Open Access Article
Open Access ArticleStepwise assembly of heterometallic, heteroleptic “triblock Janus-type” metal–organic polyhedra†
Cornelia
von Baeckmann
ab,
Sara
Ruiz-Relaño
ab,
Inhar
Imaz
ab,
Marcel
Handke
ab,
Judith
Juanhuix
 c,
Felipe
Gándara
c,
Felipe
Gándara
 d,
Arnau
Carné-Sanchez
d,
Arnau
Carné-Sanchez
 *ab and
Daniel
Maspoch
*ab and
Daniel
Maspoch
 *abe
*abe
aCatalan Institute of Nanoscience and Nanotechnology (ICN2), CSIC, and The Barcelona Institute of Science and Technology, Campus UAB, Bellaterra, Barcelona 08193, Spain. E-mail: arnau.carne@icn2.cat; daniel.maspoch@icn2.cat
bDepartament de Química, Facultat de Ciències Universitat Autònoma de Barcelona, Bellaterra 08193, Spain
cAlba Synchrotron Light Facility, Cerdanyola del Vallès, Barcelona 08290, Spain
dMaterials Science Institute of Madrid (ICMM), Consejo Superior de Investigaciones Científicas (CSIC), Calle Sor Juana Inés de la Cruz, 3, Madrid 28049, Spain
eICREA, Pg. Lluís Companys 23, Barcelona 08010, Spain
First published on 13th February 2023
Abstract
Increasing the chemical complexity of metal–organic cages (MOCs) or polyhedra (MOPs) demands control over the simultaneous organization of diverse organic linkers and metal ions into discrete caged structures. Herein, we show that a pre-assembled complex of the archetypical cuboctahedral MOP can be used as a template to replicate such caged structure, one having a “triblock Janus-type” configuration that is both heterometallic and heteroleptic.
Discrete metal–organic assemblies featuring empty cavities, generally known as coordination-/metal–organic cages (MOCs)1,2 or metal–organic polyhedra (MOPs),3 are receiving great attention due to their rich structural versatility and potential applications in areas such as molecular separation, catalysis, gas storage and biomedical applications.4–7 To date, most of these assemblies have been constructed from one type of organic linker and one type of metal ion. This is mainly due to the inherent difficulty of controlling the assembly of multiple ions and linkers into such discrete caged structures.8 However, there is an increasing interest in developing more complex, multicomponent MOCs that may present emerging properties arising from the intra-MOC interactions between different adjacent building blocks, such as increased chemical stability, tandem catalysis, optical or magnetic properties.9,10 In this context, multicomponent assembly strategies for the construction of cages with two or more metal ions (known as multimetallic cages), or with two or more organic linkers (known as heteroleptic cages), have recently been proposed.11,12 Specifically, heteroleptic cages have been assembled through the use of shape complementary linkers,13,14 asymmetric linkers,15–17 and coordination sphere engineering.18 Interestingly, heterometallic cages19 are even rarer than heteroleptic ones. The most common route to them entails the use of heterotopic ligands, which can bind two different metal ions according to their different coordination preferences.20–22 Alternatively, pre-synthesized hetero-bimetallic clusters can be employed.23
Herein, we report a new multicomponent assembly strategy that uses a pre-assembled metal–organic fragment of a known caged structure as a building unit. We envisioned that such a pre-assembled fragment could template the formation of the parent cage when combined with complementary organic linkers and/or metal ions. We reasoned that, if these complementary organic linkers and metal ions were different to those comprising the metal–organic fragment, then our templated synthesis would enable assembly of heteroleptic and bimetallic versions of the parent caged structure. To validate our hypothesis, we applied our strategy to the archetypical cuboctahedral Cu24bdc2424 or Rh24bdc2425 (where bdc stands for 1,3-benzenedicarboxylic acid) MOP, aiming to assemble heteroleptic and/or bimetallic cuboctahedral caged structures having a “triblock Janus-type” configuration.
We began with a retrosynthetic analysis to identify a repetitive, non-interconnected, metal–organic fragment within the cuboctahedral MOP. We found the Rh2(bdc)4 fragment in the central part (Fig. 1, in magenta) of the cage. It is a Rh(II) paddlewheel complex comprising four bdc linkers, each showing a free carboxylic acid group. We reasoned that four of these pre-synthesized Rh2(bdc)4 complexes should template the formation of the cuboctahedral MOP when connected via 16 additional metal ions and 8 bdc linkers (Fig. 1; in cyan). This assembly involves three different components that ultimately provide access to cuboctahedral MOP whose central portion differs in composition from their two lateral portions, analogously to the well-known, “triblock Janus-type” particles.26 Accordingly, the cuboctahedral structure of the resulting MOPs will be divided in three distinct parts that exhibit two different chemistries.
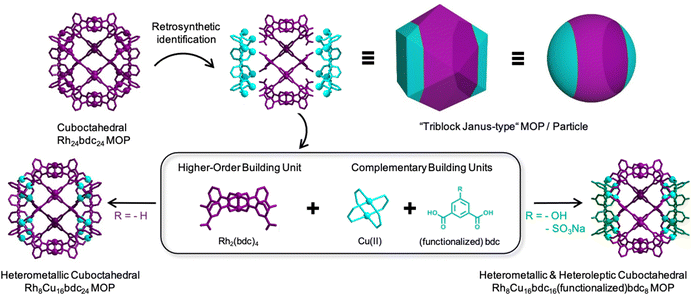 | ||
| Fig. 1 Schematic illustration of the multicomponent assembly strategy used to synthesize heteroleptic and heterometallic, “triblock Janus-type”, MOPs. | ||
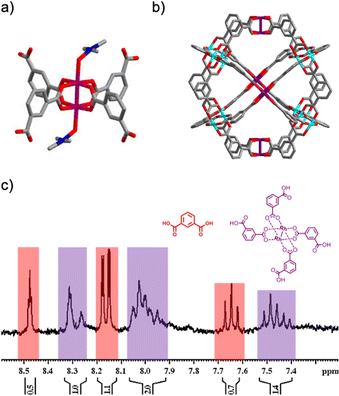
Having identified the Rh2(bdc)4 fragment, we next proceeded with its synthesis. As this complex contains free carboxylic acid groups, we used a protection/deprotection strategy for one of the two carboxylic groups in the bdc linker.27 In the first step, the carboxylic acid group was protected using 2-(trimethylsilyl)ethanol (see synthetic details in the ESI,† and Fig. S5 and S6). This step was essential, as it prevented reaction of the two carboxylic acid groups of each bdc with Rh(II), thus promoting formation of the protected Rh2(bdc)4 and consequently, precluding generation of homometallic MOPs or extended structures. The protected Rh2(bdc)4 complex was synthesized by reacting the half-protected bdc linker with Rh(II) acetate in chlorobenzene at 150 °C overnight. The resulting complex was purified through column chromatography, and its formation was confirmed by nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS) (Fig. S7 and S8, ESI†). Finally, the four carboxylic acid groups of the complex were deprotected by treatment with tetra-n-butylammonium fluoride at room temperature overnight. The formation of Rh2(bdc)4 with the four deprotected carboxylic acid groups was confirmed by single-crystal X-ray diffraction (SCXRD, Fig. 2a), 1H NMR and MS (Fig. S9–S11, ESI†).
With the complex in hand, we first used it as a building block to assemble a three-component, bimetallic cuboctahedral MOP. Based on the expected stoichiometry between the three components in the final caged structure, Rh2(bdc)4 was reacted with 4 mol. eq. of Cu(NO3)2 and 2 mol. eq. of bdc in dimethylacetamide (DMA) at room temperature for 1 week to obtain a green solid, herein named HET-MOP-1 (where HET stands for heteroleptic and/or heterometallic). The solid was washed with DMA and diethyl ether to remove unreacted reagents and left to dry in open air (yield: 13%). To confirm the MOP structure, we performed N2 and CO2 adsorption of the obtained solid (Fig. S12, ESI†), observing a Brunauer–Emmett–Teller (BET) surface area of 411 m2 g−1 and a maximum uptake at 1 bar and 298 K of 1.03 mmol CO2 g−1. Importantly, the solid could be redissolved in dimethylformamide (DMF), which suggested that the product of the reaction is not an extended framework but a discrete molecule. Exposure of the DMF solution in the presence of 4-tert-butylpyridine to ether vapors let to formation of crystals suitable for SCXRD, which revealed formation of the targeted, bimetallic, cuboctahedral HET-MOP-1, of formula [Rh8Cu16(bdc)24] (Fig. 2b). However, in this structure, the position of each metal could not be elucidated, due to the high symmetry of the cage, whereby Rh(II) and Cu(II) can occupy the same crystallographic positions (Fig. S13, ESI†), and where all paddlewheels are topologically equivalent, corresponding to the only kind of vertex present in the cuboctahedron.28,29 Considering this, a satisfactory refinement was obtained when the occupancy of each metal site was fixed to 1/3 for Rh(II) and 2/3 for Cu(II), as expected from the molecular formula of HET-MOP-1. Note that the powder-XRD pattern matched the calculated pattern, confirming the purity of the sample (Fig. S14, ESI†).
Next, we further confirmed the bimetallic character of the cuboctahedral HET-MOP-1 by matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) spectrometry, from which the solubilized HET-MOP-1 showed a peak at m/z = 5779.8, which is in very good agreement with the expected molecular mass ([M + H+] = 5779.4) (Fig. S15, ESI†). Also, high resolution SEM/EDX performed on several single crystals confirmed the presence of both Rh and Cu in the expected proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Rh/Cu) (Fig. S16, ESI†). Furthermore, EDX mapping performed on several single crystals of HET-MOP-1 revealed the homogeneous distribution of both metal ions through the crystal (Fig. S17, ESI†). In addition, UV-Vis analysis of the DMF solution of HET-MOP-1 revealed the presence of two broad bands centered at 588 nm and 746 nm, which were ascribed to the λmax of both Rh(II)- and Cu(II)-based paddlewheels, respectively (Fig. S18, ESI†).30,31 Finally, to further demonstrate the integrity of Rh2(bdc)4 throughout the self-assembly process, HET-MOP-1 was disassembled into its initial components by exposing it to mild acidic conditions at room temperature. The titration of a DMF solution of HET-MOP-1 with HCl was monitored through UV-Vis (Fig. S19, ESI†). Among the two types of paddlewheels forming HET-MOP-1, titration revealed that the Cu(II) one could be fully disassembled, whereas the Rh(II) paddlewheel could not be, as it was maintained even upon addition of 12 mol. eq. of acid. This disassembly experiment further confirms the absence of metal exchange between the Rh2(bdc)4 and Cu(II) ions during the synthesis of the HET-MOP-1, which is ascribed to inertness of the equatorial position of Rh(II)-carboxylate paddlewheel structures.10 The 1H-NMR spectrum of the acid-treated HET-MOP-1 (in DMSO-d6) further confirmed selective disassembly of the MOP into the deprotected Rh2(bdc)4 complex and bdc linkers at a molar ratio of 1
2 (Rh/Cu) (Fig. S16, ESI†). Furthermore, EDX mapping performed on several single crystals of HET-MOP-1 revealed the homogeneous distribution of both metal ions through the crystal (Fig. S17, ESI†). In addition, UV-Vis analysis of the DMF solution of HET-MOP-1 revealed the presence of two broad bands centered at 588 nm and 746 nm, which were ascribed to the λmax of both Rh(II)- and Cu(II)-based paddlewheels, respectively (Fig. S18, ESI†).30,31 Finally, to further demonstrate the integrity of Rh2(bdc)4 throughout the self-assembly process, HET-MOP-1 was disassembled into its initial components by exposing it to mild acidic conditions at room temperature. The titration of a DMF solution of HET-MOP-1 with HCl was monitored through UV-Vis (Fig. S19, ESI†). Among the two types of paddlewheels forming HET-MOP-1, titration revealed that the Cu(II) one could be fully disassembled, whereas the Rh(II) paddlewheel could not be, as it was maintained even upon addition of 12 mol. eq. of acid. This disassembly experiment further confirms the absence of metal exchange between the Rh2(bdc)4 and Cu(II) ions during the synthesis of the HET-MOP-1, which is ascribed to inertness of the equatorial position of Rh(II)-carboxylate paddlewheel structures.10 The 1H-NMR spectrum of the acid-treated HET-MOP-1 (in DMSO-d6) further confirmed selective disassembly of the MOP into the deprotected Rh2(bdc)4 complex and bdc linkers at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as expected from the molecular formula of HET-MOP-1 (Fig. 2c and Fig. S20 and S21, ESI†).
2, as expected from the molecular formula of HET-MOP-1 (Fig. 2c and Fig. S20 and S21, ESI†).
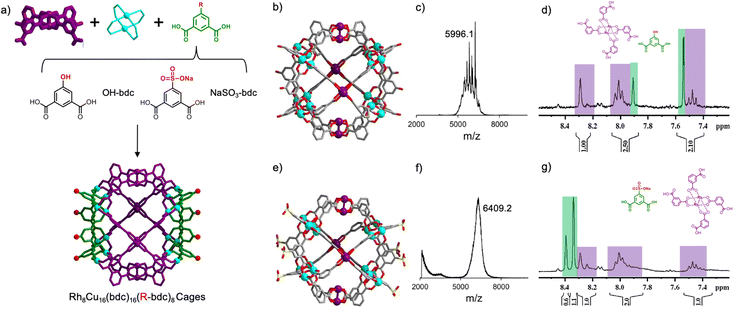
We then envisioned incorporating a functionalized bdc linker into the self-assembly process to assemble cuboctahedral MOPs that would be both bimetallic and heteroleptic. With the use of functionalized bdc linkers, we expected to introduce such functional groups into both side portions of the “triblock Janus-type” MOPs. We imagined replacing the complementary bdc with functionalized versions of it, at its 5-position (Fig. 3a). Thus, we decided to run a co-assembly reaction of Cu(II), Rh2(bdc)4 and either 5-hydroxyl-isophthalic acid (OH-bdc) or 5-sulfoisophthalic acid sodium salt (NaSO3-bdc). In a first assembly, OH-bdc, Cu(NO3)2 and Rh2(bdc)4 were reacted in DMA at room temperature for 24 hours, after which a green precipitate (hereafter named HET-MOP-2) was obtained, which was isolated and washed with DMA and ether (yield: 14%). The green solid was solubilized in DMF and exposed to ether vapours to obtain crystals suitable for SCXRD. Next, a second assembly reaction was performed under similar conditions with respect to the stoichiometry of the different building blocks (85 °C for 48 hours), except that NaSO3-bdc was used instead of OH-bdc. This led to a similar product, named HET-MOP-3 (yield: 25%). Here, single crystals suitable for SCXRD analysis were directly harvested from the solvothermal reaction.
SCXRD confirmed formation of two cuboctahedral cages built up from two types of linkers (bdc and either OH-bdc or NaSO3-bdc) and two types of metal ions [Cu(II) and Rh(II)] (Fig. 3b and e). Analysis of the crystal structures of HET-MOP-2 and HET-MOP-3, which followed the same principles as for HET-MOP-1, confirmed occupancy values at each metal site of 1/3 for Rh(II) and of 2/3 for Cu(II). Similarly, based on these symmetric effects, the electron density of all functional groups of the linkers are a sum of hydrogen (for bdc) and OH (for OH-bdc) or NaSO3 (for NaSO3-bdc). Here, the molecular formula suggested a ratio of 1/3 R-bdc (R = OH, NaSO3) to 2/3 bdc, which were confirmed during the refinement process. Powder-XRD patterns further confirmed the purity of the samples (Fig. S22 and S28, ESI†). We then sought further evidence of formation of the above heterometallic and heteroleptic cages, through MALDI-TOF (Fig. 3c and f), which revealed a peak at m/z = 5996.6 (for HET-MOP-2) and m/z = 6409.2 (for HET-MOP-3), which are in agreement with the corresponding postulated formulas: [Rh8Cu16(bdc)16(OH-bdc)8] ([M + H3O+ + DMF] = 5996.3); and [Rh8Cu16(bdc)16(NaSO3-bdc)8] ([M + H+] = 6409.6). Consistently, mild acidic disassembly of both HET-MOP-2 and HET-MOP-3 at room temperature revealed the expected molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Rh2(bdc)4 complex/R-bdc) (Fig. 3d and g) (for UV/Vis analysis see Fig. S23 and S29, ESI†). Identification of Rh2(bdc)4 in this disassembly process clearly evidenced preservation of this complex throughout the self-assembly process, thus excluding any possible ligand exchange. Acidic disassembly at high temperature (T = 100 °C) enabled full disassembly of both HET-MOP-2 and HET-MOP-3 into their corresponding free linkers (Fig. S24 and S30, ESI†). 1H-NMR spectra of the resulting solutions revealed molar ratios of 1
2 (Rh2(bdc)4 complex/R-bdc) (Fig. 3d and g) (for UV/Vis analysis see Fig. S23 and S29, ESI†). Identification of Rh2(bdc)4 in this disassembly process clearly evidenced preservation of this complex throughout the self-assembly process, thus excluding any possible ligand exchange. Acidic disassembly at high temperature (T = 100 °C) enabled full disassembly of both HET-MOP-2 and HET-MOP-3 into their corresponding free linkers (Fig. S24 and S30, ESI†). 1H-NMR spectra of the resulting solutions revealed molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 for HET-MOP-2 (bdc/OH-bdc) and HET-MOP-3(bdc/NaSO3-bdc), which are in good agreement with the expected values (1
0.5 for HET-MOP-2 (bdc/OH-bdc) and HET-MOP-3(bdc/NaSO3-bdc), which are in good agreement with the expected values (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5). High-resolution SEM/EDX analysis and EDX mapping of single crystals confirmed the expected Cu
0.5). High-resolution SEM/EDX analysis and EDX mapping of single crystals confirmed the expected Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rh ratio (2/3 Cu and 1/3 Rh) and the homogeneous distribution of both metals within the same crystal for both HET-MOP-2 and HET-MOP-3 (Fig. S25, S26, S31 and S32, ESI†). Finally, we found that HET-MOP-2 is porous to N2 (BET surface area = 359 m2 g−1) and CO2 (uptake at 1 bar and 298 K = 1.2 mmol CO2 g−1), whereas HET-MOP-3 is only porous to CO2 (uptake at 1 bar and 298 K = 1.2 mmol CO2 g−1; Fig. S27 and S33, ESI†).
Rh ratio (2/3 Cu and 1/3 Rh) and the homogeneous distribution of both metals within the same crystal for both HET-MOP-2 and HET-MOP-3 (Fig. S25, S26, S31 and S32, ESI†). Finally, we found that HET-MOP-2 is porous to N2 (BET surface area = 359 m2 g−1) and CO2 (uptake at 1 bar and 298 K = 1.2 mmol CO2 g−1), whereas HET-MOP-3 is only porous to CO2 (uptake at 1 bar and 298 K = 1.2 mmol CO2 g−1; Fig. S27 and S33, ESI†).
In conclusion, we have developed a stepwise synthetic method that employs pre-assembled complexes of a known cage to replicate it with a multicomponent character. The value of our method stems from the fact that the pre-assembled Rh2(bdc)4 complex determines the position of both the additional inorganic and organic building blocks in the final cuboctahedral structure. This enables convergence of four different molecules (two types of metal ions + two types of organic linkers) into a single, well-defined caged structure. We are confident that this degree of structural control in discrete metal–organic assemblies should pave the way to achieving greater chemical complexity in cages, expanding the catalog of synthetically accessible cages, and optimizing cages for functional applications such as catalysis and gas storage.
This work was supported by the Spanish MINECO (project RTI2018-095622-B-I00), the Catalan AGAUR (project 2021 SGR 00458), the CERCA Program/Generalitat de Catalunya, and the MCIN/AEI/10.13039/501100011033. ICN2 is supported by the Severo Ochoa program from the Spanish MINECO (grant SEV-2017-0706). The project that gave rise to these results received the support of a fellowship (LCF/BQ/PR20/11770011) from “la Caixa” Foundation (ID 100010434). C.v.B. thanks the Austrian Science Fund (FWF), Erwin Schrödinger fellowship for supporting the project J 4637.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Zarra, D. M. Wood, D. A. Roberts and J. R. Nitschke, Chem. Soc. Rev., 2015, 44, 419–432 RSC.
- M. Yoshizawa, J. K. Klostermann and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS PubMed.
- D. J. Tranchemontagne, Z. Ni, M. O’Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2008, 47, 5136–5147 CrossRef CAS PubMed.
- D. Zhang, T. K. Ronson, Y.-Q. Zou and J. R. Nitschke, Nat. Rev. Chem., 2021, 5, 168–182 CrossRef CAS.
- A. J. Gosselin, C. A. Rowland and E. D. Bloch, Chem. Rev., 2020, 120, 8987–9014 CrossRef CAS PubMed.
- S. K. Samanta and L. Isaacs, Coord. Chem. Rev., 2020, 410, 213181 CrossRef CAS PubMed.
- B. S. Pilgrim and N. R. Champness, ChemPlusChem, 2020, 85, 1842–1856 CrossRef CAS PubMed.
- T. Tateishi, M. Yoshimura, S. Tokuda, F. Matsuda, D. Fujita and S. Furukawa, Coord. Chem. Rev., 2022, 467, 214612 CrossRef CAS.
- D. Nam, J. Kim, E. Hwang, J. Nam, H. Jeong, T.-H. Kwon and W. Choe, Matter, 2021, 4, 2460–2473 CrossRef CAS.
- A. Khobotov-Bakishev, C. von Baeckmann, B. Ortín-Rubio, L. Hernández-López, A. Cortés-Martínez, J. Martínez-Esaín, F. Gándara, J. Juanhuix, A. E. Platero-Prats, J. Faraudo, A. Carné-Sánchez and D. Maspoch, J. Am. Chem. Soc., 2022, 144, 15745–15753 CrossRef CAS PubMed.
- H. Li, Z.-J. Yao, D. Liu and G.-X. Jin, Coord. Chem. Rev., 2015, 293, 139–157 CrossRef.
- S. Pullen and G. H. Clever, Acc. Chem. Res., 2018, 51, 3052–3064 CrossRef CAS PubMed.
- K. Wu, D. Zhang, C. Drechsler, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2021, 60, 6403–6407 CrossRef CAS PubMed.
- S. Saha, I. Regeni and G. H. Clever, Coord. Chem. Rev., 2018, 374, 1–14 CrossRef CAS.
- S. Pullen, J. Tessarolo and G. H. Clever, Chem. Sci., 2021, 12, 7269–7293 RSC.
- S. C. Bete and M. Otte, Angew. Chem., Int. Ed., 2021, 60, 18582–18586 CrossRef CAS PubMed.
- W. M. Bloch and G. H. Clever, Chem. Commun., 2017, 53, 8506–8516 RSC.
- B. Chen, J. J. Holstein, A. Platzek, L. Schneider, K. Wu and G. H. Clever, Chem. Sci., 2022, 13, 1829–1834 RSC.
- L. S. Lisboa, D. Preston, C. J. McAdam, L. J. Wright, C. G. Hartinger and J. D. Crowley, Angew. Chem., Int. Ed., 2022, 61, e202201700 CrossRef CAS PubMed.
- Y.-Y. Zhang, W.-X. Gao, L. Lin and G.-X. Jin, Coord. Chem. Rev., 2017, 344, 323–344 CrossRef CAS.
- J.-F. Ayme, J. E. Beves, C. J. Campbell and D. A. Leigh, Angew. Chem., Int. Ed., 2014, 53, 7823–7827 CrossRef CAS PubMed.
- J. P. Carpenter, T. K. Ronson, F. J. Rizzuto, T. Héliot, P. Grice and J. R. Nitschke, J. Am. Chem. Soc., 2022, 144, 8467–8473 CrossRef CAS PubMed.
- J. M. Teo, C. J. Coghlan, J. D. Evans, E. Tsivion, M. Head-Gordon, C. J. Sumby and C. J. Doonan, Chem. Commun., 2016, 52, 276–279 RSC.
- M. Eddaoudi, J. Kim, J. B. Wachter, H. K. Chae, M. O’Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2001, 123, 4368–4369 CrossRef CAS PubMed.
- S. Furukawa, N. Horike, M. Kondo, Y. Hijikata, A. Carné-Sánchez, P. Larpent, N. Louvain, S. Diring, H. Sato, R. Matsuda, R. Kawano and S. Kitagawa, Inorg. Chem., 2016, 55, 10843–10846 CrossRef CAS PubMed.
- A. H. Gröschel, A. Walther, T. I. Löbling, J. Schmelz, A. Hanisch, H. Schmalz and A. H. E. Müller, J. Am. Chem. Soc., 2012, 134, 13850–13860 CrossRef PubMed.
- S. Matasunaga, K. Hasada, K. Sugiura, N. Kitamura, Y. Kudo, N. Endo and W. Mori, Bull. Chem. Soc. Jpn., 2012, 85, 433–438 CrossRef.
- D. J. Tranchemontagne, Z. Ni, M. O’Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2008, 47, 5136–5147 CrossRef CAS PubMed.
- M. O’Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef PubMed.
- P. Mosae Selvakumar, S. Nadella, J. Sahoo, E. Suresh and P. S. Subramanian, J. Coord. Chem., 2013, 66, 287–299 CrossRef CAS.
- A. Carné-Sanchez, J. Albalad, T. Grancha, I. Imaz, J. Juanhuix, P. Larpent, S. Furukawa and D. Maspoch, J. Am. Chem. Soc., 2019, 141, 4094–4102 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. CCDC 2190388, 2190389, 2184149, and 2190390. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06815j |
| This journal is © The Royal Society of Chemistry 2023 |