Open Access Article
Open Access ArticleElectrophilicity of neutral square-planar organosilver(III) compounds†
Daniel
Joven-Sancho
 a,
Luca
Demonti
b,
Antonio
Martín
a,
Luca
Demonti
b,
Antonio
Martín
 a,
Nathalie
Saffon-Merceron
c,
Noel
Nebra
a,
Nathalie
Saffon-Merceron
c,
Noel
Nebra
 *b,
Miguel
Baya
*b,
Miguel
Baya
 a and
Babil
Menjón
a and
Babil
Menjón
 *a
*a
aInstituto de Síntesis Química y Catálisis Homogénea (iSQCH), CSIC-Universidad de Zaragoza, Zaragoza 50009, Spain. E-mail: b.menjon@csic.es
bLaboratoire Hétérochimie Fondamentale et Appliquée (LHFA), Université Paul Sabatier, CNRS, 118 Route de Narbonne, Toulouse 31062, France. E-mail: noel.nebra-muniz@univ-tlse3.fr
cInstitut de Chimie de Toulouse ICT-UAR2599, Université de Toulouse UPS, CNRS, 118 route de Narbonne, Toulouse 31062, France
First published on 24th February 2023
Abstract
Neutral Ag(III) complexes stabilised with just monodentate ligands are here unambiguously established. In a series of square-planar (CF3)3Ag(L) compounds with hard and soft Group 15 donor ligands, L, the metal center has been found to exhibit substantial acidity favouring apical coordination of an additional ligand under no coordination constraints.
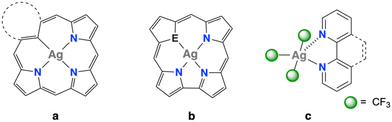
Neutral silver(III) compounds are rare chemical species.1 Aside from the binary compounds2,3 AgF3 and Ag2O3—both exhibiting extended structures‡—, all known neutral Ag(III) compounds are stabilised by polydentate ligands (Chart 1). Thus, macrocryclic ligands such as confused porphyrins, carbaporphyrinoids, corroles or carbacorroles are especially suited.4 Two additional kinds of molecular compounds have been isolated with bidentate ligands: the dithiolate complex (CF3)2Ag(S&S) (S&S = diethyldithiocarbamate) of unknown structure5 and the five-coordinate (CF3)3Ag(N&N) compounds, where N&N = bpy or phen (Chart 1c).6,7 In this work, neutral Ag(III) complexes stabilised with just monodentate ligands are exemplified in the solvento-complex (CF3)3Ag(NCMe) (1) and some of its derivatives with Group 15 donor ligands. Evidence for substantial electrophilic character at the metal centre is also given.
The solvento-complex (CF3)3Ag(NCMe) has remained intriguing for more than two decades. Previous efforts to prepare it under different experimental conditions5,8 gave compounds with significant discrepancies in their 19F NMR spectroscopic properties (Table S1 in the ESI†). In one case,5 the compound was claimed to have been isolated as a colourless oil in 8.2% estimated yield. This claim, however, is at odds with the observed properties of the gold homologous species (CF3)3Au(NCMe), which we isolated as a white solid.9 Even more intriguing is the purported failure to replace the MeCN molecule by other ligands.5,8 Here, we provide a reliable procedure to prepare the neutral organosilver(III) compound (CF3)3Ag(NCMe) (1), which we first isolate as a white solid.
We have found that by treating the anionic chloride complex10 [(CF3)3AgCl]− with TlClO4 in MeCN (Scheme 1), the neutral organosilver(III) compound (CF3)3Ag(NCMe) (1) is formed quantitatively on a spectroscopic basis (19F NMR; Fig. S1 in the ESI†). After the appropriate workup, the desired compound is isolated as a deliquescent white solid in moderate yield due to its high solubility in most organic media.
 | ||
| Scheme 1 Synthetic procedures to prepare the neutral organosilver(III) compounds 1–5 (dmap = 4-dimethylaminopyridine). | ||
Compound 1 has square-planar (SP-4) geometry (Fig. 1a; Table S3 in the ESI†) as established by single-crystal X-ray diffraction methods (sc-XRD). The observed Ag–N distance (209.05(16) pm) is significantly shorter than those found for the basal Ag–N bonds in the five-coordinate complexes (CF3)3Ag(bpy) (214.3(3) pm) and (CF3)3Ag(phen) (213.9(2) pm).6 In addition to the different coordination number (4 vs. 5), the bond shortening observed here can also be attributed to the lack of strain in 1 and to the different hybridisation of the N-donor atom in each case (sp vs. sp2).
 | ||
| Fig. 1 Displacement-ellipsoid diagram (50% probability) of the SP-4 neutral (CF3)3Ag(L) complexes as found in single crystals of 1 (a), 2 (b), 3 (c), and 4 (d). Crystallographic details and relevant structural parameters are given in the ESI.† | ||
In contrast to previous reports,5,8 we found that the MeCN molecule in 1 can be easily replaced by other donors, both soft and hard, as actually expected for a typical labile ligand.11 Thus, a set of neutral (CF3)3Ag(L) compounds [L = py (2), dmap (3), PPh3 (4), AsPh3 (5)] has been prepared by simple exchange processes (Scheme 1). All these new compounds have been isolated and spectroscopically characterised (ESI†). The NMR spectra of the phosphine complex 4 are particularly rich due to the presence of various nuclei with ½ nuclear spin (Fig. S4–S6 in the ESI†). Structural characterisation by sc-XRD (Fig. 1) reveals that these neutral complexes exhibit SP-4 structure, as in the parent complex 1. Except for the phosphine complex 4 (Table S13 in the ESI†), the Ag–C distance trans to the neutral L ligand is substantially shorter than in the mutually trans Ag–CF3 due to the different trans influence of the CF3 group and the L ligand.12 As an example, in the dmap complex 3 (Table S11 in the ESI†), the Ag–C bond distance in the CF3–Ag–L axis (205.2(4) pm) is significantly shorter than the average value in the CF3–Ag–CF3 line: 209.3(4) pm.
The most salient feature in this set of compounds is the observed tendency to associate an additional ligand at the apical position. This feature is clearly seen in the arsine complex (CF3)3Ag(AsPPh3)·OCMe2 (5·OCMe2) and exemplified in the case of the pyridine complex (CF3)3Ag(py) (2), which has also been crystallised as the MeCN and py solvates: 2·NCMe and 2·py (Fig. 2). In all these cases, the solvent molecule enters the coordination sphere of silver. The additional bond is substantially longer than a standard covalent bond, but much shorter than the sum of the corresponding van der Waals radii.
 | ||
| Fig. 2 Displacement-ellipsoid diagram (50% probability) of the solvates (CF3)3Ag(L)·L′ as found in single crystals of 2·NCMe (a), 2·py (b), and 5·OCMe2 (c). The Ag⋯L′ distance is indicated in each case together with the corresponding penetration index, p(AB).13 Crystallographic details and relevant structural parameters are given in the ESI.† | ||
This kind of interaction is best described making use of the descriptor recently introduced by Álvarez and coworkers, namely the penetration index.13 This descriptor§, p(AB), provides a measure of the interpenetration of the electron clouds of the involved atoms A/B and is especially suited for interactions lying between typically covalent and van der Waals bonding interactions, i.e. somewhere between the intra- and intermolecular dominions. By using this tool, we obtain p(AB) values ranging from 70% to 76% for the apical interaction in our solvate-complexes (CF3)3Ag(L)·L′ (Fig. 2). This means that the apical Ag⋯E interaction is quite similar in all cases, regardless of the different nature of the donor atom (E = N or O) or its precise hybridisation (sp or sp2 in the case of N). Of key importance is also the fact that the Ag⋯E line virtually coincides with the normal to the best basal plane, the deviation being < 4° in all cases (precise values given in the ESI†). Thus, the position of the apical donor atom E matches both metrical and angular criteria to indicate a genuine Ag⋯E interaction.
We also performed theoretical calculations on various (CF3)3Ag(L)/L′ systems in the gas phase (L′ = NCMe, Me2CO) in order to evaluate the influence of the crystal lattice on establishing the apical interaction. The referred systems were modelled under no symmetry or environmental restraints. We found that in these isolated systems, the additional ligand L′ also enters the metal coordination sphere (Fig. S9 and S10 in the ESI†) as the result of a stabilising (exothermic) interaction (Table S16 in the ESI†). In the calculated energy minima, the L′ ligand is also located at the apical site near the basal plane normal. The calculated Ag⋯L′ distance in the 2·NCMe molecule (276.0 pm) shows excellent agreement with that experimentally observed in the crystal (278.4(3) pm). The agreement is less satisfactory in the case of 5·OCMe2 (Ag⋯L′ = 276.0 (theor.) vs. 265.3(1) (exptl.) pm), but still reasonable. Following our calculations, it becomes clear, that the neutral (CF3)3Ag(L) complexes exhibit a significant tendency to associate an additional ligand at the apical site. The resulting interaction is not just a solid-state effect, but evidences an intrinsic electrophilic character of the silver(III) centre (Chart 2). The loose apical interaction has little effect on the geometry of the basal unit. This is in contrast with the anionic halide systems [(CF3)2AgX2]− previously observed, where the association of an additional X− anion resulted in marked structural changes.14 Regardless of the extent of distortion observed in each system, it can be concluded that the electrophilic character of the Ag(III) centre seems to be a general feature in SP-4 complexes. It would therefore be advisable to take it into account when trying to trace reliable reaction mechanisms, as this might lead to a better understanding of the chemical processes.
Compound 2·py bears an interesting relationship with (CF3)3Ag(bpy),6 since the two pyridine rings are detached in the former, whereas they are linked in the latter. A detailed comparison of their structures is therefore in order. In both cases, the basal Ag–N distance is indistinguishable within the experimental error: 213.1(5) vs. 214.3(3) pm. These values are just marginally longer than in the non-solvated SP-4 complex 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210.0(6) pm (Table S5 in the ESI†). By contrast, the apical Ag–N distance is substantially longer where the two rings are disconnected, 263.6(5) vs. 245.4(3) pm. This shortening can be assigned to the geometric constraint imposed by the chelating bpy ligand and involves an increment in the penetration index p(AB) from 76% in 2·py (Fig. 2b) to 86% in (CF3)3Ag(bpy). We can now attribute the apical interaction to a common effect, namely the electrophilicity of the Ag(III) centre (Chart 2). The apical interaction is incipient in 2·py and tighter in (CF3)3Ag(bpy).
210.0(6) pm (Table S5 in the ESI†). By contrast, the apical Ag–N distance is substantially longer where the two rings are disconnected, 263.6(5) vs. 245.4(3) pm. This shortening can be assigned to the geometric constraint imposed by the chelating bpy ligand and involves an increment in the penetration index p(AB) from 76% in 2·py (Fig. 2b) to 86% in (CF3)3Ag(bpy). We can now attribute the apical interaction to a common effect, namely the electrophilicity of the Ag(III) centre (Chart 2). The apical interaction is incipient in 2·py and tighter in (CF3)3Ag(bpy).
In this work, we first isolate the solvento-complex (CF3)3Ag(NCMe) (1), which has obvious synthetic potential. It provides a convenient entry to a series of (CF3)3Ag(L) complexes including the first documented compound with Ag(III)–As bond (5: L = AsPh3). These neutral, square-planar Ag(III) complexes show a conspicuous tendency to uptake an additional ligand at the apical position (Fig. 2). Our calculations demonstrate that this feature is not just a solid-state effect, but rather has its origin in an intrinsic electrophilic character of the Ag(III) centre (Chart 2).
This work was supported by the Spanish MICIU/FEDER (Project PID2021-122869NB-I00), the CNRS, the Université Paul Sabatier, the Agence Nationale de la Recherche (ANR-JCJC-20-CE07-0023), and the Gobierno de Aragón (Grupo E17_20R). BIFI (Instituto de Biocomputación y Física de Sistemas Complejos) and CESGA (Centro de Supercomputación de Galicia) are acknowledged for allocation of computational resources. D. J.-S. thanks the Spanish MICIU for a grant (BES-2016–078732). L. D. is thankful to the French MESR for PhD funding.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. Malischewski, in Comprehensive Organometallic Chemistry IV, ed. P. L. Holland, Elsevier, 2021, vol. 1, ch. 5, pp. 109–134 Search PubMed; (b) A. Higelin and S. Riedel, in Modern Synthesis Processes and Reactivity of Fluorinated Compounds, ed. H. Groult, F. Leroux and A. Tressaud, Elsevier, Amsterdam, 2017, vol. 3, ch. 19, pp. 561–586 Search PubMed; (c) S. Riedel, in Comprehensive Inorganic Chemistry II, eds. E. V. Antipov, A. M. Abakumov and A. V. Shevelkov, Elsevier, Amsterdam, 2013, vol. 2, ch. 8, pp. 187–221 Search PubMed; (d) S. Riedel and M. Kaupp, Coord. Chem. Rev., 2009, 253, 606 CrossRef CAS.
- B. Žemva, K. Lutar, A. Jesih, W. J. Casteel, Jr., A. P. Wilkinson, D. E. Cox, R. B. von Dreele, H. Borrmann and N. Bartlett, J. Am. Chem. Soc., 1991, 113, 4192 CrossRef.
- (a) B. Standke and M. Jansen, Z. Anorg. Allg. Chem., 1986, 535, 39 CrossRef CAS; (b) B. Standke and M. Jansen, Angew. Chem., Int. Ed. Engl., 1985, 24, 118 CrossRef.
- (a) M. Toganoh and H. Furuta, Chem. Rev., 2022, 122, 8313 CrossRef CAS PubMed; (b) S. Ganguly and A. Ghosh, Acc. Chem. Res., 2019, 52, 2003 CrossRef CAS PubMed; (c) T. D. Lash, Chem. Rev., 2017, 117, 2313 CrossRef CAS PubMed; (d) B. Szyszko and L. Latos-Grażyński, Chem. Soc. Rev., 2015, 44, 3588 RSC; (e) T. D. Lash, Chem. – Asian J., 2014, 9, 682 CrossRef CAS PubMed; (f) C. Brückner, J. Chem. Educ., 2004, 81, 1665 CrossRef.
- D. Naumann, W. Tyrra, F. Trinius, W. Wessel and T. Roy, J. Fluorine Chem., 2000, 101, 131 CrossRef CAS.
- L. Demonti, N. Saffon-Merceron, N. Mézailles and N. Nebra, Chem. – Eur. J., 2021, 27, 15396 CrossRef CAS PubMed.
- Z. Lu, S. Liu, Y. Lan, X. Leng and Q. Shen, Organometallics, 2021, 40, 1713 CrossRef CAS.
- R. Eujen, B. Hoge and D. J. Brauer, Inorg. Chem., 1997, 36, 1464 CrossRef CAS PubMed.
- A. Pérez-Bitrián, M. Baya, J. M. Casas, L. R. Falvello, A. Martín and B. Menjón, Chem. – Eur. J., 2017, 23, 14918 CrossRef PubMed.
- D. Joven-Sancho, M. Baya, L. R. Falvello, A. Martín, J. Orduna and B. Menjón, Chem. – Eur. J., 2021, 27, 12796 CrossRef CAS PubMed.
- (a) S. F. Rach and F. E. Kühn, Chem. Rev., 2009, 109, 2061 CrossRef CAS PubMed; (b) B. N. Storhoff and H. C. Lewis, Jr., Coord. Chem. Rev., 1977, 23, 1 CrossRef CAS.
- (a) A. G. Algarra, V. V. Grushin and S. A. Macgregor, Organometallics, 2012, 31, 1467 CrossRef CAS; (b) P. Sgarbossa, A. Scarso, G. Strukul and R. A. Michelin, Organometallics, 2012, 31, 1257 CrossRef CAS; (c) T. G. Appleton, H. C. Clark and L. E. Manzer, Coord. Chem. Rev., 1973, 10, 335 CrossRef CAS.
- D. M. Gil, J. Echeverría and S. Alvarez, Inorg. Chem., 2022, 61, 9082 CrossRef CAS PubMed.
- D. Joven-Sancho, M. Baya, A. Martín, J. Orduna and B. Menjón, Angew. Chem., Int. Ed., 2021, 60, 26545 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, spectroscopic, computational and X-ray diffraction details (.pdf); atomic coordinates of the optimized structures (.xyz). CCDC 2226904–2226910. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00493g |
| ‡ The structures of AgF3 (80477-ICSD)2 and Ag2O3 (59193-ICSD)3 are isomorphous with their corresponding gold homologues. |
| § The penetration index, p(AB), of two given atoms A and B is defined as follows: p(AB) = 100·[ν(A) + ν(B) − d(AB)]·[ν(A) + ν(B) − r(A) − r(B)]−1, where d(AB) is the actual distance between the involved atoms, and r(A)/ν(A) and r(B)/ν(B) are their respective covalent/van der Waals radii.13 |
| This journal is © The Royal Society of Chemistry 2023 |