Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Beryllium-centred C–H activation of benzene†
Kyle G.
Pearce
 ,
Michael S.
Hill
,
Michael S.
Hill
 * and
Mary F.
Mahon
* and
Mary F.
Mahon
Department of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: msh27@bath.ac.uk
First published on 6th January 2023
Abstract
Reaction of BeCl2 with the dilithium diamide, [{SiNDipp}Li2] ({SiNDipp} = {CH2SiMe2NDipp}2), provides the dimeric chloroberyllate, [{SiNDippBeCl}Li]2, en route to the 2-coordinate beryllium amide, [SiNDippBe]. Lithium or sodium reduction of [SiNDippBe] in benzene, provides the relevant organoberyllate products, [{SiNDippBePh}M] (M = Li or Na), via the presumed intermediacy of transient Be(I) radicals.
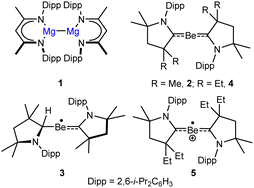
Despite a reputation as the most toxic non-radioactive element, the coordination, bioinorganic and organometallic chemistry of beryllium has begun to attract more concerted attention.1–3 More specifically, a flurry of reports have invigorated interest in the lower oxidation state chemistry of the lightest alkaline earth element.4–7 Although the theoretical viability of Be(I)–Be(I)-bonded species analogous to Jones and co-workers’ landmark magnesium(I) derivatives (e.g.1)8,9 is yet to be realised, exploitation of neutral cyclic alkyl(amino)carbene (CAAC) ligands has led to the isolation of several compounds in which a formal oxidation state <+2 may be assigned to the Be atom. The first examples of charge neutral Be(0) (2) and Be(I) (3) compound types were provided by Braunschweig and co-workers through the respective potassium and lithium reduction of CAAC adducts of BeCl2 and organoberyllium chloride precursors.10,11 Although the stability of these compounds has been attributed to strong three-centre-two-electron back-bonding between the Be atom and the carbene ligands, recent calculations have argued that the π-acidic properties of CAACs lend an assignment of a more conventional Be2+ oxidation state.12 Gilliard's exploitation of the Be(0) species 4, however, as a reducing agent and to access the radical cation, 5, highlight the latent reactivity of such species.13,14
Related observations by the Gilliard group have implied that the alkali metal reduction of carbene-ligated halides also proceeds through the transient formation of Be(I) radicals.15 Similarly, Jones and co-workers had earlier inferred that a short-lived blue colour produced during Li reduction of [(MesBDI)BeI] (MesBDI = HC{(Me)CN-C6H2Me3-2,4,6}2) was indicative of transient beryllium radical formation.16 In contrast, our own study of the potassium reduction of [(BDI)BeCl] (BDI = HC{(Me)CNDipp}2; Dipp = 2,6-di-isopropylphenyl) provided no evidence for Be-centred reduction. Rather, a mixture of compounds arising from H-atom transfer between two BDI ligands was suggested to indicate a ligand-based reduction step.17
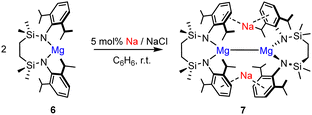 | (1) |
We have recently reported that the Mg(I) species, [{SiNDipp}MgNa]2 (7; [{SiNDipp} = {CH2SiMe2N(Dipp)}2] may be prepared by sodium reduction of the magnesium(II) precursor 6 (eqn (1)).18 This approach sought to circumvent conventional salt elimination as a driving force for the reduction and to make deliberate use of the redox-innocence of the {SiNDipp} dianion and the robust Na-aryl interactions of 7. In this contribution we report our initial observations arising from attempts to apply a similar protocol to the isolation of analogous low oxidation state beryllium species.
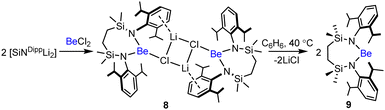
A reaction of the dilithium diamide, [{SiNDipp}Li2], with BeCl2 in hexane provided, after work-up, compound 8 as a colourless solid (Scheme 1). A C6D6 solution of compound 8 displayed 1H and 13C NMR spectra diagnostic of a C2-symmetric structure. The incorporation of lithium in compound 8 was apparent from the corresponding 7Li NMR spectrum (δLi −6.6 ppm) and a broad (ω1/2 = 314 Hz) 9Be NMR signal was observed at δBe 8.2 ppm. While tetrahedrally coordinated Be centres have been shown to display chemical shifts in the range δBe = −2 to 8 ppm, this resonance lies to the far low field extreme and the breadth of the signal is more redolent of a lower symmetry environment.19 Consistent with these solution observations, single crystal X-ray diffraction analysis identified compound 8 as a centrosymmetric lithium diamidochloroberyllate, in which the Be1/Be11 centres occupy distorted trigonal environments provided by the chelated {SiNDipp} ligands and Cl1/Cl11 (Fig. S42, ESI†). Dimer propagation is achieved by μ2-Cl-Li-Cl bridging, while each lithium coordination sphere is augmented by η6-interactions with a single N-Dipp substituent of the {SiNDipp} ligands.
The 1H and 13C NMR spectra of 8 did not allow for any discrimination between the N-Dipp environments, however, and these solid-state interactions are evidently not retained in solution at room temperature. Further investigation of the solution dynamics of compound 8 was hampered by its apparent conversion on extended storage in C6D6 to a new species (9). This transformation could be accelerated to completion within 16 hours by heating at temperatures ≥40 °C, a process accompanied by the emergence of a broad (ω1/2 = 266 Hz) resonance in the 9Be NMR spectrum at even lower field (δBe 11.6 ppm) than that arising from 8.
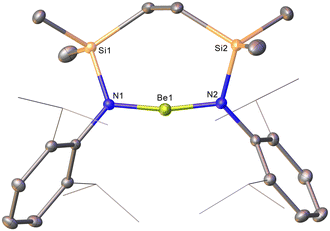
The origin of these observations was resolved by the isolation of colourless single crystals of 9 from benzene suitable for X-ray diffraction analysis (Fig. 1). The solid-state structure of 9 confirmed the elimination of LiCl from compound 8 and the production of the initially targeted two-coordinate beryllium analogue of compound 6. Structurally characterised examples of two-coordinate amidoberyllium monomers are limited to [Be{N(SiMe3)2}2] and 2,6-Mes2-C6H3BeN(SiMe3)2.20,21 The more directly comparable homoleptic diamide displayed effectively identical Be–N bond lengths [1.525(2), 1.519(2) Å] to those of compound 9 [N1–Be1 1.515(2), N2–Be1 1.519(2) Å]. In contrast to the almost linear coordination geometry at Be in [Be{N(SiMe3)2}2] [N1–Be1–N2 178.73(16)°], however, the amide donors of 9 are perturbed to subtend a more acute angle of 168.49(15)°. While we attribute this contrast to the greater constraints placed on the local geometry at beryllium by the seven-membered chelate structure of 9, it is notable that this angle is still significantly more obtuse than the N–Mg–N angles observed in either of the previously reported structures of the analogous magnesium derivative 6.18 In these cases, the coordination spheres of the Mg atoms were augmented by dihapto interactions with a molecule of either benzene [N–Mg–N 132.52(10)°] or toluene [133.92(11)°] solvent, which are, in turn, facilitated by the longer Mg–N bonds [ca. 1.95 Å]. On this basis, therefore, the {SiNDipp} ligand of 9 may be assessed to provide significantly greater steric encumbrance of the beryllium atom of 9 than the magnesium centre of its heavier group 2 congener.
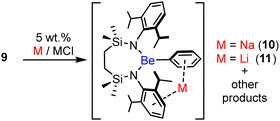
A likely consequence of this enhanced kinetic protection was made apparent by initial attempts to effect the sodium reduction of 9 under the reaction conditions applied to the synthesis of compound 7 (eqn (1)). Although the formation of 7 was facile and proceeded smoothly at room temperature, no discernible changes could be observed to the resultant 1H NMR spectrum when a C6D6 solution of 9 was reacted with an excess of 5 wt% Na/NaCl under similar conditions.22 Further assessment after heating at 100 °C, however, evidenced the generation of multiple new species, albeit the presence of two predominant compounds was characterised by the emergence of new multiplet isopropyl methine signals at δH 3.90 and 4.40 ppm. Although the chemical shift (δBe = 11.8 ppm) of the corresponding 9Be NMR spectrum was only marginally different to that of 8, the additional broadening incurred (ω1/2 = 327 Hz) was consistent with a further adjustment to the group 2 element symmetry. Filtration of the resultant solution after two days under these conditions and slow evaporation of the reaction solvent provided a mixture of materials comprising a small but significant quantity of colourless single crystals of compound 10-d that were suitable for X-ray diffraction analysis (Fig. 2).
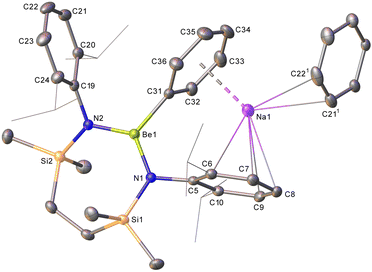
Compound 10-d is a sodium diamidophenylberyllate resulting from the apparent activation of a C–D bond of C6D6 (Scheme 2). Like those of 8, the Be atoms occupy distorted trigonal coordination environments while the sodium cations are encapsulated by the C5-containing Dipp group of the Be-chelating {SiNDipp} dianion and the phenyl substituent. Despite these interactions, the Be–Cipso distance [C31–Be1 1.779(4) Å] is only marginally elongated in comparison to those observed [ca. 1.75 Å] in other 3-coordinate and terminally-bonded phenylberyllium derivatives.23–26 The respective Na-to-centroid distances [2.566, 2.449 Å] are also typical of such polyhapto Na–arene interactions,27 while further η2 contacts [Na1–C211 2.783(4), Na1–C221 2.825(4) Å] to an adjacent N-Dipp substituent dictate that 10 describes a 1-dimensional polymer propagated along the crystallographic a-axis.
The C6D6 solvent was confirmed as the source of the phenyl substituent of compound 10-d by performance of an analogous reaction in C6H6. Although it was again not possible to isolate a pure bulk sample of 10-h, its generation was apparent in the mixture of products from two mutually coupled multiplet resonances in the resultant 1H NMR spectrum at δ 6.35 and 6.20 ppm. These signals, absent from the reaction performed in perdeutero solvent, presented a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by relative integration and are, thus, assigned as the respective m- and p-C6H5 environments of the phenylberyllate unit. This supposition was confirmed by X-ray analysis of a single crystal of 10-h, which provided identical unit cell parameters to those of 10-d.
1 ratio by relative integration and are, thus, assigned as the respective m- and p-C6H5 environments of the phenylberyllate unit. This supposition was confirmed by X-ray analysis of a single crystal of 10-h, which provided identical unit cell parameters to those of 10-d.
Extension of this reduction chemistry to a reaction performed between 9 and 5 wt% Li/LiCl in C6D6 heated at 100 °C for 5 days also yielded a mixture of products. The resultant 7Li NMR spectrum presented a multiplicity of signals indicative of at least four well discriminated environments, and across a range of chemical shifts between δLica. −0.4 and −11.9 ppm. Crystallisation by slow evaporation of the reaction mixture and mechanical separation of a single crystal enabled the identification of compound 11 (Fig. S43, ESI†).
Compound 11 is a further diamidophenylberyllate derivative resulting from activation of the benzene solvent (Scheme 2). There are two crystallographically independent molecules in the asymmetric unit, which are effectively identical. The smaller lithium cation evidently prevents the formation of the additional intermolecular interactions that are a feature of the structure of compound 10. The bond lengths and angles about beryllium are otherwise only marginally perturbed across both the lithium and sodium compounds, obviating further necessary comment.
The low specificity of the reactions that provide compounds 10 and 11 precludes any definitive interpretation. Although other cooperative mechanisms derived either from transient Be(0) formation or Lewis acid-assisted benzene reduction are plausible,28 we have observed no corroborative evidence for such reactivity (see, ESI† for a summary of the control experiments performed). The precedent provided by the comparable magnesium chemistry shown in Scheme 1, therefore, leads us to suggest that the formation of 10 and 11 is a common consequence of the generation of transient beryllium(I) radicals. The formation of diamagnetic Mg–Mg bonded compounds exemplified by 1 and 7 is presumed to occur via the dimerisation of transient Mg(I) radical intermediates. Harder and co-workers have shown that a radical species may be effectively trapped by alkali metal reduction of an amidinato magnesium iodide in the presence of CAAC under solid-state (ball milling) conditions.29 Similar reduction of highly sterically encumbered β-diketiminato magnesium iodides, but performed in benzene and in the presence of N,N,N′N′-tetramethylethylenediamine, has yielded binuclear complexes with bridging antiaromatic [C6H6]2− dianions. These latter species themselves decompose when heated to provide a mixture of phenyl- and hydridomagnesium derivatives.30 Jones, Maron and co-workers have also reported that Mg(I) radical formation is induced by photoactivation of the Mg–Mg bonds of compound 1 and related variants.31 Whereas initial [C6H6]2− dianion formation was again observed in benzene solution, UV (370 nm) or blue (456 nm) light-induced photolysis in either toluene or xylene promoted regio- and chemo-selective CH bond activation, and generation of the relevant β-diketiminato magnesium-aryl and magnesium hydride derivatives. Computational studies suggested that benzene reduction is mediated via marginally preferential complexation to an initially generated triplet Mg⋯Mg ‘σ-bonded’ complex. In contrast, C–H activation of the bulkier arene substrates necessitates dissociation into two doublet (BDI)Mg(I) radical intermediates, albeit again via an initial arene dearomatisation step.
Our previous report of compound 7 has highlighted its significantly elongated Mg–Mg bond (>3.2 Å) resulting from a combination of the bulky {SiNDipp} ligands and the separation imposed by the bridging sodium cations necessary to maintain overall charge balance.18 In the current case, therefore, we suggest that the enhanced steric protection provided to beryllium by the {SiNDipp} ligand in 9 is sufficient to prevent Be–Be bond formation and enable a cascade of Be(I) radical reactivity redolent of the Mg(I) photochemistry deduced by Jones and Maron.
The identification of compounds 10 and 11 provides circumstantial evidence for this latter supposition. Completely analogous behaviour, however, also necessitates hydridoberyllate formation. Although the presence of such species remains to be authenticated, we observe that molecular beryllium hydrides are highly reactive in their own right and have been demonstrated as prone to a variety of inter- and intramolecular C–X bond activation processes.32–34 In mitigation of our hypothesis, therefore, and taking into account the necessarily harsh thermal conditions necessary for the generation of 10 and 11, we suggest that the multiplicity of additional species observed alongside both phenylberyllium derivatives is a likely reflection of similar onward Be-H-derived reactivity. We are continuing to explore these possibilities and their extension to alternative group 2 elements and bond activation processes, particularly through variation of the steric demands and structure of the supporting diamide ligand.35
KGP performed the synthesis and characterisation of the new compounds reported. MSH conceptualised the study and finalised the manuscript for submission, while MFM finalised the X-ray diffraction analyses for publication.
We acknowledge financial support from the EPSRC (research grant EP/R020752/1).
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. Naglav, M. R. Buchner, G. Bendt, F. Kraus and S. Schulz, Angew. Chem., Int. Ed., 2016, 55, 10562–10576 CrossRef CAS PubMed.
- M. R. Buchner, Chem. – Eur. J., 2019, 25, 12018–12036 CrossRef CAS PubMed.
- M. R. Buchner, Chem. Commun., 2020, 56, 8895–8907 RSC.
- C. Jones, Commun. Chem., 2020, 3, 159 CrossRef CAS.
- B. Rösch and S. Harder, Chem. Commun., 2021, 57, 9354–9365 RSC.
- L. A. Freeman, J. E. Walley and R. J. Gilliard, Nat. Synth., 2022, 1, 439–448 CrossRef.
- J. E. Walley and R. J. Gilliard Jr, Recent Adventures in Beryllium Chemistry, in Encyclopedia of Inorganic and Bioinorganic Chemistry, 2021, pp. 1–1410, DOI:10.1002/9781119951438.eibc2788.
- S. P. Green, C. Jones and A. Stasch, Science, 2007, 318, 1754–1757 CrossRef CAS PubMed.
- S. A. Couchman, N. Holzmann, G. Frenking, D. J. D. Wilson and J. L. Dutton, Dalton Trans., 2013, 42, 11375–11384 RSC.
- M. Arrowsmith, H. Braunschweig, M. A. Celik, T. Dellermann, R. D. Dewhurst, W. C. Ewing, K. Hammond, T. Kramer, I. Krummenacher, J. Mies, K. Radacki and J. K. Schuster, Nat. Chem., 2016, 8, 890–894 CrossRef CAS PubMed.
- C. Czernetzki, M. Arrowsmith, F. Fantuzzi, A. Gartner, T. Troster, I. Krummenacher, F. Schorr and H. Braunschweig, Angew. Chem., Int. Ed., 2021, 60, 20776–20780 CrossRef CAS PubMed.
- M. Gimferrer, S. Danes, E. Vos, C. B. Yildiz, I. Corral, A. Jana, P. Salvador and D. M. Andrada, Chem. Sci., 2022, 13, 6583–6591 RSC.
- G. C. Wang, L. A. Freeman, D. A. Dickie, R. Mokrai, Z. Benko and R. J. Gilliard, Chem. – Eur. J., 2019, 25, 4335–4339 CrossRef CAS PubMed.
- G. C. Wang, J. E. Walley, D. A. Dickie, S. Pan, G. Frenking and R. J. Gilliard, J. Am. Chem. Soc., 2020, 142, 4560–4564 CrossRef CAS PubMed.
- L. A. Freeman, J. E. Walley, A. D. Obi, G. C. Wang, D. A. Dickie, A. Molino, D. J. D. Wilson and R. J. Gilliard, Inorg. Chem., 2019, 58, 10554–10568 CrossRef CAS PubMed.
- S. J. Bonyhady, C. Jones, S. Nembenna, A. Stasch, A. J. Edwards and G. J. McIntyre, Chem. – Eur. J., 2010, 16, 938–955 CrossRef CAS PubMed.
- M. Arrowsmith, M. S. Hill, G. Kociok-Köhn, D. J. MacDougall, M. F. Mahon and I. Mallov, Inorg. Chem., 2012, 51, 13408–13418 CrossRef CAS PubMed.
- H. Y. Liu, R. J. Schwamm, S. E. Neale, M. S. Hill, C. L. McMullin and M. F. Mahon, J. Am. Chem. Soc., 2021, 143, 17851–17856 CrossRef CAS PubMed.
- J. K. Buchanan and P. G. Plieger, Z. Naturforsch., B: J. Chem. Sci., 2020, 75, 459–472 CrossRef CAS.
- D. Naglav, A. Neumann, D. Blaser, C. Wolper, R. Haack, G. Jansen and S. Schulz, Chem. Commun., 2015, 51, 3889–3891 RSC.
- M. Niemeyer and P. P. Power, Inorg. Chem., 1997, 36, 4688–4696 CrossRef CAS PubMed.
- J. Hicks, M. Juckel, A. Paparo, D. Dange and C. Jones, Organometallics, 2018, 37, 4810–4813 CrossRef CAS.
- J. Gottfriedsen and S. Blaurock, Organometallics, 2006, 25, 3784–3786 CrossRef CAS.
- M. Müller and M. R. Buchner, Chem. – Eur. J., 2020, 26, 9915–9922 CrossRef PubMed.
- L. R. Thomas-Hargreaves, M. Muller, N. Spang, S. I. Ivlev and M. R. Buchner, Organometallics, 2021, 40, 3797–3807 CrossRef CAS.
- L. R. Thomas-Hargreaves, C. Berthold, W. Augustinov, M. Muller, S. I. Ivlev and M. R. Buchner, Chem. – Eur. J., 2022, 28, e202200851 CAS.
- J. D. Smith, Adv. Organometal. Chem., 1999, 43, 267–348 CrossRef.
- For example mixtures of Be(0) and alkali metal have been shown to reduce ammonia; M. Müller and M. R. Buchner, Z. Naturforsch., B: J. Chem. Sci., 2020, 75, 483–489 CrossRef.
- D. Jedrzkiewicz, J. Mai, J. Langer, Z. Mathe, N. Patel, S. DeBeer and S. Harder, Angew. Chem., Int. Ed., 2022, 61, e202200511 CrossRef CAS PubMed.
- T. X. Gentner, B. Rosch, G. Ballmann, J. Langer, H. Elsen and S. Harder, Angew. Chem., Int. Ed., 2019, 58, 607–611 CrossRef CAS PubMed.
- D. D. L. Jones, I. Douair, L. Maron and C. Jones, Angew. Chem., Int. Ed., 2021, 60, 7087–7092 CrossRef CAS PubMed.
- M. Arrowsmith, M. S. Hill, G. Kociok-Köhn, D. J. MacDougall and M. F. Mahon, Angew. Chem., Int. Ed., 2012, 51, 2098–2100 CrossRef CAS PubMed.
- M. Arrowsmith, M. S. Hill and G. Kociok-Köhn, Organometallics, 2015, 34, 653–662 CrossRef CAS.
- K. J. Iversen, D. J. D. Wilson and J. L. Dutton, Dalton Trans., 2014, 43, 12820–12823 RSC.
- Note added in proof: Hicks and co-workers have very recently reported that similar reduction of magnesium and calcium diamides with KC8 in toluene gives rise to the relevant potassium hydrido-magnesiate and -calciate derivatives. In these cases, the origin of the hydride ligands was traced primarily to the arene solvent and the reaction was similarly attributed to the likely formation of short lived magnesium and calcium radical intermediates. See, J. S. McMullen, R. Huo, P. Vasko, A. J. Edwards and J. Hicks, Angew. Chem. Int. Ed., 2023, 62, e202215218 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General synthetic experimental details, NMR spectra, X-ray analysis of compounds 8–11. CCDC 2206119–2206122. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06702a |
| This journal is © The Royal Society of Chemistry 2023 |