Open Access Article
Open Access ArticlePalladium-catalyzed synthesis of benzosilacyclobutenes via position-selective C(sp3)–H arylation†
Naoya
Hamada
a,
Daigo
Hayashi
a and
Ryo
Shintani
 *ab
*ab
aDivision of Chemistry, Department of Materials Engineering Science, Graduate School of Engineering Science, Osaka University, Toyonaka, Osaka 560-8531, Japan. E-mail: shintani.ryo.es@osaka-u.ac.jp
bInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ICS-OTRI), Osaka University, Suita, Osaka 565-0871, Japan
First published on 29th June 2023
Abstract
A palladium-catalyzed synthesis of benzosilacyclobutenes has been developed via position-selective C(sp3)–H bond activation, including those having substituents at the methylene carbon on the 4-membered silacycle. The obtained products could be engaged in the palladium- or nickel-catalyzed ring-expansion reactions to give compounds possessing 6-membered silacycles.
4-Membered silacycles, silacyclobutanes and related compounds, belong to a synthetically useful class of compounds and a variety of transformations have been developed based on their ring strain and Lewis acidity.1,2 Among them, arene-fused derivatives, benzosilacyclobutenes and their analogs, have been actively utilized as synthetic intermediates of more complex organosilanes,3 but their available preparation methods are very limited. In fact, other than a recent report by Petit and coworkers where they utilized a niobium-catalyzed [2+2+2] cycloaddition,4 most of the reported compounds are prepared from 2-bromobenzyl halides and dichlorosilanes using more than a stoichiometric amount of magnesium metal.1,5 In addition, the synthesis of benzosilacyclobutenes having substituents at the methylene carbon on the 4-membered ring has been even less explored and essentially limited to bromination–metalation–nucleophilic substitution of pre-formed unsubstituted benzosilacyclobutenes (Scheme 1a).5b,6
 | ||
| Scheme 1 (a) Conventional and (b) new synthesis of benzosilacyclobutenes having substituents at the carbon on the 4-membered ring. | ||
As a new synthetic strategy of the 4-membered carbo- or heterocycles, the reactions involving a transition-metal-catalyzed intramolecular C–H bond activation can be a powerful alternative to the conventional approaches, and several effective methods have been reported to date for the synthesis of benzocyclobutenes and their analogs.7,8 However, most of them rely on the activation of methyl C–H bonds, and only a few reports have been made on the 4-membered ring formations through the activation of methylene C–H bonds.7d–f In this context, herein we describe the development of a palladium-catalyzed 4-membered ring-forming intramolecular C(sp3)–H bond arylation of 2-(alkylsilyl)aryl triflates,8a–d enabling the synthesis of substituted benzosilacyclobutenes at the methylene carbon of the silacycle (Scheme 1b).
Initially, we conducted a reaction of 2-naphthyl triflate 1a having butyldicyclohexylsilyl group at 1-position in the presence of a catalytic amount of Pd(OAc)2/PPh3 with Et2NH as the base in DMF at 80 °C. Under these conditions, desired naphthosilacyclobutene 2a was obtained in a moderate yield of 40% along with some uncyclized butenyldicyclohexyl(1-naphthyl)silanes (Table 1, entry 1). The change of ligand to PCy3 led to a significant improvement to give compound 2a in 74% yield (entry 2), while the use of bulkier P(tBu)3 led to a decrease of the reaction efficiency (entry 3). On the other hand, no desired product 2a was observed by using binap as the ligand (entry 4), and the ferrocene-based bisphosphine ligands such as dppf and dtbpf were found to be similarly ineffective as P(tBu)3 (entries 5 and 6).9
| Entry | Ligand | Yield of 2aa (%) | Yield of side productsa (%) |
|---|---|---|---|
| a Determined by 1H NMR against internal standard. b PR3·HBF4/Et2NH was used. c 2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl. d 1,1′-Bis(diphenylphosphino)ferrocene. e 1,1′-Bis(di-tert-butylphosphino)ferrocene. | |||
| 1 | PPh3 | 40 | 8 |
| 2 | PCy 3 | 74 | 2 |
| 3 | P(tBu)3b | 21 | 3 |
| 4 | binapc | 0 | 0 |
| 5 | dppfd | 27 | 2 |
| 6 | dtbpfe | 16 | 4 |
Under the conditions in Table 1, entry 2, several alkyl groups were installed at the alkyl carbon on the silacycle of compounds 2 by changing the R group of substrates 1 (Scheme 2). For example, in addition to propyl group, naphthosilacyclobutenes having isobutyl group or alkyl groups containing aryl, silyloxy, and carbozolyl groups could be obtained in reasonably high yields (2b–2g). Regarding the ‘spectator’ groups on silicon, sterically demanding aryl groups could also be employed instead of cyclohexyl group as shown for the synthesis of compound 2h, but simple phenyl group was more reactive toward C–H bond activation over an alkyl group for substrate 1x to give the corresponding benzonaphthosilole preferentially instead of the desired naphthosilacyclobutene (data not shown).10 In addition to naphthosilacyclobutenes, substituted benzosilacyclobutenes could also be synthesized by using phenyl triflate derivatives in the present catalysis. Thus, although 3-unsubstituted aryl triflates such as 1y did not undergo this reaction, various 3-substituted substrates 1 could be employed to give corresponding benzosilacyclobutenes 2 in moderate to high yields (2i–2o). 4-Silyl-5-indolyl triflate 1p could also be converted to product 2p albeit with lower efficiency, but substrate 1z having trifluoromethyl group at 3-position was not applicable. The structures of 2g and 2p were unambiguously confirmed by X-ray crystallographic analysis.11
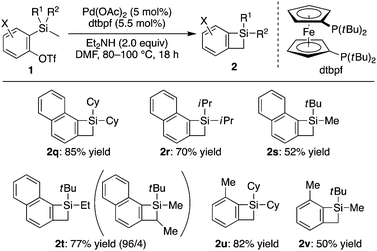
The present catalysis was further extended to the synthesis of compounds 2 with no substituents at the carbon on the 4-membered ring by employing substrates having methyl group on silicon. Although the use of PCy3 as the ligand gave almost no desired product 2q for the reaction of 2-naphthyl triflate 1q having dicyclohexyl(methyl)silyl group at 1-position, a high yield of 85% was realized by employing dtbpf, a ferrocene-based bulky bisphosphine ligand (Scheme 3). Under these conditions, the product yields were found to be higher for substrates having bulkier substituents on silicon, and a gradual decrease in the yield was observed by changing the substituents from dicyclohexyl (2q) to diisopropyl (2r) and to (tert-butyl)(methyl) (2s). It is worth noting that a C–H bond of methyl group was selectively activated over a methylene C–H bond or a methyl C–H bond of ethyl group on silicon as demonstrated for the synthesis of 2t. In addition to naphthosilacyclobutenes, benzosilacyclobutenes 2u and 2v could also be synthesized with similar tendency.
With a series of naphtho- and benzosilacyclobutenes in hand, we briefly examined their reactivity by applying them to some reported transformations. For example, the reaction of naphthosilacyclobutene 2a with dimethyl acetylenedicarboxylate (3a) in the presence of Pd(PPh3)4 (5 mol%) proceeded via selective C(aryl)–Si bond cleavage to give ring-expanded naphthosilacyclohexadiene 4aa in 53% yield (Scheme 4a).3b A similar result was obtained with benzosilacyclobutene 2i to give benzosilacyclohexadiene 4ia in 52% yield. On the other hand, compound 2q having no substituent at the alkyl carbon on the 4-membered ring showed a higher reactivity to give product 4qa in a nearly quantitative yield. The reaction of 2q with diethyl acetylenedicarboxylate (3b) also gave 4qb in a high yield, but the use of methyl propiolate (3c) resulted in a lower yield of 4qc as expected from the literature precedent.3b Furthermore, these could be employed in a nickel-catalyzed ring-expansion reaction with aldehydes as well.3e,i The reaction of 2a or 2i with benzaldehyde (5a) proceeded with relatively high diastereoselectivity (89/11–95/5) to give corresponding dihydronaphtho- or dihydrobenzooxasiline 6aa or 6ia through the cleavage of a C(aryl)–Si bond (Scheme 4b). The relative configuration of the major diastereomer was determined to be cis by X-ray crystallographic analysis for both 6aa and 6ia.11 A higher yield of 87% was achieved for the reaction of unsubstituted 2q to give ring-expanded product 6qa. E-Cinnamaldehyde (5b) could also be used for the reaction of 2q to give compound 6qb in 82% yield.
A proposed catalytic cycle of the present catalysis toward benzosilacyclobutenes is illustrated in Scheme 5a. Oxidative addition of aryl triflate of 1 to palladium(0) gives arylpalladium species A. This then undergoes C–H bond activation of the alkyl carbon adjacent to silicon to give 5-membered palladacycle B.12 Carbon–carbon bond-forming reductive elimination leads to the formation of product 2 along with regeneration of palladium(0) species. As shown in Table 1, side products for the reaction of 1a are butenyldicyclohexyl(1-naphthyl)silanes, and the formation of these compounds can be explained by the pathways shown in Scheme 5b. Instead of reductive elimination from intermediate B, 1,4- or 1,5-palladium migration from A-a gives alkylpalladium species C-a or D-a,13 and subsequent β-hydrogen elimination would lead to the observed side products.
To gain insights into the reaction mechanism of the present catalysis, we conducted some control experiments. When the reaction of 1s-d3 having tert-butyl(methyl)(methyl-d3)silyl group was conducted under the conditions in Scheme 3, C–H bond activated product 2s-d3 and C–D bond activated product 2s-d2 were obtained in the ratio of 7.5/1 (Scheme 6a). We also carried out a competition between 1q and 1q-d3 to determine their relative reactivity, and found that C–H bond activated product 2q and C–D bond activated product 2q-d2 were obtained in the ratio of 1.2/1 at an early stage of the reaction (Scheme 6b). These results indicate that the C–H(D) bond activation step (A → B in Scheme 5a) is not the turnover-limiting step and occurs after the irreversible oxidative addition step.14 Although we have not been able to determine the turnover-limiting step by kinetic experiments due to the existence of an induction period at the beginning of the reaction, 4-membered ring-forming reductive elimination step could be the turnover-limiting step, considering that this step generates a significant ring strain.7d,f It is also worth noting that the substituent effects observed in Scheme 2 could be explained by the difficulty of the 4-membered ring formation. As illustrated in Scheme 5c, the methyl group at 3-position of 1i would facilitate the reductive elimination from intermediate B-i to reduce the steric repulsion between the methyl group and the silyl group. On the other hand, 3-unsubstituted 1y does not have this effect in intermediate B-y. Along this line, the lack of reactivity of 1z having trifluoromethyl group at 3-position might be due to the favorable interaction between the fluorine atoms and the silicon atom, which could retard the formation and/or subsequent reductive elimination of palladacycle B-z. Furthermore, the decrease of the product yield by reducing the steric bulk of the silicon substituents in Scheme 3 is also consistent with these explanations.
In summary, we developed a palladium-catalyzed synthesis of benzosilacyclobutenes from 2-(alkylsilyl)aryl triflates via position-selective C(sp3)–H bond activation. Although the applicable substrates need to meet the steric requirement, various benzosilacyclobutenes could be synthesized including those substituted at the methylene carbon on the 4-membered silacycle. The obtained products could be employed in the palladium- or nickel-catalyzed ring-expansion reactions to give benzosilacyclohexadienes or dihydrobenzooxasilines possessing 6-membered silacycles. Future studies will be directed toward further expansion of this process for the synthesis of various functional organosilicon compounds.
Support has been provided in part by JSPS KAKENHI Grant Number JP20H02741 (Grant-in-Aid for Scientific Research (B)). We thank Dr Hiroyasu Sato at Rigaku Corporation and Mr Hirokazu Moniwa and Mr Donghyeon Lee at Osaka University for the X-ray crystallographic analysis.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For reviews: (a) J. Huang, F. Liu, X. Wu, J.-Q. Chen and J. Wu, Org. Chem. Front., 2022, 9, 2840 RSC; (b) Q.-C. Mu, J. Chen, C.-G. Xia and L.-W. Xu, Coord. Chem. Rev., 2018, 374, 93 CrossRef CAS; (c) N. V. Ushakov and E. S. Finkelshtein, Russ. Chem. Rev., 2013, 82, 205 CrossRef; (d) M. Ishikawa, A. Naka and H. Kobayashi, Coord. Chem. Rev., 2017, 335, 58 CrossRef CAS.
- (a) H. Sakurai and T. Imai, Chem. Lett., 1975, 891 CrossRef CAS; (b) Y. Takeyama, K. Oshima and K. Utimoto, Tetrahedron Lett., 1990, 31, 6059 CrossRef CAS; (c) A. G. Myers, S. E. Kephart and H. Chen, J. Am. Chem. Soc., 1992, 114, 7922 CrossRef CAS; (d) S. E. Denmark, B. D. Griedel and D. M. Coe, J. Org. Chem., 1993, 58, 988 CrossRef CAS; (e) S. E. Denmark, B. D. Griedel, D. M. Coe and M. E. Schnute, J. Am. Chem. Soc., 1994, 116, 7026 CrossRef CAS; (f) K. Matsumoto, K. Oshima and K. Utimoto, J. Org. Chem., 1994, 59, 7152 CrossRef CAS; (g) K. Hirano, H. Yorimitsu and K. Oshima, J. Am. Chem. Soc., 2007, 129, 6094 CrossRef CAS PubMed; (h) R. Shintani, K. Moriya and T. Hayashi, J. Am. Chem. Soc., 2011, 133, 16440 CrossRef CAS PubMed; (i) N. Ishida, S. Okumura, T. Kawasaki and M. Murakami, Angew. Chem., Int. Ed., 2018, 57, 11399 CrossRef CAS PubMed; (j) Y. Qin, J.-L. Han, C.-W. Ju and D. Zhao, Angew. Chem., Int. Ed., 2020, 59, 8481 CrossRef CAS PubMed.
- (a) R. Okazaki, K.-T. Kang and N. Inamoto, Tetrahedron Lett., 1981, 22, 235 CrossRef CAS; (b) Y. Takeyama, K. Nozaki, K. Matsumoto, K. Oshima and K. Utimoto, Bull. Chem. Soc. Jpn., 1991, 64, 1461 CrossRef CAS; (c) K. Uenishi, I. Imae, E. Shirakawa and Y. Kawakami, Macromolecules, 2002, 35, 2455 CrossRef CAS; (d) Y. Kakihana, K. Uenishi, I. Imae and Y. Kawakami, Macromolecules, 2005, 38, 6321 CrossRef CAS; (e) K. Hirano, H. Yorimitsu and K. Oshima, Org. Lett., 2006, 8, 483 CrossRef CAS PubMed; (f) N. Agenet, J.-H. Mirebeau, M. Petit, R. Thouvenot, V. Gandon, M. Malacria and C. Aubert, Organometallics, 2007, 26, 819 CrossRef CAS; (g) N. Ishida, S. Okumura and M. Murakami, Chem. Lett., 2018, 47, 570 CrossRef CAS; (h) W.-T. Zhao, F. Gao and D. Zhao, Angew. Chem., Int. Ed., 2018, 57, 6329 CrossRef CAS PubMed; (i) J. Huo, K. Zhong, Y. Xue, M. Lyu, Y. Ping, W. Ouyang, Z. Liu, Y. Lan and J. Wang, Chem. – Eur. J., 2022, 28, e202200191 CAS; (j) Q. Wang, K.-B. Zhong, H. Xu, S.-N. Li, W.-K. Zhu, F. Ye, Z. Xu, Y. Lan and L.-W. Xu, ACS Catal., 2022, 12, 4571 CrossRef CAS; (k) X.-C. Wang, B. Li, C.-W. Ju and D. Zhao, Nat. Commun., 2022, 13, 3392 CrossRef CAS PubMed; (l) S. Chen, X. He, C. Jin, W. Zhang, Y. Yang, S. Liu, Y. Lan, K. N. Houk and X. Shen, Angew. Chem., Int. Ed., 2022, 61, e202213431 CAS.
- C. Simon, M. Amatore, C. Aubert and M. Petit, Org. Lett., 2015, 17, 844 CrossRef CAS PubMed.
- (a) H. Gilman and W. H. Atwell, J. Am. Chem. Soc., 1964, 86, 5589 CrossRef CAS; (b) K.-T. Kang, H.-Y. Song and H.-C. Seo, Chem. Lett., 1985, 617 CrossRef CAS; (c) H. J. R. de Boer, O. S. Akkerman and F. Bickelhaupt, J. Organomet. Chem., 1987, 321, 291 CrossRef CAS ; See also: ; (d) L. E. Gusel’nikov, V. V. Volkova, E. N. Buravtseva, A. S. Redchin, N. Auner, B. Herrschaft, B. Solouki, G. Tsantes, Y. E. Ovchinnikov, S. A. Pogozhikh, F. M. Dolgushin and V. V. Negrebetsky, Organometallics, 2002, 21, 1101 CrossRef.
- For other isolated examples: (a) T. J. Barton and B. L. Groh, Organometallics, 1985, 4, 575 CrossRef CAS; (b) M. Trommer, G. E. Miracle, B. E. Eichler, D. R. Powell and R. West, Organometallics, 1997, 16, 5737 CrossRef CAS; (c) D. Yan, J. Mohsseni-Ala, N. Auner, M. Bolte and J. W. Bats, Chem. – Eur. J., 2007, 13, 7204 CrossRef CAS PubMed; (d) M. Ahmad, A.-C. Gaumont, M. Durandetti and J. Maddaluno, Angew. Chem., Int. Ed., 2017, 56, 2464 CrossRef CAS PubMed.
- For selected examples: (a) G. Dyker, Angew. Chem., Int. Ed. Engl., 1994, 33, 103 CrossRef; (b) M. Chaumontet, R. Piccard, N. Audic, J. Hitce, J.-L. Peglion, E. Clot and O. Baudoin, J. Am. Chem. Soc., 2008, 130, 15157 CrossRef CAS PubMed; (c) S. Rousseaux, M. Davi, J. Sofack-Kreutzer, C. Pierre, C. E. Kefalidis, E. Clot, K. Fagnou and O. Baudoin, J. Am. Chem. Soc., 2010, 132, 10706 CrossRef CAS PubMed; (d) C. E. Kefalidis, M. Davi, P. M. Holstein, E. Clot and O. Baudoin, J. Org. Chem., 2014, 79, 11903 CrossRef CAS PubMed; (e) X. Yang, G. Shan, Z. Yang, G. Huang, G. Dong, C. Sheng and Y. Rao, Chem. Commun., 2017, 53, 1534 RSC; (f) P. A. Provencher, J. F. Hoskin, J. J. Wong, X. Chen, J.-Q. Yu, K. N. Houk and E. J. Sorensen, J. Am. Chem. Soc., 2021, 143, 20035 CrossRef CAS PubMed; (g) B. Xu, D. Ji, L. Wu, L. Zhou, Y. Liu, Z.-M. Zhang and J. Zhang, Chemistry, 2022, 8, 836 CrossRef CAS ; For a review: ; (h) O. Baudoin, Acc. Chem. Res., 2017, 50, 1114 CrossRef CAS PubMed ; See also: ; (i) A. K. Sadana, R. K. Saini and W. E. Billups, Chem. Rev., 2003, 103, 1539 CrossRef CAS PubMed.
- For recent reviews on C–H bond activation under transition-metal catalysis: (a) B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B.-F. Shi, Chem. Rev., 2021, 121, 14957 CrossRef CAS PubMed; (b) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Q. Yu, Chem. Rev., 2017, 117, 8754 CrossRef CAS PubMed; (c) N. Dastbaravardeh, M. Christakakou, M. Haider and M. Schnürch, Synthesis, 2014, 1421 Search PubMed; (d) O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902 RSC; (e) P. Wedi and M. van Gemmeren, Angew. Chem., Int. Ed., 2018, 57, 13016 CrossRef CAS PubMed; (f) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603 RSC; (g) C. Liu, J. Yuan, M. Gao, S. Tang, W. Li, R. Shi and A. Lei, Chem. Rev., 2015, 115, 12138 CrossRef CAS PubMed.
- Essentially no C–N coupling products with Et2NH were observed in any entries presumably due to the low basicity of the reaction conditions.
- (a) M. Shimizu, K. Mochida and T. Hiyama, Angew. Chem., Int. Ed., 2008, 47, 9760 CrossRef CAS PubMed; (b) R. Shintani, H. Otomo, K. Ota and T. Hayashi, J. Am. Chem. Soc., 2012, 134, 7305 CrossRef CAS PubMed.
- CCDC Deposition Numbers 2237529–2237532 contain the supplementary crystallographic data for this paper†.
- Y. Liang, W. Geng, J. Wei, K. Ouyang and Z. Xi, Org. Biomol. Chem., 2012, 10, 1537 RSC.
- For recent reviews on 1,n-metal migration reactions: (a) M.-Y. Li, D. Wei, C.-G. Feng and G.-Q. Lin, Chem. – Asian J., 2022, 17, e202200456 CAS; (b) J. Corpas, P. Mauleón, R. G. Arrayás and J. C. Carretero, ACS Catal., 2021, 11, 7513 CrossRef CAS; (c) X. Dong, H. Wang, H. Liu and F. Wang, Org. Chem. Front., 2020, 7, 3530 RSC; (d) A. Rahim, J. Feng and Z. Gu, Chin. J. Chem., 2019, 37, 929 CrossRef CAS.
- E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2237529–2237532. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00442b |
| This journal is © The Royal Society of Chemistry 2023 |