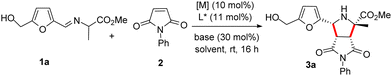Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective transformations of 5-hydroxymethylfurfural via catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides†
Christian
Cristóbal
a,
César
Corral
a,
Juan C.
Carretero
 abc,
Maria
Ribagorda
abc,
Maria
Ribagorda
 *ab and
Javier
Adrio
*ab and
Javier
Adrio
 *abc
*abc
aDepartamento de Química Orgánica, Facultad de Ciencias, Universidad Autónoma de Madrid, Cantoblanco, Madrid 28049, Spain. E-mail: javier.adrio@uam.es; maria.ribagorda@uam.es
bInstitute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, Madrid 28049, Spain
cCentro de Innovación en Química Avanzada (ORFEO-CINQA), Spain
First published on 21st March 2023
Abstract
A catalytic asymmetric 1,3-dipolar cycloaddition between iminoesters derived from 5-hydroxymethylfurfural (HMF) and different activated alkenes is reported. Excellent levels of diastereo and enantioselectivity were obtained when Fesulphos/CuI complex was used as catalyst. This metodology provides an effective and sustainable access to challenging enantioenriched heterocyclic scaffolds and represents one of the rare examples of catalytic asymmetric transformations using HMF as a starting material.
The use of renewable starting materials derived from biomass instead of those arising from petrochemicals for the synthesis of fine chemicals, represents a synthetic challenge in modern organic chemistry.1 In this context, numerous research groups have focused their efforts in converting biomass into high value-added products. A remarkable example of these sustainable starting materials is the 5-hydroxymethylfurfural (HMF), which is obtained by dehydration of hexoses.2 HMF offers a great synthetic potential due to its high functionalization including a furanic ring, an aldehyde, and an alcohol. Consequently, a plethora of synthetic transformations of HMF including oxidation, reduction, alkylation, aldol condensation, reductive or oxidative cleavage, reductive amination, Baylis–Hillman reaction, Diels–Alder cycloaddition, and C-H activation have been developed in the last years.3 However, the use of HMF as starting material for the preparation of enantioenriched products has been scarcely studied.4
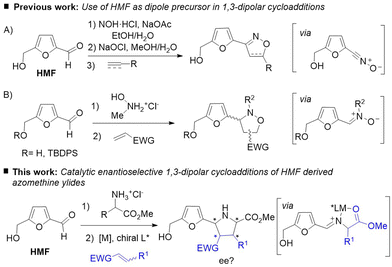
1,3-Dipolar cycloadditions have become a fundamental tool in organic synthesis with important applications in natural product synthesis, chemical biology, and material science.5 Aldehydes have been extensively used as precursors of different 1,3-dipoles such as nitrones, nitrile oxides or azometine ylides. However, as far as we are aware, only two examples of the use of HMF as dipole precursor in 1,3-dipolar cycloadditions have been reported. In 2007 Amarasekara and co-workers6 described a procedure for the generation of nitrile oxides from HMF and subsequent 1,3-dipolar cycloaddition with a variety of alkenes and alkynes to afford furanyl isoxazoles (Scheme 1A). More recently, Queneau and co-workers7 reported an efficient procedure for the preparation of 3-furanyl isoxazolidines by 1,3-dipolar cycloaddition of HMF derived nitrones with various dipolarophiles. Good yields were obtained either in stepwise or multicomponent approaches using isopropanol as solvent. However, modest control of the regio and diastereoselectivity was observed in most of the examples (Scheme 1B). Nevertheless, to the best of knowledge, the use of HMF as dipole precursor in catalytic asymmetric 1,3-dipolar cycloadditions remains to be described.
On the other hand, the pyrrolidine ring is a privileged structure presents in numerous natural products and biologically active molecules.8 Furthermore, proline derivatives have shown special applicability as organocatalysts.9 One of the most useful procedures for the synthesis of enantioenriched pyrrolidines is the metal catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with activated olefins. Over the course of the last two decades a great effort has been devoted to the development of new catalytic systems based on the use of transition metal complexes of a variety of bidentate and monodentate chiral ligands.10 With this set of efficient catalyst in hands, the research in this area has expanded the scope of the cycloaddition enabling the preparation of new types of heterocyclic moieties. On these grounds, and following our interest in dipolar cycloadditions,11 we decided to investigate the behaviour of iminoesters derived from HMF in metal catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides. This reaction could open a practical enantioselective access to highly valuable 2-furyl pyrrolidines from a common biomass derived starting material.
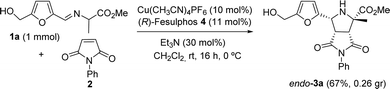
The azomethine ylide precursor 1a required to carry out the cycloaddition was obtained in quantitative yield by condensation of commercially available alanine methyl ester hydrochloride with HMF using Et3N as base in CH2Cl2.12 N-Phenylmaleimide 2 was selected as model dipolarophile for the optimization of the reaction conditions. Initially, the reaction was performed in the presence of Cu(CH3CN)4 as metal source, Fesulphos (4) as ligand, and Et3N as base in CH2Cl2, conditions commonly used by our research group in related cycloadditions.11c,d,12 We were pleased to find that reaction is compatible with the presence of the free hydroxyl group of 1a. Moreover, the expected cycloadduct 3a was obtained with high conversion, excellent diastereocontrol (only the endo isomer was observed by 1H-NMR) and good enantioselectivity (84% ee, Table 1, entry 1). A lower yield and enantioselectivity was observed with AgOAc as metal source (entry 2). The use of other solvents (such as toluene or THF) or bases (KOtBu) led to poorer results (entries 3–5). Next, we evaluated the effect of other ligands on the reaction (entries 6–9). The bulky DTBM-Segphos (5) did not give the desired cycloadduct (entry 6) while a 50% yield but null enantioselectivity was observed using Segphos (6) (entry 7). Lower conversions and enantioselectivities were also obtained using FePhox (7) or BTFM-Garphos (8) ligands (entries 8–9). Gratifyingly, the desired cycloadduct was isolated in 65% yield and 95% ee when the reaction was performed at 0 °C (entry 10). A significant drop in conversion and asymmetric induction was observed when the catalyst loading was reduced to 5 mol% (entry 11). The relative and absolute configuration of adduct 3a was unequivocally stablished by X-ray crystallographic analysis.13
| Entry | [M] | L* | Solvent | Base | Yieldb (%) | dr (%) | eed (%) |
|---|---|---|---|---|---|---|---|
a Cu(CH3CN)4PF6.
b Isolated yield after chromatographic purification.
c Determined by 1H-NMR in the crude reaction mixture.
d ee determined by HPLC.
e Reaction performed at 0 °C.
f 5 mol% of catalyst.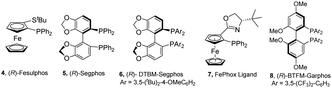
|
|||||||
| 1 | CuPF6a | 4 | CH2Cl2 | Et3N | 70 | ≥98 | 84 |
| 2 | AgOAc | 4 | CH2Cl2 | Et3N | 60 | ≥98 | 43 |
| 3 | CuPF6a | 4 | toluene | Et3N | 0 | — | — |
| 4 | CuPF6a | 4 | THF | Et3N | 14 | ≥98 | 78 |
| 5 | CuPF6a | 4 | CH2Cl2 | KOtBu | 63 | ≥98 | 68 |
| 6 | CuPF6a | 5 | CH2Cl2 | Et3N | 0 | — | — |
| 7 | CuPF6a | 6 | CH2Cl2 | Et3N | 50 | ≥98 | 0 |
| 8 | CuPF6a | 7 | CH2Cl2 | Et3N | 42 | ≥98 | 47 |
| 9 | CuPF6a | 8 | CH2Cl2 | Et3N | 14 | ≥98 | 59 |
| 10e | CuPF6a | 4 | CH2Cl2 | Et3N | 65 | ≥98 | 95 |
| 11f | CuPF6a | 4 | CH2Cl2 | Et3N | 40 | ≥98 | 70 |
With the optimized reaction conditions on hands, we then investigated the scope of this cycloaddition regarding the substitution at the iminoester (Scheme 2). A remarkably broad range of azomethine ylide precursors with alkyl or aryl substituents at the α position performed well to afford the corresponding α-quaternary proline derivatives in good yields (67–85%), excellent diastereoselectivity (only the endo adduct was observed by 1H-NMR of the crude mixture) and very high enantioselectivity (86–99% ee). Thus, excellent results were obtained with iminoesters derived from alkyl amino acids such as 2-aminobutyric acid (1b), leucine (1c) or phenyl alanine (1d).
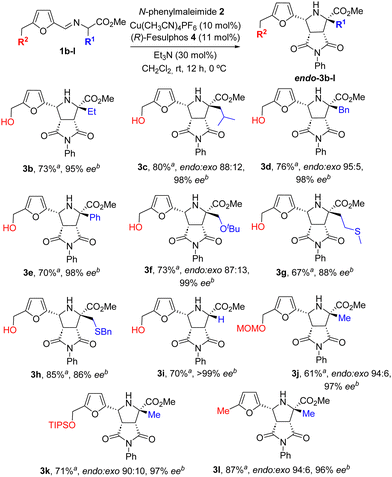 | ||
| Scheme 2 Scope of the reaction regarding the azomethine ylide precursor. aIsolated yield after chromatographic purification. bee determined by HPLC. | ||
The cycloaddition was also compatible with the Schiff base prepared from phenylglycine (3e). Proline derivatives with either ether (3f) or thioether (3g and 3h) functional groups at the substituent at C-2 could also be prepared with high yield diastereo and enantioselectivity. The α-unsubstituted iminoester 1i, derived from methyl glycinate was also a suitable substrate in the cycloaddition. The MOM and TIPS protected HMF iminoesters 1j and 1k showed a similar reactivity giving rise the endo-adducts 3j and 3k in good yields and excellent enantioselectivities.14 Moreover, the cycloaddition of iminoester 1l derived from 5-methylfurfural also takes place with excellent yield diatereo and enantioselectivity (cycloadduct 3l).
Next, to explore deeply the scope of this [3+2] asymmetric cycloaddition we extend the study to different dipolarophiles under the optimized reaction conditions. Diactivated acyclic alkenes, such as dimethyl fumarate, dimethyl maleate, gave the desired adducts with high enantioselectivities (93–83% ee) albeit with poor yield and diastereoselectivity (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) exo 4
exo 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) than in the cycloaddition with maleimide (Scheme 3 adducts 9,11). The diastereslectivity improved significantly when alanine derived iminoester was used as dipole precursor (adduct 10). Interestingly, almost complete diastereoselectivity and excellent enantioselectivity was obtained when monoactivated methyl acrylate was used as dipolarophile (cycloadduct 12). The reaction with 2-ethylacroleine, which allow the formation of a quaternary stereocenter at C-4 (adduct endo-13), is of particular relevance. Chalcone proved to be also an efficient dipolarophile (adduct 14). An inversion of the diastereoselectivity was observed in the case of 4-methoxy-β-nitrostyrene (adduct 15).15
1) than in the cycloaddition with maleimide (Scheme 3 adducts 9,11). The diastereslectivity improved significantly when alanine derived iminoester was used as dipole precursor (adduct 10). Interestingly, almost complete diastereoselectivity and excellent enantioselectivity was obtained when monoactivated methyl acrylate was used as dipolarophile (cycloadduct 12). The reaction with 2-ethylacroleine, which allow the formation of a quaternary stereocenter at C-4 (adduct endo-13), is of particular relevance. Chalcone proved to be also an efficient dipolarophile (adduct 14). An inversion of the diastereoselectivity was observed in the case of 4-methoxy-β-nitrostyrene (adduct 15).15
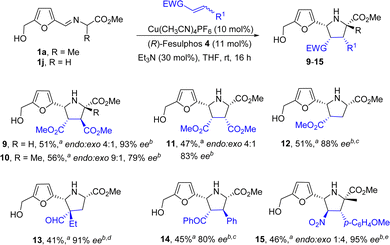 | ||
| Scheme 3 Scope of the cycloaddition with regard to dipolarophile. aIsolated yield after chromatographic purification. bee determined by HPLC. cReaction at 0 °C. dIn CH2Cl2. eReaction at −15 °C. | ||
Next, to illustrate the robustness of this methodology a scale up cycloaddition of the HMF derived iminoester 1a (1 mmol scale) with N-phenyl maleimide 2 was performed using standard conditions. The corresponding adduct endo-3a was isolated in 67% yield (Scheme 4) maintaining the excellent diastereo and enantioslectivity.
Pyrroles are among the most valuable heteroaromatic compounds and are present in a vast number of natural products and biologically active compounds.16 Furanyl-pyrroles could be readily prepared from HMF derived iminoester 1a in a one-pot procedure using commercially available 1,2-bis-phenylsulfonyl ethylene (16) as dipolarophile.17In situ aromatization of the bis-sulfone adduct by double basic elimination of the sulfone moieties afforded the corresponding bisheterocycle 17 in reasonable yield (Scheme 5).
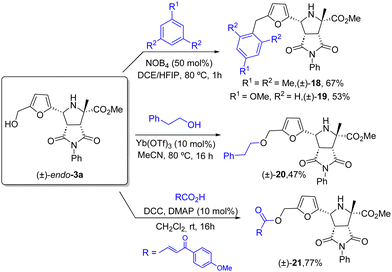
The cycloadducts obtained in this work offer a chance for additional transformations towards appealing products. For example, the HMF derived adduct endo-3a can be used as alkylating agent in Friedel–Crafts reactions.18 Thus, the reaction with mesitylene or anisole catalyzed by nitrosonium tretrafluorborate provided 18 and 19 in 67 and 53% yield respectively (Scheme 6). Ether 20 was prepared by Yb(OTf)3-catalyzed reaction between pyrrolidine endo-3a and 2-phenylethanol.19 The DCC promoted esterification between 3a and the corresponding carboxylic acid in the present of DMP as base furnished the ester 21 in 77% yield20 (Scheme 6).
A practical procedure for the enantioselective preparation of furyl-pyrrolidines from bio based 5-hydroxymethylfurfural (HMF) has been developed by catalytic asymmetric 1,3-dipolar cycloaddition. The stereocontrol exerted by the CuI/Fesulphos catalytic system allowed the access to different furyl-pyrrolidines with excellent diastereo and enantioselectivities. Remarkably, the system is compatible with the free hydroxyl group present in the HMF moiety. The synthetic utility of this methodology was demonstrated by further hydroxyl derivatizations.
We thank FEDER/Ministerio de Ciencia, Innovación y Universidades–Agencia Estatal de Investigación (Grants PGC2018-098660-B-I00 and PID2021-1248553NB-100, MICINN (Grant PID2020-113059GB-C22) and Comunidad Autónoma de Madrid co-financed by the European Structural and investment fund for financial support (Grant B2017/BMD- 3867 RENIMCM). C. C. thanks CAM for a research contract (PEG-2020-AI/IND-18195).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed; (b) S. Chen, R. Wojcieszak, F. Dumeignil, E. Marceau and S. Royer, Chem. Rev., 2018, 118, 11023–11117 CrossRef CAS PubMed; (c) C. Mondelli, G. Gözaydin, N. Yan and J. Pérez-Ramírez, Chem. Soc. Rev., 2020, 49, 3764–3782 RSC.
- G. Trapasso, G. Mazzi, B. Chicharo, M. Annatelli, D. Dalla Torre and F. Arico, Org. Process Res. Dev., 2022, 26, 2830–2838 CrossRef CAS PubMed.
- (a) A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC; (b) F. A. Kucherov, K. I. Galkin, E. G. Gordeev and V. P. Ananikov, Green Chem., 2017, 19, 4858–4864 RSC; (c) F. A. Kucherov, L. V. Romashov, K. I. Galkin and V. P. Ananikov, ACS Sustainable Chem. Eng., 2018, 6, 8064–8092 CrossRef CAS; (d) W. Fan, C. Verrier, Y. Queneau and F. Popowycz, Curr. Org. Synth., 2019, 16, 583–614 CrossRef CAS PubMed; (e) S. Dutta and N. S. Bhat, ACS Omega, 2021, 6, 35145–35172 CrossRef CAS PubMed; (f) F. Chacon-Huete, C. Messina, B. Cigana and P. Forgione, ChemSusChem, 2022, 15, e202200328 CrossRef CAS PubMed; (g) Y. Wang, H. Wang, X. Kong and Y. Zhu, ChemSusChem, 2022, 15, e202200421 CAS; (h) A. A. Turkin, E. V. Makshina, B. Sels and F. Bert, ChemSusChem, 2022, 15, e202200412 CrossRef CAS PubMed; (i) Z. Li, Y. Jiang, Y. Li, H. Zhang, H. Li and S. Yang, Catal. Sci. Technol., 2022, 12, 1902–1921 RSC; (j) Z. Jiang, Y. Zeng, D. Hu, R. Guo, K. Yan and R. Luque, Green Chem., 2023, 25, 871–892 RSC.
- (a) Y.-L. Su, Z.-Y. Han, Y.-H. Li and L.-Z. Gong, ACS Catal., 2017, 7, 7917–7922 CrossRef CAS; (b) P.-F. Koh and T.-P. Loh, Green Chem., 2015, 17, 3746–3750 RSC.
- (a) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Towards Heterocycles and Natural Products, eds. A. Padwa and W. H. Pearson, Wiley, New York, 2003 Search PubMed; (b) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366–5412 CrossRef CAS PubMed; (c) M. Breugst and H.-U. Reissig, Angew. Chem., Int. Ed., 2020, 59, 12293–12307 CrossRef CAS PubMed.
- A. S. Amarasekara, O. Edigin and W. Hernandez, Lett. Org. Chem., 2007, 4, 306–308 CrossRef CAS.
- L. Wang, C. Verrier, M. Ahmar and Y. Queneau, Green Chem., 2020, 22, 7907–7912 RSC.
- (a) G. L. Petri, M. V. Raimondi, V. Spanò, R. Holl, P. Barraja and A. Montalbano, Top. Curr. Chem., 2021, 34, 379–425 Search PubMed; (b) M. Kuhnert, A. Blum, H. Steuber and W. E. Diederich, J. Med. Chem., 2015, 58, 4845–4850 CrossRef CAS PubMed; (c) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed; (d) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS PubMed; (e) J. P. Michael, Nat. Prod. Rep., 2008, 25, 139–165 RSC.
- (a) B. List, Tetrahedron, 2002, 58, 5573–5590 CrossRef CAS; (b) D. W. C. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS PubMed; (c) S. K. Panday, Tetrahedron: Asymmetry, 2011, 22, 1817–1847 CrossRef CAS.
- For recent reviews, see: (a) L. Wei, X. Chang and C. J. Wang, Acc. Chem. Res., 2020, 53, 1084–1100 CrossRef CAS PubMed; (b) J. Adrio and J. C. Carretero, Chem. Commun., 2019, 55, 11979–11991 RSC; (c) I. Arrastia, A. Arrieta and F. P. Cossio, Eur. J. Org. Chem., 2018, 5889–5904 CrossRef CAS PubMed; (d) X. Fang and C.-J. Wang, Org. Biomol. Chem., 2018, 16, 2591–2601 RSC; (e) B. Bdiri, B.-J. Zhao and Z.-M. Zhou, Tetrahedron: Asymmetry, 2017, 28, 876–899 CrossRef CAS; (f) H. A. Döndas, M. G. Retamosa and J. M. Sansano, Synthesis, 2017, 2819–2851 CrossRef; (g) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366–5412 CrossRef CAS PubMed; (h) J. Adrio and J. C. Carretero, Chem. Commun., 2014, 50, 12434–12446 RSC; (i) Y. Xing and N.-X. Wang, Coord. Chem. Rev., 2012, 256, 938–952 CrossRef CAS; (j) J. Adrio and J. C. Carretero, Chem. Commun., 2011, 47, 6784–6794 RSC.
- (a) C. Cristóbal, D. Gaviña, I. Alonso, M. Ribagorda, J. C. Carretero, C. del Pozo and J. Adrio, Chem. Commun., 2022, 58, 7805–7808 RSC; (b) J. Corpas, A. Ponce, I. McLean, J. Adrio and J. C. Carretero, Synthesis, 2022, 4673–4682 CAS; (c) A. Molina, S. Díaz-Tendero, J. Adrio and J. C. Carretero, Chem. Commun., 2020, 56, 5050–5053 RSC; (d) J. Corpas, A. Ponce, J. Adrio and J. C. Carretero, Org. Lett., 2018, 20, 3179–3182 CrossRef CAS PubMed; (e) A. Pascual-Escudero, A. de Cózar, F. Cossío, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2016, 55, 15334–15338 CrossRef CAS PubMed.
- S. Cabrera, R. Gómez-Arrayas and J. C. Carretero, J. Am. Chem. Soc., 2005, 127, 16394–16395 CrossRef CAS PubMed.
- CCDC-2223031 contains the supplementary crystallographic data for this paper. http://www.ccdc.cam.ac.uk/data_request/cif.
- The stereochemistry of adduct 3k was confirmed by correlation with 3a obtained by deprotection of TIPS ether to the corresponding alcohol using TBAF in CH2Cl2.
- This type of cycloadditions using β-nitrostyrene as dipolarophile usually proceeds with exo selectivity, see ref. 12.
- (a) V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2014, 43, 4633–4657 RSC; (b) R. Khajuria, S. Dhamb and K. K. Kapoor, RSC Adv., 2016, 6, 37039–37066 RSC; (c) S. C. Philkhana, F. O. Badmus, I. C. Dos Reis and R. Kartika, Synthesis, 2021, 1531–1555 CrossRef CAS PubMed.
- (a) A. López-Pérez, R. Robles-Machín, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2007, 46, 9261–9264 CrossRef PubMed; (b) J. Adrio, R. Robles-Machín, A. López-Pérez, M. González-Esguevillas and J. C. Carretero, Chem. – Eur. J., 2010, 16, 9864–9873 CrossRef PubMed.
- L. Bering, K. Jeyakumar and A. P. Antonchick, Org. Lett., 2018, 20, 3911–3914 CrossRef CAS PubMed.
- H. Quiroz-Florentino, R. I. Hernández-Benítez, J. A. Avina, E. Burgueno-Tapia and J. Tamariz, Synthesis, 2011, 1106–1112 CAS.
- H. Zhou, W. Liu, C. Sun, C. Peng, J. Wang, Q. Wang and C. Yang, Heterocycles, 2013, 87, 1711–1726 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2223031. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00499f |
| This journal is © The Royal Society of Chemistry 2023 |