Open Access Article
Open Access ArticleSynthesis of tetrahydrocarbazoles through a radical cation [4+2] cycloaddition reaction of 2-vinylindoles†
Elisa
Brambilla
 a,
Elisa
Moretti
a,
Mirko
Magni
a,
Elisa
Moretti
a,
Mirko
Magni
 b,
Giorgio
Abbiati
b,
Giorgio
Abbiati
 a and
Valentina
Pirovano
a and
Valentina
Pirovano
 *a
*a
aDipartimento di Scienze Farmaceutiche, Sezione di Chimica Generale e Organica “A. Marchesini”, Università degli Studi di Milano, Via G. Venezian 21, 20133, Milano, Italy. E-mail: valentina.pirovano@unimi.it
bDepartment of Enviromental Science and Policy, Università degli Studi di Milano, Via Celoria 2, 20133, Milano, Italy
First published on 17th February 2023
Abstract
A redox umpolung strategy for the synthesis of complex tetrahydrocarbazoles is reported. The reaction involves a visible light promoted radical cation [4+2] cycloaddition between 2-vinylindoles and conjugated alkenes that proceeds with good yields and diastereoselectivity.
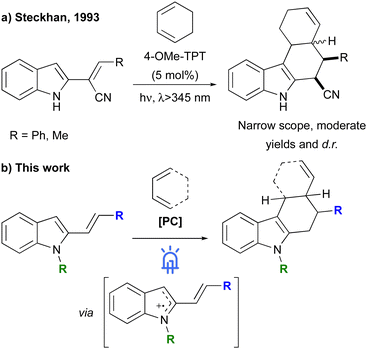
For more than thirty years, 2-vinylindoles have represented an incomparable four-atom building block for the synthesis of polycyclic indole scaffolds, such as carbazoles and tetrahydrocarbazoles, via [4+2] cycloaddition reactions.1 Their easy preparation from commercially available substrates, flexible substitutions on both the indole and vinyl moieties and versatile reactivity in the presence of suitable 2C partners, in fact, have made these substrates an ideal synthon to efficiently build up molecular complexity. Starting from the pioneering works of Pindur, in which tetrahydrocarbazoles were prepared from 2-vinylindoles through thermal Diels–Alder reactions with α,β-unsaturated carbonyl compounds2 many efforts have been devoted to the development of catalytic [4+2] cycloadditions characterised by broad substrate scope, mild reaction conditions and high levels of stereoselectivity. Examples of [4+2] cycloadditions employing electron-poor alkenes as two carbon units were reported, by our and by other research groups, under Lewis acid3 and organo-catalysis.4 Furthermore, we also expanded the applicability of these reactions to other unsaturated systems such as N-allenamides5 and propargylic esters6 under gold catalysis. However, all reported examples of [4+2] cycloadditions of electron-rich 2-vinylindoles require the use of an electron-poor partner. A strategy to overcome this limitation could be represented by the development of a radical [4+2] cycloaddition reaction.7 In this case, in fact, the single electron oxidation of the 2-vinylindole would generate its radical cation providing the required difference in electron density for the cycloaddition with neutral alkenes.8 An example of this reactivity has been reported in 1993 by Steckhan, which studied a radical [4+2] cycloaddition promoted by a triarylpyrylium salt, between 2-vinylindoles bearing a CN group at the α-vinyl position and 1,3-cyclohexadiene (Scheme 1a).8c Despite its innovativeness, the substrate scope of this reaction was very limited and the corresponding tetrahydrocarbazoles were obtained with modest yields and diasteroselectivities. For this reason and, considering our research interests in the synthesis of complex indole derivatives through [4+n] cycloadditions of 2-vinylindoles, we focused our attention on the development of a radical cation [4+2] cycloaddition between ready-available 2-vinylindoles and conjugated alkenes under visible light photoredox catalytic conditions (Scheme 1b).
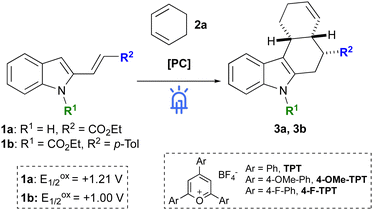
The results that we obtained from the screening of the reaction conditions are summarized in Table 1 (see ESI† for the full screening table). A preliminary electrochemical investigation by cyclic voltammetry was aimed at ranking the selected substrates according to their half-wave potentials of the first oxidation reaction (Eox1/2) to estimate their thermodynamic capability to be oxidized by commonly employed photocatalysts.
| Entry | 1 | [PC] (5 mol%) | Solvent | 3, (%)bc |
|---|---|---|---|---|
a Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol, 2 equiv.), catalyst (5 mol%), in the stated solvent (2 ml, 0.1 M) at rt for 18 h under 40 W blue led irradiation (λmax = 440 nm).
b Isolated yield.
c
d.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
d Degradation of 1a.
e 4 equivalents of 2a were used. (Eox1/2) values vs. SCE in CH3CN. 1.
d Degradation of 1a.
e 4 equivalents of 2a were used. (Eox1/2) values vs. SCE in CH3CN.
|
||||
| 1 | 1a | TPT | CH3CN | —d |
| 2 | 1b | TPT | CH3CN | 32 |
| 3 | 1b | TPT | DCE | 42 |
| 4 | 1b | 4-OMe-TPT | DCE | 39 |
| 5 | 1b | 4-F-TPT | DCE | 26 |
| 6 | 1b | TPT | CH3NO2 | 48 |
| 7 | 1b | TPT | DCE/HFIP (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
48 |
| 8 | 1b | TPT | CH3NO2/HFIP (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
57 |
| 9e | 1b | TPT | CH3NO2/HFIP (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
71 |
Considering previous reports from Steckhan on radical cycloaddition reactions,7e,8a,c,d we verified the activity of pyrylium salts to promote the formation of tetrahydrocarbazoles 3. Employing 5 mol% of triphenylpyrylium tetrafluoroborate (TPT) as a catalyst we observed a complex mixture of unidentified products when 1a was used as a substrate. Conversely, vinylindole 1b afforded tetrahydrocarbazole 3b in a modest 32% yield and with complete diastereoselectivity (d.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (entry 2). Interestingly, despite the modest yield, vinylindole 1b was completely consumed at the end of the reaction and a series of unidentified side-products were observed in the crude mixture together with 3b. Furthermore, dimerization of 1,3-CHD or [2+2] cycloaddition products were never detected. Changing the reaction solvent and employing DCE instead of CH3CN led to a slight increase of the yield (entry 3). Aryl substituted pyrylium salts were then tested in DCE but neither 4-OMe-TPT nor 4-F-TPT led to better results (entries 4–5). An interesting increase of the yield up to 48% was instead observed with CH3NO2 as a solvent (entry 6) or when HFIP was added as a co-solvent9 with DCE (entry 7). The combination of CH3NO2 and HFIP (10
1), (entry 2). Interestingly, despite the modest yield, vinylindole 1b was completely consumed at the end of the reaction and a series of unidentified side-products were observed in the crude mixture together with 3b. Furthermore, dimerization of 1,3-CHD or [2+2] cycloaddition products were never detected. Changing the reaction solvent and employing DCE instead of CH3CN led to a slight increase of the yield (entry 3). Aryl substituted pyrylium salts were then tested in DCE but neither 4-OMe-TPT nor 4-F-TPT led to better results (entries 4–5). An interesting increase of the yield up to 48% was instead observed with CH3NO2 as a solvent (entry 6) or when HFIP was added as a co-solvent9 with DCE (entry 7). The combination of CH3NO2 and HFIP (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
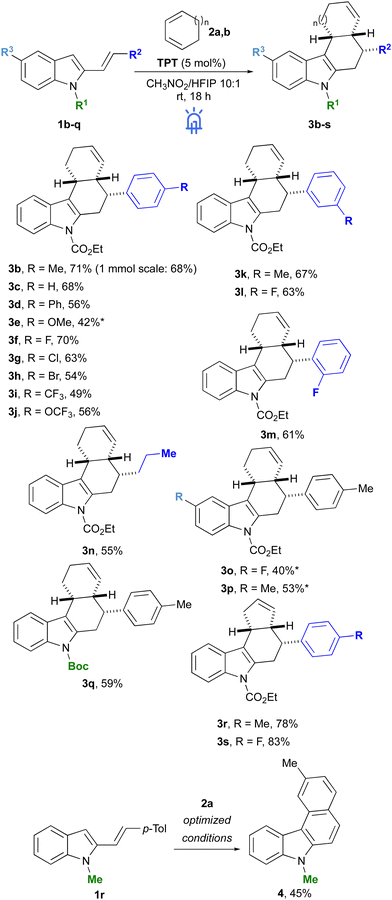
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.1 M) afforded 3b in 57% yield (entry 8), while a satisfying 71% yield was finally obtained increasing the equivalents of 2a up to 4 (entry 9). Having the optimized conditions in hand, we examined the reactivity of vinylindoles 1b-q with 1,3-CHD 2a (Scheme 2). In all cases, products 3b-q were formed as single diastereoisomers (d.r. > 20
1, 0.1 M) afforded 3b in 57% yield (entry 8), while a satisfying 71% yield was finally obtained increasing the equivalents of 2a up to 4 (entry 9). Having the optimized conditions in hand, we examined the reactivity of vinylindoles 1b-q with 1,3-CHD 2a (Scheme 2). In all cases, products 3b-q were formed as single diastereoisomers (d.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Modification of the nature of 2-vinylindole was generally well tolerated and applying the optimised reaction conditions we were able to synthesise a library of tetrahydrocarbazoles 3b-q with good yields. In particular, 4-styryl substituted vinylindoles bearing electron-donating and electron-withdrawing substituents afforded the corresponding products 3d-e and 3f-j with yields ranging from 42 to 70%. Notably, 4-OMe substituted 2-vinylindole 1e gave the worst result despite the prolonged reaction time of 48 h. Substituents in the 3- or 2-position of the aryl group were also tolerated, and the corresponding carbazole derivatives 3k-m were isolated with satisfactory yields. Interestingly, an alkyl-substituted vinylindole 1n could be employed in the radical cycloaddition reaction to afford product 3n in 55% yield. Next, we evaluated the effect of various substituents at the 5-C position of indole scaffolds. In this case the reaction gave the desired products 3o-p, albeit in lower yields and after a prolonged reaction time (48 h). Finally, a Boc protecting group could be used as an alternative to CO2Et to prepare Boc-derivative 3q, while N-methyl substituted vinylindole 1r did not give the expected tetrahydrocarbazole under standard conditions but an intramolecular cyclization product 4 in moderate yield. Variations on 1,3-conjugated diene were more complicated. We tried several cyclic and acyclic substrates, however, only cyclopentadiene (2b) reacted with 2-vinylindoles 1b and 1f affording the corresponding tetrahydrocarbazoles 3r and 3s in 78% and 83% yield, respectively, and as a single diastereoisomer. Conversely, 1,3-cycloheptadiene and 2-(trimethylsiloxy)-1,3-cyclohexadiene gave no reaction under the optimised conditions, while α-terpinene, isoprene and 2,3-dimethyl-1,3-butadiene led to complex mixtures mainly of unreacted 1b and unidentified side-products. Similarly, not all the tested 2-vinylindoles gave successful results: α-substituted and disubstituted vinylindoles as well as a vinylindole having R2 = SMe were not reactive or led to a complex mixture of products (see ESI†).
1). Modification of the nature of 2-vinylindole was generally well tolerated and applying the optimised reaction conditions we were able to synthesise a library of tetrahydrocarbazoles 3b-q with good yields. In particular, 4-styryl substituted vinylindoles bearing electron-donating and electron-withdrawing substituents afforded the corresponding products 3d-e and 3f-j with yields ranging from 42 to 70%. Notably, 4-OMe substituted 2-vinylindole 1e gave the worst result despite the prolonged reaction time of 48 h. Substituents in the 3- or 2-position of the aryl group were also tolerated, and the corresponding carbazole derivatives 3k-m were isolated with satisfactory yields. Interestingly, an alkyl-substituted vinylindole 1n could be employed in the radical cycloaddition reaction to afford product 3n in 55% yield. Next, we evaluated the effect of various substituents at the 5-C position of indole scaffolds. In this case the reaction gave the desired products 3o-p, albeit in lower yields and after a prolonged reaction time (48 h). Finally, a Boc protecting group could be used as an alternative to CO2Et to prepare Boc-derivative 3q, while N-methyl substituted vinylindole 1r did not give the expected tetrahydrocarbazole under standard conditions but an intramolecular cyclization product 4 in moderate yield. Variations on 1,3-conjugated diene were more complicated. We tried several cyclic and acyclic substrates, however, only cyclopentadiene (2b) reacted with 2-vinylindoles 1b and 1f affording the corresponding tetrahydrocarbazoles 3r and 3s in 78% and 83% yield, respectively, and as a single diastereoisomer. Conversely, 1,3-cycloheptadiene and 2-(trimethylsiloxy)-1,3-cyclohexadiene gave no reaction under the optimised conditions, while α-terpinene, isoprene and 2,3-dimethyl-1,3-butadiene led to complex mixtures mainly of unreacted 1b and unidentified side-products. Similarly, not all the tested 2-vinylindoles gave successful results: α-substituted and disubstituted vinylindoles as well as a vinylindole having R2 = SMe were not reactive or led to a complex mixture of products (see ESI†).
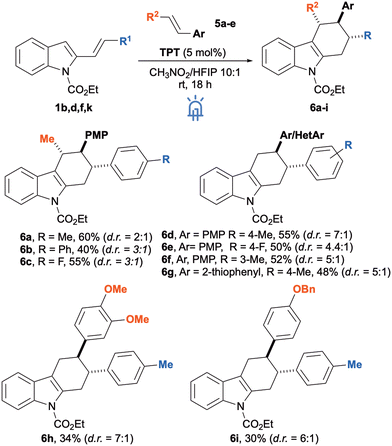
As a continuation, we searched for alternative 2C partners to expand the scope of the cycloaddition reaction with 2-vinylindoles and, among all the possibilities, styrenes were selected because of their wide use in radical cycloadditions (albeit as oxidable species)7i–l,10 (Scheme 3). Vinylindoles 1b, 1d and 1f smoothly reacted under the optimised conditions with 2 equivalents of trans-anethole (5a) affording the corresponding tetrahydrocarbazoles 6a-c with moderate yields and d.r. ranging from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 6a up to 3
1 for 6a up to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 6b and 6c. 4-Methoxystyrene (5b) could also be employed and yielded carbazoles 6d-f when reacted with the corresponding 4- or 3-arylsubstituted vinylindoles 1b, 1f and 1k, while 2-vinylthiophene (5c) gave 6g by its reaction with 1b. Interestingly, in this case, the yields were still moderate but d.r. increased up to 7
1 for 6b and 6c. 4-Methoxystyrene (5b) could also be employed and yielded carbazoles 6d-f when reacted with the corresponding 4- or 3-arylsubstituted vinylindoles 1b, 1f and 1k, while 2-vinylthiophene (5c) gave 6g by its reaction with 1b. Interestingly, in this case, the yields were still moderate but d.r. increased up to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for product 6d. Other styrenes such as 3,4-dimethoxy or benzyloxy derivatives 5d-e were also tested and led to the formation of products 6h and 6i even if in lower yields.
1 for product 6d. Other styrenes such as 3,4-dimethoxy or benzyloxy derivatives 5d-e were also tested and led to the formation of products 6h and 6i even if in lower yields.
Considering the observed reactivity and previous mechanistic investigations on radical cation cycloaddition reactions,11 including transformations on indole derivatives,8,12 the mechanism that we propose for the synthesis of tetrahydrocarbazoles 3 is reported in Scheme 4. Single electron oxidation, promoted by the excited state of the triphenylpyrylium tetrafluoroborate (TPT+*; E1/2 = TPT+*/TPT˙ = 2.28 V vs. SCE)13 leads to the formation of indoxyl radical cation I (1b, Eox1/2 = 1.00 V vs. SCE). Radical attack of the C3 position of indole on 1,3-CHD (2a, Eox1/2 = 1.54 V vs. SCE)14 gives radical intermediate II, which cyclises on exocyclic double bond to afford III. Aromatisation of the indole core to IV, followed by single electron reduction finally leads to product 3b. Considering this last step, reduction of IV to 3b might be promoted by the photocatalyst (TPT˙) with concurrent regeneration of the active species (TPT+), or, as for analogous transformations,15 might likely proceed through a radical chain propagation mechanism that involves the oxidation of a second equivalent of 1b to I. We believe that a similar mechanism could also be invoked for the reaction of 2-vinylindoles with styrenes. A comparison between the oxidation potentials of 1b (1.00 V vs. SCE) and trans-anethole or 4-methoxy styrene (1.24 V and 1.29 V vs. SCE, respectively)14,16 suggests the preferential oxidation of 1b to I rather than the formation of a styryl radical cation from 5. Finally, a difference in the reactivity of 1a and 1b could be explained by cyclic voltammetry studies. Despite being both oxidisable by the catalyst (1a, Eox1/2 = 1.21 V vs. SCE), CV revealed different voltammetric patterns that thus imply a different reactivity (see ESI† for detailed description).
In conclusion we have designed a radical cation [4+2] cycloaddition of 2-vinylindoles promoted by an organic photoredox catalyst. The single electron oxidation of these substrates to the corresponding indoxyl radical cations allows for their reaction with otherwise unreactive conjugated alkenes. Therefore, through a redox umpolung strategy we were able to efficiently synthesise a new series of tetrahydrocarbazoles.
Prof. Elisabetta Rossi is acknowledged for helpful suggestions and revision of the manuscript. Donatella Nava is acknowledged for NMR analyses. Mass spectrometry analyses were performed at the Mass Spectrometry facility of the Unitech COSPECT at the University of Milan (Italy).
Conflicts of interest
There are no conflicts to declare.Notes and references
- For selected reviews see: (a) U. Pindur, Adv. Nitrogen Heterocycl., 1995, 1, 121 CrossRef CAS; (b) R. F. Kester, S. J. Berthel and F. Firooznia, Top. Heterocycl. Chem., 2010, 26, 327 CrossRef CAS; (c) E. Rossi, G. Abbiati and V. Pirovano, Eur. J. Org. Chem., 2017, 4512 CrossRef CAS; (d) Y. A. A. M. Elshaier, M. T. M. Nemr, M. S. Refaey, W. A. A. Fadalyd and A. Barakat, New J. Chem., 2022, 46, 13383 RSC.
- M. Eitel and U. Pindur, J. Org. Chem., 1990, 55, 5368 CrossRef CAS.
- (a) G. Abbiati, V. Canevari, D. Facoetti and E. Rossi, Eur. J. Org. Chem., 2007, 517 CrossRef CAS; (b) V. Pirovano, G. Abbiati, M. Dell’Acqua, D. Facoetti, M. Giordano and E. Rossi, Synlett, 2012, 2913 CAS; (c) F. Tan, H.-G. Cheng, W. Wu, K.-R. Ding and W.-J. Xiao, Chem. – Asian J., 2012, 7, 493 CrossRef CAS PubMed; (d) V. Pirovano, M. Dell’Acqua, D. Facoetti, S. Rizzato, G. Abbiati and E. Rossi, Eur. J. Org. Chem., 2013, 6267 CrossRef CAS; (e) B. Ouyang, T. Yu, R. Luo and G. Lu, Org. Biomol. Chem., 2014, 12, 4172 RSC; (f) E. Rossi, V. Pirovano, M. Negrato, G. Abbiati and M. Dell’Acqua, Beilstein J. Org. Chem., 2015, 11, 1997 CrossRef CAS PubMed.
- (a) X.-F. Wang, J.-R. Chen, Y.-J. Cao, H.-G. Cheng and W.-J. Xiao, Org. Lett., 2010, 12, 1140 CrossRef CAS PubMed; (b) C. Gioia, L. Bernardi and A. Ricci, Synthesis, 2010, 161 CAS; (c) C. Zheng, Y. Lu, J. Zhang, X. Chen, Z. Chai, W. Ma and G. Zhao, Chem. – Eur. J., 2010, 16, 5853 CrossRef CAS PubMed; (d) F. Tan, F. Li, X.-X. Zhang, X.-F. Wang, H.-G. Cheng, J.-R. Chen and W.-J. Xiao, Tetrahedron, 2011, 67, 446 CrossRef CAS; (e) Y.-J. Cao, H.-G. Cheng, L.-Q. Lu, J.-J. Zhang, Y. Cheng, J.-R. Chen and W.-J. Xiao, Adv. Synth. Catal., 2011, 353, 617 CrossRef CAS; (f) X. Tian, N. Hofmann and P. Melchiorre, Angew. Chem., Int. Ed., 2014, 53, 2997 CrossRef CAS PubMed; (g) Y. Wang, M.-S. Tu, L. Yin, M. Sun and F. Shi, J. Org. Chem., 2015, 80, 3223 CrossRef CAS PubMed; (h) J.-W. Ren, Z.-F. Zhou, J.-A. Xiao, X.-Q. Chen and H. Yang, Eur. J. Org. Chem., 2016, 1264 CrossRef CAS.
- (a) V. Pirovano, L. Decataldo, E. Rossi and R. Vicente, Chem. Commun., 2013, 49, 3594 RSC; (b) V. Pirovano, M. Borri, G. Abbiati, S. Rizzato and E. Rossi, Adv. Synth. Catal., 2017, 359, 1912 CrossRef CAS.
- V. Pirovano, E. Arpini, M. Dell'Acqua, R. Vicente, G. Abbiati and E. Rossi, Adv. Synth. Catal., 2016, 358, 403 CrossRef CAS.
- (a) D. J. Bellville, D. W. Wirth and N. L. Bauld, J. Am. Chem. Soc., 1981, 103, 718 CrossRef CAS; (b) N. L. Bauld, D. J. Bellville, R. Pabon, R. Chelsky and G. Green, J. Am. Chem. Soc., 1983, 105, 2378 CrossRef CAS; (c) C. R. Jones, B. J. Allman, A. Mooring and B. Spahic, J. Am. Chem. Soc., 1983, 105, 652 CrossRef CAS; (d) R. A. Pabon, D. J. Bellville and N. L. Bauld, J. Am. Chem. Soc., 1983, 105, 5158 CrossRef CAS; (e) J. Mlcoch and E. Steckhan, Angew. Chem., Int. Ed. Engl., 1985, 24, 412 CrossRef; (f) N. L. Bauld, D. J. Bellville, B. Harirchian, K. T. Lorenz, R. A. Pabon Jr., D. W. Reynolds, D. D. Wirth, H.-S. Chiou and B. K. Marsh, Acc. Chem. Res., 1987, 20, 371 CrossRef CAS; (g) N. Akbulut, D. Hartsough, J.-I. Kim and G. B. Schuster, J. Org. Chem., 1989, 54, 2549 CrossRef CAS; (h) C. S. Sevov and O. Wiest, J. Org. Chem., 2008, 73, 7909 CrossRef CAS PubMed; (i) S. Lin, M. A. Ischay, C. G. Fry and T. P. Yoon, J. Am. Chem. Soc., 2011, 133, 19350 CrossRef CAS PubMed; (j) S. M. Stevenson, M. P. Shores and E. M. Ferreira, Angew. Chem., Int. Ed., 2015, 54, 6506 CrossRef CAS PubMed; (k) K. Nakayama, N. Maeta, G. Horiguchi, H. Kamiya and Y. Okada, Org. Lett., 2019, 21, 2246 CrossRef CAS PubMed; (l) K. Tanaka, M. Kishimoto, Y. Tanaka, Y. Kamiyama, Y. Asada, M. Sukekawa, N. Ohtsuka, T. Suzuki, N. Momiyama, K. Honda and Y. Hoshino, J. Org. Chem., 2022, 87, 3319 CrossRef CAS PubMed.
- For radical cation Diels-Alder reactions with indoles see (a) A. Gieseler, E. Steckhan, O. Wiest and F. Enoch, J. Org. Chem., 1991, 56, 1405 CrossRef CAS; (b) O. Wiest, E. Steckhan and F. Grein, J. Org. Chem., 1992, 57, 4034 CrossRef CAS; (c) O. Wiest and E. Steckhan, Angew. Chem., Int. Ed. Engl., 1993, 32, 901 CrossRef; (d) C. F. Gürtler, E. Steckhan and S. Blechert, J. Org. Chem., 1996, 61, 4136 CrossRef PubMed; (e) C. F. Gurtler, S. Blechert and E. Steckhan, Chem. – Eur. J., 1997, 3, 447 CrossRef CAS; (f) U. Haberl, E. Steckhan, S. Blechert and O. Wiest, Chem. – Eur. J., 1999, 5, 2859 CrossRef CAS; (g) S. P. Pitre, J. C. Scaiano and T. P. Yoon, ACS Catal., 2017, 7, 6440 CrossRef CAS PubMed.
- (a) L. Eberson, M. P. Hartshorn, O. Persson and F. Radner, Chem. Commun., 1996, 2105 RSC; (b) N. Shida, Y. Imada, S. Nagahara, Y. Okada and K. Chiba, Commun. Chem., 2019, 2, 24 CrossRef; (c) I. Colomer, A. E. R. Chamberlain, M. B. Haughey and T. J. Donohoe, Nat. Rev. Chem., 2017, 1, 0088 CrossRef CAS.
- For selected examples see (a) D. Wei, Y. Li and F. Liang, Adv. Synth. Catal., 2016, 358, 3887 CrossRef CAS; (b) K. Wang, L.-G. Meng, Q. Zhanga and L. Wang, Green Chem., 2016, 18, 2864 RSC; (c) L. Wang, F. Wu, J. Chen, D. A. Nicewicz and Y. Huang, Angew. Chem., Int. Ed., 2017, 56, 6896 CrossRef CAS PubMed.
- (a) M. Martiny, E. Steckhan and T. Esch, Chem. Ber., 1993, 126, 1671 CrossRef CAS; (b) W. Yueh and N. L. Bauld, J. Phys. Org. Chem., 1996, 9, 529 CrossRef CAS; (c) N. P. Schepp and L. J. Johnston, J. Am. Chem. Soc., 1996, 118, 2872 CrossRef CAS; (d) R. Pérez-Ruiz, L. R. Domingo, M. C. Jiménez and M. A. Miranda, Org. Lett., 2011, 13, 5116 CrossRef PubMed.
- U. Haberl, E. Steckhan, S. Blechert and O. Wiest, Chem. – Eur. J., 1999, 5, 2859 CrossRef CAS.
- N. J. Gesmundo and D. A. Nicewicz, Beilstein J. Org. Chem., 2014, 10, 1272 CrossRef PubMed.
- H. G. Roth, N. A. Romero and D. A. Nicewicz, Synlett, 2016, 714 CAS.
- M. A. Cismesia and T. P. Yoon, Chem. Sci., 2015, 6, 5426 RSC.
- Y. J. Jang, H. An, S. Choi, J. Hong, S. H. Lee, K.-H. Ahn, Y. You and E. J. Kang, Org. Lett., 2022, 24, 4479 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06723d |
| This journal is © The Royal Society of Chemistry 2023 |