273 CrossRef CAS.
- (a) P. Wang, J. Wang, L. Wang, D. Li, K. Wang, Y. Liu, H. Zhu, X. Liu, D. Yang and R. Wang, Adv. Synth. Catal., 2018, 360, 401 CrossRef CAS; (b) J.-J. Wang, H. Yang, B.-B. Gou, L. Zhou and J. Chen, J. Org. Chem., 2018, 83, 4730 CrossRef CAS PubMed.
- For selected examples: (a) J. Dhineshkumar, P. Samaddar and K. R. Prabhu, Chem. Commun., 2016, 52, 11084 RSC; (b) R.-Q. Xu, P. Yang, H.-F. Tu, S.-G. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 15137 CrossRef CAS PubMed; (c) D. Sarkar, M. K. Ghosh and N. Rout, Org. Biomol. Chem., 2016, 14, 7883 RSC; (d) Z. Zhang, Q. Sun, D. Xu, C. Xia and W. Sun, Green Chem., 2016, 18, 5485 RSC; (e) Y.-F. Wang, J.-J. Shao, B. Wang, M.-M. Chu, S.-S. Qi, X.-H. Du and D.-Q. Xu, Adv. Synth. Catal., 2018, 360, 2285 CrossRef CAS; (f) M. Uyanik, N. Sahara and K. Ishihara, Eur. J. Org. Chem., 2019, 27 CrossRef CAS; (g) J.-C. Yi, Z.-J. Wu and S.-L. You, Eur. J. Org. Chem., 2019, 5736 CrossRef CAS; (h) Y. Ge, C. Qin, L. Bai, J. Hao, J. Liu and X. Luan, Angew. Chem., Int. Ed., 2020, 59, 18985 CrossRef CAS PubMed; (i) H. Egami, T. Rouno, T. Niwa, K. Masuda, K. Yamashita and Y. Hamashima, Angew. Chem., Int. Ed., 2020, 59, 14101 CrossRef CAS PubMed; (j) E. Lee, Y. Hwang, Y. B. Kim, D. Kim and S. Chang, J. Am. Chem. Soc., 2021, 143, 6363 CrossRef CAS PubMed; (k) X.-Y. Huang, Q. Zheng, L.-M. Zou, Q. Gu, T. Tu and S.-L. You, ACS Catal., 2022, 12, 4545 CrossRef CAS; (l) K. Zhao, P. Kohnke, Z. Yang, X. Cheng, S.-L. You and L. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202207518 CAS.
- Q. Cheng, Y. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 3496 CrossRef CAS PubMed.
- C.-X. Zhuo and S.-L. You, Adv. Synth. Catal., 2014, 356, 2020 CrossRef CAS.
- (a) L. Yao and K. Ishihara, Chem. Sci., 2019, 10, 2259 RSC; (b) L. Yao, K. Takeda, K. Ando and K. Ishihara, Chem. Sci., 2023, 14, 2441 RSC.
- Q.-X. Zhang, Q. Gu and S.-L. You, Org. Lett., 2022, 24, 8031 CrossRef CAS PubMed.
- C. Goux, M. Massacret, P. Lhoste and D. Sinou, Organometallics, 1995, 14, 4585 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2234267. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00568b |
| This journal is © The Royal Society of Chemistry 2023 |