 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent developments in visible light induced polymerization towards its application to nanopores
Claire
Förster
and
Annette
Andrieu-Brunsen
 *
*
Macromolecular Chemistry – Smart Membranes, Technische Universität Darmstadt, 64287, Darmstadt, Germany. E-mail: annette.andrieu-brunsen@.tu-darmstadt.de
First published on 19th January 2023
Abstract
Visible light induced polymerizations are a strongly emerging field in recent years. Besides the often mild reaction conditions, visible light offers advantages of spatial and temporal control over chain growth, which makes visible light ideal for functionalization of surfaces and more specifically of nanoscale pores. Current challenges in nanopore functionalization include, in particular, local and highly controlled polymer functionalizations. Using spatially limited light sources such as lasers or near field modes for light-induced polymer functionalization is envisioned to allow local functionalization of nanopores and thereby improve nanoporous material performance. These light sources are usually providing visible light while classical photopolymerizations are mostly based on UV-irradiation. In this review, we highlight developments in visible light induced polymerizations and especially in visible light induced controlled polymerizations as well as their potential for nanopore functionalization. Existing examples of visible light induced polymerizations in nanopores are emphasized.
Introduction
Polymer functionalization of nanoscale pores advanced in the last years. This advance has been driven by the resulting material properties but as well by new possibilities in the field of polymerization itself, as e.g. the increasing number of reported visible light induced polymerizations.1–3 Polymer functionalized nanopores, for example, allow for specific control of molecular transport which is relevant in separation, energy conversion, sensing, or release.2,4–10 Especially, gating of molecular transport using stimuli-responsive polymers in nanopores has been demonstrated for all possible stimuli from pH, ion interaction to redox or even magnetic gating.7,11 However, for nanopore transport beyond gating, controlled- and local (polymer-) functionalization is one essential tool aiming to control polymer amount, chain composition, and local gradient formation for various monomers. Such asymmetric design of porous materials has been shown to result in Janus materials for side selective separation or increased transport performance.3,12 For example, theoretical studies from Huang and Szleifer demonstrated the relevance of controlled and local nanopore functionalization, as well as polymer sequence design of nanopores allowing transport direction.13,14An experimental implementation of local polymer functionalization including polymer sequence design has to be based on controlled (living) polymerizations. The general concept of controlled (living) polymerizations was first realized for anionic polymerizations in 1956.15 A more robust way to control polymer functionalization tolerating a large variety of different monomers can be realized by controlled radical polymerizations. Often-used controlled radical polymerizations are atom transfer radical polymerization (ATRP),16,17 reversible addition fragmentation chain transfer polymerization (RAFT),18 nitroxide-mediated radical polymerization19,20 (NMP) and iniferter initiated polymerization.21–23 In recent years these polymerizations were increasingly often initiated using visible light irradiation including spatial and temporal control over chain growth for example at planar surfaces. Furthermore, these polymerizations allow for mild reaction conditions, since the use of light often eliminates the need for high temperatures.24
Although, most polymerization initiators absorb UV-light, visible light, here defined for wavelength between 380–750 nm, is an interesting trigger. Among others, it is cheap, abundant in nature, and lasers usually operate in the visible wavelength range.25 Furthermore, initiation by visible light is often considered to be sustainable as it allows to use sunlight and bears some aspects of green chemistry as it is abundant in nature.26–28 Thereby, both, catalyst-free approaches and polymerization with the use of photo catalysts were realized, to date.29–31
Besides visible light irradiation, also high throughput processes are an emerging field of research, in context of polymer synthesis.32–35 In particular visible light can be used for realization of high throughput processes.33,36,37 Data driven material design needs large data and sample sets as shown for data driven nanopore application in the context of sensing.3,38 Thus, automated synthesis is essential and of potentially increasing importance in future. This renders visible light induced polymerization with a potential for automated process design interesting.
In this review different visible light induced polymerization techniques are discussed in more detail, focusing on their potential for nanopore functionalization and especially for (nano)local and automated polymer nanopore functionalization. In addition, especially examples for visible light induced polymerizations in nanopores are highlighted.
Free radical polymerizations initiated by visible light
Visible light induced free radical polymerizations receive increasing attention within the last decade. In general, two classes of photoinitiators are used for visible light induced, free radical polymerizations: Type I photoinitiators generate radicals by irradiation to a single-molecule bond cleavage. Type II photoinitiators generate radicals by interaction with a second molecule due to a bimolecular reaction after irradiation.39,40 For visible light irradiation mostly type II photoinitiators are used.39But especially in the last decade also type I photoinitiators were developed for visible light induced polymerizations.41 For example He et al.42 recently used the type I photoinitiator dimethyl 1,4-dibenzoylformate (DM-BD-F) under 405 nm irradiation to polymerize acrylate monomers. But also wavelength up to 600 nm were used for visible light induced polymerizations initiated by type I photoinitiators, as demonstrated by the group of Haas using triacylstannenolates as initiator.43 Currently, especially the broadening of the applicable wavelengths range, water solubility, automated fabrication technology as well as absorption and bleaching behaviour of type I photoinitiators for visible light induced polymerizations are investigated.41 In the following two aspects are highlighted in more detail: dye-sensitized polymerizations which allow to use a broad wavelength range as well as to polymerize in water and the application of visible-light induced free radical polymerizations for additive manufacturing as automated fabrication method.
With respect to broadening the wavelength range and allowing polymerization in water an interesting free radical visible light induced polymerization, based on type II photoinitiators, is the so-called dye-sensitized photopolymerization. Dye-sensitized polymerizations allow polymerization at basically the entire visible light wavelength range depending on the selected dye photosensitizer absorption. Lalevèe and Fouassier summarized trends in dye sensitized radical polymerizations in their book “Dyes and Chromophores in Polymer Science” (2015).44 Briefly, by using two-component photo initiating systems (Fig. 1a),45 consisting of a photosensitizer (dye) and a coinitiator (e.g. a tertiary amine) polymerizations using various dyes and thus at different wavelengths are accessible.46 For example cyanine dyes together with irradiation at a wavelength range of 510–570 nm47 were addressed. While using methylene blue red light with a wavelength around 660 nm was used to initiate this radical polymerization.48 But also sunlight was used for polymerization using various dyes.49 Deeb et al.45 performed a nanoscale dye-sensitized visible-light induced photopolymerization using surface plasmons generated by silver nanoparticles (Fig. 1b). They show nanoscale polymer formation in the areas of surface plasmon enhancement by selecting an irradiation energy below a so-called threshold energy. In further work, it was demonstrated that polymerization is indeed initiated via the photochemical mechanism and not by local temperature increase or hot electrons under the applied low irradiation energy.50 Our research group48,51,52 applied dye-sensitized visible-light induced photopolymerizations using a dye-tertiary amine photosensitizer, photoinitiator combination for mesoporous silica functionalization resulting in polymer-dominated gating of ionic mesopore accessibility. By using surface plasmons localized polymer functionalization in mesoporous layers was achieved (Fig. 1c).48 Besides using visible light and implementing local control on polymer placement, automation of polymerization is of relevance especially in data-driven material design approaches which need large data sets. Automation of local polymer structuring using visible light induced free radical polymerizations was realized using direct laser writing (DLW) in combination with photoresists. This combination let to the development of additive manufacturing of three dimensional polymer materials.53–55 The combination of visible light induced, free radical polymerization with 3D printing is a rapidly growing research area.41,56,57 For example, Lalevèe and colleagues58 used ketone derivatives for a free radical polymerization under 405 nm light and demonstrated 3D printing. Breloy et al.46 used methacrylated quinizarin derivatives to achieve complex 3D biosourced structures under 405 nm light irradiation for 3D-photoprinting technology. Also using 405 nm, Zhao and colleagues59 used dealkaline lignin for digital light processing 3D printing. Zhang et al.60 used blue, green, yellow, and red LEDs to initiate a free radical polymerization and furthermore, demonstrated 3D printing by using a polychromatic visible light (400–730 nm) 3D printer. In nanoporous materials DLW or 3D printing in combination with free radical polymerizations have not been demonstrated to date.
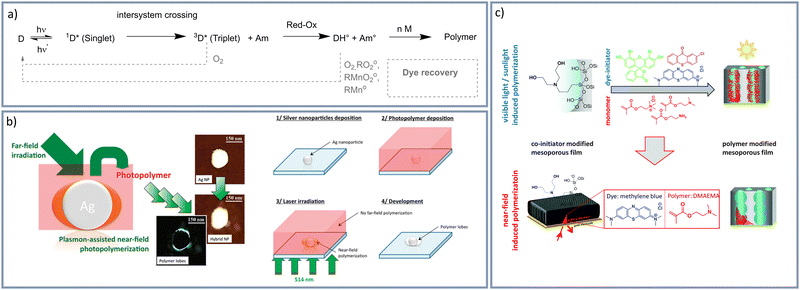 | ||
| Fig. 1 (a) Photopolymerization by dye (D)-amine (Am)-System.45 Adapted with permission from J. Am. Chem. Soc., 2011, 133, 10535–10542.45 Copyright 2021 American Chemical Society. (b) Plasmon-assisted near field photopolymerization by Deeb et al.45 Reprinted (adapted) with permission from J. Am. Chem. Soc., 2011, 133, 10535–10542.45 Copyright 2021 American Chemical Society. (c) Plasmon near-field induced surface polymer modification of mesoporous thin silica films.48 Reprinted with permission Chem. Commun., 2015, 51, 11697–11700.48 Copyright 2015 Royal Society of Chemistry. | ||
Visible light induced ATRP
Besides, the above mentioned free radical polymerization ATRP, as one of the most widely used methods of controlled radical polymerization,16,61 was applied to surface polymer grafting using porous silica in 1997 by Huang and Wirth.62 In 2012 Kruk63 summarized polymer functionalization of mesoporous silica using ATRP. For example, Fu et al.64 reported the adjustment of pore sizes in mesoporous silica particles due to increasing polymer amount using ATRP. The group of Schönherr65 recently analyzed the confinement effects in anodic alumina nanopores and demonstrated the effect of curvature and pore diameter effect on the polymerization kinetics using SI-ATRP. ATRP was as well used to functionalize mesoporous silica66–70 and ion track etched pores71–73 with different polyelectrolytes to study e.g. the resulting permselective ion transport in such polyelectrolyte functionalized mesoporous thin films. In the last years research has been directed, among others, towards visible light induced ATRP. The majority of studies focuses on solution polymerization. Thereby often small amounts of metal catalysts have been used. But also metal-free visible light induced ATRPs based entirely on organic catalysts have been reported. Since 2014 photoinduced organocatalyzed ATRP gained increasing interest.74 Konkolewicz et al.75 used a few ppm of copper catalyst for a photoinduced ATRP under blue and violet LED irradiation and even sunlight whereby at 631 nm no successful ATRP at was observed. Also in the presence of ppm copper catalysts Ciftci et al.76 managed a visible light induced ATRP using Mn2(CO)10 as photocatalyst. Pan et al.77 described an ATRP, which was induced at 392 nm. Upon 380 nm irradiation, a controlled radical polymerization using Ir(ppy)3 (ppy = 2-pyridylphenyl) as photoredoxcatalyst was reported by Treat et al.78 Because of the robust nature of the catalyst the polymerization allows even monomers with acid groups to be polymerized. Ir(ppy)3 as photoredoxcatalyst was as well applied by the group of Zhu79 performing a visible light induced ATRP. Zhu et al. managed to separate and recycle the catalyst after polymerization. Besides metal organic complexes, organic photocatalysts were used for visible light induced ATRPs. Matyjaszewski and coworkers80 reported a green light induced ATRP using a combination of Eosin Y and a copper catalyst to achieve ATRP in contact with air. In 2014 visible light induced, metal free ATRPs were reported by the groups of Hawker81 and Theriot.82 But already in 2012 the group of Yagci83 described the control of molecular weight and distribution using bis (2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO), Eosin Y, and Erythrosin B at 400–500 nm light for ATRP. Liu et al.84 used fluorescein as organocatalyst, the group of Yagci85 used reducible dyes and amine as well as alkylhalides, and Wang et al.86 described an ATRP with an organic semiconductor based visible light activated photo catalyst. In 2018 Xu et al.87 demonstrated the suitability of metal-free, visible light induced ATRP for the polymerization of acrylamides. Very recently Qiao et al.88 used carbon quantum dots as catalyst for a visible light induced ATRP at 405 nm. Using these carbon quantum dots a high monomer conversion of more than 90% in one minute was achieved, making polymerization suitable for 3D printing.88 Although most of the visible light induced ATRP were performed at wavelengths below 600 nm, there have been studies on ATRP using 630 nm89 or even using near infrared irradiation.90These initial developments on visible light induced ATRP in solution were also transferred to surface grafting of polymers using visible light induced ATRPs. Bansal et al.91 reported a surface initiated ATRP (SI-ATRP) under visible light irradiation using tetrasulfonated copper(II) phthalocyanine (CuPcS) as catalyst (Fig. 2a). Under visible light irradiation CuPcS is reduced to Cu(I) complex by a one-electron transfer process.91 To achieve polymer grafting in a first step Bansal et al.91 functionalized TiO2-particels with (3-aminopropyl)triethoxysilane and 2-bromoisobutyryl bromide, which was used as initiator for the surface initiated polymerization. The polymerization was then carried out using visible light irradiation and a varying irradiation time of 5–24 h at room temperature. Also on surfaces Jiang et al. prepared self-healing nanocomposite hydrogels using a SI-PET (photoinduced electron/energy transfer)-ATRP under visible light irradiation for 15 h.92 Fan et al.93 also performed a SI-PET-ATRP to prepare self-healing nanocomposite hydrogels. As in solution, on surfaces and in pores metal free ATRPs are carried out in recent years. The group of Hawker94 reported a metal free SI-ATRP under visible light at 405 nm and room temperature. They used N-phenyl phenothiazine (PTH) as catalyst and an irradiation time from 0.5–4 h. The group of Matyjaszewski95 used PTH as photocatalyst while functionalizing 16 nm and 120 nm silica nanoparticles with poly(methyl methacrylate) using a wavelength of 365 nm. Also using irradiation of 365 nm and PTH as catalyst SI-ATRP's to functionalize mesoporous silica polymer nanocomposites96 and SBA-1597 were reported. A visible light induced SI-ATRP in mesoporous silica was as well achieved by Ma et al.98 while using 380 nm light to functionalize SBA-15 (Fig. 2b). The group of Yagci99 analyzed the photoinitiation mechanism of photoinduced metal free ATRP using PTH (Fig. 2c).
 | ||
| Fig. 2 (a) SI-ATRP under visible light irradiation on TiO2 particles using CuPcS as photo catalyst by Bansal et al.91 Adapted with the permission from RSC Adv., 2015, 5, 21189–21196.91 Copyright 2015 Royal society of chemistry. (b) SI-ATRP under visible light irradiation on SBA-15 using PTH as photo catalyst by Ma et al.98 Adapted with permission from Polymers, 2017, 9, 58.98 Copyright 2017 by the authors; licensee MDPI, Basel, Switzerland. (c) Mechanism of the metal free photo imitated ATRP using PTH as photo catalyst by the group of Yagci.99 Reprinted with the permission from Polym. Chem., 2016, 7, 6039–6043.99 Copyright 2016 Royal society of chemistry. | ||
Also performing a metal-free SI-ATRP on SBA-15 the group of Zhang100,101 used fluorescein as photo catalyst and triethylamine as electron donor under blue light irradiation. Another example of pore functionalization using visible light induced ATRP was performed by Meng et al.,102 while using the photo catalyst fac-[Ir(ppy)3] they functionalized microporous polypropylene membrane (MPPM) surfaces with methacrylates and diblock copolymer brushes. Local polymer-reinitiation at the nanoscale has recently been demonstrated by Soppera and Colleagues103 using a visible light induced ATRP and surface plasmons at 532 nm.
Numerous visible light induced ATRP's have been performed at different wavelengths, both with and without metal catalysts. The listed examples clearly show that visible light induced ATRP is well studied in solution, and that it is increasingly studied on surfaces, while it becomes recently a research topic also in porous materials.
Visible light induced RAFT and Iniferter initiated polymerizations
Besides ATRP, RAFT is a versatile controlled radical polymerization mechanism as well applied to porous materials and in recent years combined with visible light irradiation. The classical RAFT polymerization was reported in 1988 by the Commonwealth Scientific and Industrial Research Organization (CSIRO) research group.18 One advantage of RAFT polymerization is the large variety of different monomers which are compatible with the RAFT mechanism. Because of the high tolerance to different functional groups the polymerization can even be performed in aqueous solution. The RAFT process uses thiocarbonylthio-compounds to control the polymerization. Basis of the polymerization is the reversible degenerative chain transfer.104 The mechanism of RAFT consists mainly of two reactions: the pre-equilibrium and the main equilibrium.RAFT agents show characteristic colors, which indicate their absorbance in the visible light region such as the yellow trithiocarbonates and dithiobenzoates being red. When the RAFT agents are exposed to suitable light, carbon-centered and sulfur-centered radicals are generated. The carbon-centered radicals are usually responsible for initiating the polymerization.105 The photolytic stability of various RAFT agents under blue light was analyzed by the group of Qiao.106 They reported that the stability of the RAFT agent (i.e. thiocarbonylthio-compounds) depends on the structure of the fragmenting group (R-). The reactivity of the carbon-centered radical depends on the photolytic cleavage.106
Light induced RAFT is usually realized either by the PET107 or the iniferter (initiator–transferagent–terminator21) approach.108 Thereby, PET-RAFT is a visible light induced variant of the RAFT polymerization, which was developed in 2014 by the group of Boyer.107 Interestingly, this PET-RAFT provides oxygen tolerance under ambient conditions and can be performed either using a transition metal-based or an organic photoredox catalyst such as e.g. Ru(bpy)3Cl2, ZnTPP or Eosin Y, respectively.109 The alternative approach of iniferter initiated polymerization using RAFT agents to polymerize upon visible light irradiation were first investigated by Otsu.110 Already in 1958 Otsu and Nayatani111 used thiuram disulfides to polymerize styrol in solution discussing two types of iniferters: an asymmetric A–B type and a symmetric C–C type iniferter,110,112 where the A–B type is preferred for better reactivity control, and e.g. preparation of block-co-polymers.110 For further details on iniferter initiated polymerizations we recommend the article by Otsu.110
As already observed for ATRP a significant number of visible light induced RAFT and iniferter initiated polymerizations were performed in solution and on planar surfaces. Especially the relatively recently reported PET-RAFT seems to be promising for controlled visible light induced polymer functionalization. Depending on the required wavelength, different catalysts can be used, both on metal and organic basis. In comparison to PET-RAFT polymerization, which can be initiated up to 850 nm,113 iniferter initiated polymerizations are usually initiated at a wavelength below 400 nm. Whereas, in recent years iniferter initiated polymerizations using visible light were also described. On the other hand, iniferter initiated polymerization don’t require any additional photo catalyst, which might be advantageous in the context of nanopore polymer functionalization with respect to reagent availability in these nanopores during polymerization.
In 2014 visible light induced PET-RAFT using Ir(ppy)3 as photocatalyst was reported.107 Since then zinc tetraphenylporphyrin (ZnTPP) was frequently used as metal-based photo catalyst in visible light induced PET-RAFT.36,114–118 Very recently Wanasinghe et al.116 used both Ir(ppy)3 and ZnTPP to analyze bulk swelling ratios and homogeneity of polymer networks, whereby better results were reported using ZnTPP. Corrigan et al.119 used ZnTPP as photoredox catalyst for a PET-RAFT polymerization at low-intensity yellow light irradiation, ambient temperatures, and in an open reaction vessel in the presence of oxygen. Also by using ZnTPP Shanmugam et al.120 polymerized styrene, (meth)acrylates, and (meth)acrylamides using various visible light wavelengths between 435–655 nm to activate the trithiocarbonate compounds. A variety of wavelengths has been used in combination with ZnTPP as ZnTPP has serval absorption maxima at 422 nm, 520 nm, 570 nm, and 600 nm (Fig. 3a). Shanmugam et al.120 used 2-(n-butyltrithiocarbonate)-propionic acid (BTPA) as trithiocarbonate to analyze the polymerization rates upon tuning the light wavelength. This study showed that the polymerization with yellow light was the fastest and the polymerization speed decreased from yellow > green > orange > red > blue light whereby the emission of the yellow lamp was centered on the maximum absorption at 570 nm. The group of Pester109 used ZnTPP for an oxygen tolerant, surface initiated PET-RAFT (SI-PET-RAFT) under visible light (405 or 590 nm, see Fig. 3b). In this study two different RAFT agents: 2-(dodecylthiocarbonothioylthio)-2-methylpropanoic acid (DDMAT) and 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (CPADB) were investigated. A higher monomer tolerance for CPADB-functionalized surfaces in combination with the photo catalyst Ir(ppy)3, showing a strong redox-potential, was described. Furthermore, the synthesis of block copolymers was demonstrated. The group of Pan121 also used the RAFT agent CPADB for a visible light induced PET-RAFT and demonstrated a temporal control over the polymerization and polymer formation by switching the light on and off (Fig. 3c). Additionally, the effects of catalyst concentration and light intensity on the polymerization rate were analyzed showing a gradual increase of monomer conversion with catalyst concentration and faster polymerization rate with increasing light intensity. Applying blue light irradiation (460 nm, irradiation time 24 h) Yeow et al.122 used a ruthenium-based photoredox catalyst (Ru(bpy)3Cl2) to activate a photoinduced PET-RAFT.
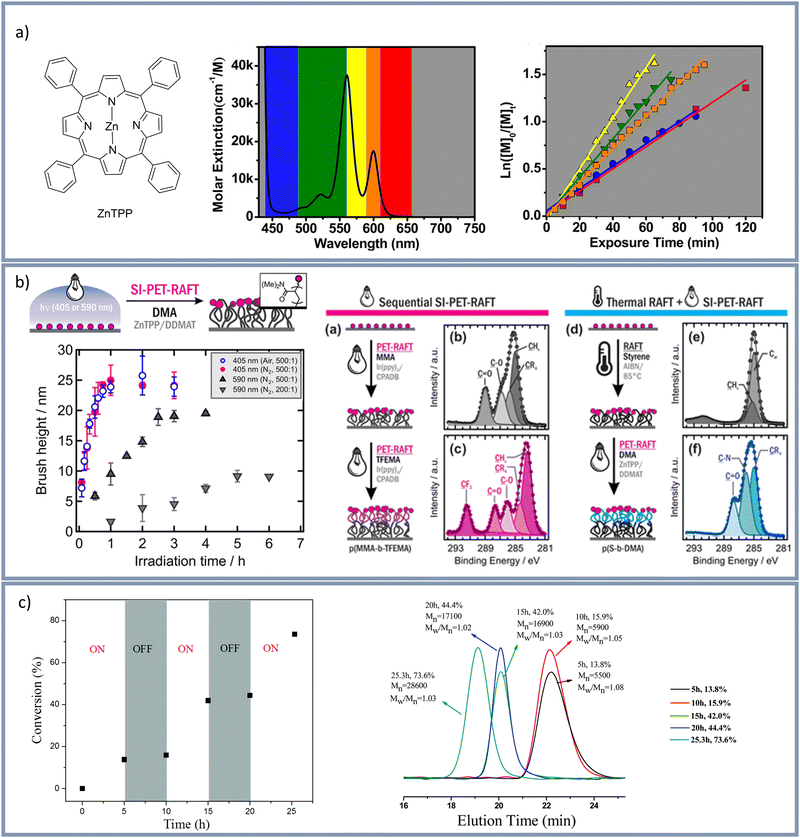 | ||
| Fig. 3 (a) Molecular structure of the photo catalyst ZnTPP (left). Molar extinction spectrum of ZnTPP with the corresponding LED colors (middle) and plot of ln([M]0/[M]t) vs. exposure time depending on different light wavelengths (right) by Shanmugam et al.120 Reprinted with the permission from J. Am. Chem. Soc., 2015, 137, 9174–9185.120 Copyright 2015 American Chemical Society. (b) Surface-initiated PET-RAFT by group of Pester109 Polymer brush thickness vs. irradiation time under inert gas and under air and diblock copolymer synthesis by SI-PET-RAFT. Reprinted with the permission from ACS Macro Lett., 2019, 8, 374–380.109 Copyright 2019 American Chemical Society. (c) Visible light mediated RAFT by the group of Pan121 using PTH as photo catalyst, demonstrating “ON/OFF” control over polymerization. Reproduced with the permission from Mater. Chem. Front., 2017, 1, 1200–1206121 with permission from the Chinese Chemical Society (CCS), Institute of Chemistry of Chinese Academy of Sciences (IC), and the Royal Society of Chemistry. | ||
But not only metal-based catalysts are suitable for PET-RAFT induced by visible light. Recently, organic catalysts such as Eosin Y123,124 and fluorescein125 were applied to initiate RET-RAFT polymerizations. Using organic catalysts for a SI-PET-RAFT Kuzmyn et al.124 prepared antifouling polymer brushes on gold surfaces using three different monomers, namely oligo(ethylene glycol) methyl ether methacrylate (MeOEGMA), carboxybetaine methacrylamide (CBMA), and N-(2-hydroxypropyl) methacrylamide (HPMA). In 2016 Shanmugam et al.113 used Bacteriochlorophyll a as organic photoredox catalyst and demonstrated the first PET-RAFT under near-infrared/far-red irradiation (850 nm und 780 nm). Very recently Bellotti and Simounutti126 summarized theoretical basics, as well as industrial applications for PET-RAFT.
Unlike the classical RAFT or the PET-RAFT, the iniferter initiated polymerization does not require any additional catalyst which makes it an interesting candidate for polymerization in confined space. This might be an advantage for nanopore functionalization as nanopore accessibility to reagent might be an essential factor for polymerization control. Based on the visible light irradiation of trithiocarbonates (e.g. RAFT agents) the polymerization is initiated. Trithiocarbonate compounds possesses thiocarbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) groups. The absorption characteristics of thiocarbonyl-groups are slightly shifted to longer wavelengths, as compared to the absorption of carbonyl (C
S) groups. The absorption characteristics of thiocarbonyl-groups are slightly shifted to longer wavelengths, as compared to the absorption of carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups. Thiocarbonyl groups show an absorption band in UV range at approximately 320 nm (spin allowed) and a second absorption band in the visible light range between 400–550 nm (spin-forbidden). Because of the absorption band at approximately 400–550 nm, compounds with thiocarbonyl groups, for example trithiocarbonate, react to visible light irradiation.29
O) groups. Thiocarbonyl groups show an absorption band in UV range at approximately 320 nm (spin allowed) and a second absorption band in the visible light range between 400–550 nm (spin-forbidden). Because of the absorption band at approximately 400–550 nm, compounds with thiocarbonyl groups, for example trithiocarbonate, react to visible light irradiation.29
For the first time, in 2015 the group of Qiao29 used the iniferter benzyldodecyl carbonotrithioate (TTC-1) for a light-triggered radical polymerization via visible light irradiation at approximately 460 nm in the absence of exogenous photoinitiators or catalysts (Fig. 4a). In this study the synthesis of well-defined poly(acrylamides) and poly(acrylates) was demonstrated. Furthermore, Qiao et al. showed that thermal induction of the polymerization could be excluded although the applied LED's heated the reaction mixture. In another example, as well from 2015, the group of Qiao127 used trithiocarbonate for a photo-controlled radical polymerization under visible light (460 nm) to generate cross-liked star polymers. Furthermore, Rubens et al.25 used a blue light source to initiate a polymerization, which was also controlled by trithiocarbonate. This study demonstrated full monomer conversion within one hour or even shorter reaction time. Therefore, no notable degradation of the trithiocarbonate was observed. The group of Boyer30 reported a photo-controlled radical polymerization using polymerization-induced self-assembly with 4-cyano-4-((dodecylsulfanylthiocarbonyl)sulfanyl)pentanoic acid (CDTPA) as iniferter under blue (460 nm) and green (530 nm) light irradiation (Fig. 4b). Probably due to differing degrees of polymerization control using two different light wavelengths, block-copolymer nanoparticles with different morphologies were obtained. In another example CDTPA was also used to synthesize polymeric nanomaterials with various morphologies using visible light irradiation and the organic photoredox catalyst PTH.121
 | ||
| Fig. 4 (a) Visible light induced photo-controlled radical polymerization using the Iniferter TTC-1 for the first time in absence of exogenous photoinitiators or catalysts by the group of Qiao.29 Reprinted with the permission from Macromolecules, 2015, 48, 3864–3872.29 Copyright 2015 American Chemical Society (b) Synthesis of complex polymeric architectures using CDTPA as Iniferter under blue (460 nm) and green (530 nm) light irradiation by the group of Boyer.30 Reprinted with the permission from ACS Macro Lett., 2016, 5, 558–564.30 Copyright 2016 American Chemical Society. (c) Mesopore functionalization using CDTPA and SBDTTC as iniferter under visible light irradiation by our group.130 Reprinted with the permission from Adv. Funct. Mater., 2021, 31, 2009732.130 Copyright 2021 John Wiley & Sons, Inc. | ||
Despite the recent dynamic developments in visible light induced RAFT including PET-RAFT and iniferter initiated polymerizations in solution and on planar surfaces examples on nanopore functionalization using visible light induced RAFT remain scarce up to date: The group of Wei128 used 4-cyano-4-(ethylsulfanylthiocarbonylsulfanyl)pentanoic acid (CEP) for RAFT polymerizations under 480 nm light to functionalized mesoporous silica nanoparticles with zwitterionic polymers. Recently, the first PET-RAFT in mesopores was reported by Joshi and Nebhani.129 After synthesizing mesoporous silica particles with an in-built RAFT agent, a visible light induced PET-RAFT was performed using a 50 W LED and Eosin Y as photo catalyst. This PET-RAFT in mesopores was achieved by stepwise co-condensation, using different hydrophobic and hydrophilic monomers. Our research group recently reported an asymmetric mesopore functionalization using a visible light induced PET-RAFT with ZnTPP as photo catalyst.130 In another example of our research group a layer-selective functionalization of mesoporous double layered films using the iniferter 4-(N,N-diethyldithiocarbamoylmethyl)benzoic acid (BDC) under UV-light irradiation was demonstrated.131 A visible light induced mesopore functionalization was also performed using two different iniferter approaches: Using the iniferter S-p-trimeth-oxysilylbenzyl-S′-dodecyltrithiocarbonate (SBDTTC) for a grafting from approach and CDTPA for a grafting through approach (Fig. 4c).132 Using these two visible light sensitive iniferters in combination with localized surface plasmon resonance from alloy Ag/Au nanoparticles as optical near field method 3D local polymer functionalization was demonstrated.132
Ionic and other controlled polymerizations initiated by visible light
Many examples on visible light induced cationic polymerizations have been reported. One reason for this is probably related to the tolerance of ambient oxygen and water. Most of the reported visible light induced cationic polymerizations are based on onium salt initiators.133,134 Examples of typical onium salts for photoinitiated cationic polymerizations are shown in Fig. 5a.133 Mechanistically this polymerization is based on the decay of the excited state of the onium salt after photoexcitation. Thereby, both, a homolytic and a heterolytic cleavage of the onium salt is possible, whereby highly reactive aryl cations and aryliodine cation radicals are generated. For more information on the mechanism of photoinduced cationic polymerization and the mechanism we refer to the highlight-article “The Discovery and Development of Onium Salt Cationic Photoinitiators” from Crivello in 1999.134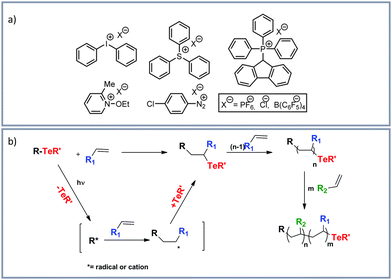 | ||
| Fig. 5 (a) Typical onium salts for photoinitated cationic polymerizations by Michaudel et al.133 Reprinted with the permission from Angew. Chem., 2017, 129, 9798–9808.133 Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Photoinduced organotellurium-mediated polymerization by Kaya et al.138 Reprinted with the permission from Polym. Chem., 2018, 9, 5639–5643.138 Copyright 2018 Royal Society of Chemistry. | ||
Regarding reported examples on cationic salt-based polymerizations, Yilmaz et al.135 performed a free radical promoted cationic polymerization under irradiation of visible light from 430–490 nm by using thioxanthone-fluorene carboxylic acid or thioxanthone-carbazole as photocatalysts and diphenyliodonium hexafluorophosphate or silver hexa-fluorophosphate as cationic salts. Sari et al.136 described a surface initiated free radical promoted cationic polymerization on tetrakis(2,4,6-trimethylbenzoyl)silane (TTBS) at room temperature and visible light (>400 nm) in presence of the onium salts diphenyliodonium hexafluorophosphate and triphenylsulfonium hexafluorophosphate. Also in the presence of onium salts Yilmaz et al.137 reported a visible light (>400 nm) induced cationic polymerization by fullerene sensitization. Kaya et al.138 used organotellurium compounds in the presence of diphenyliodonium hexafluorophosphate for polymerizations initiated by visible light and sunlight (organotellurium-mediated polymerization, Fig. 5b). Crivello139 reported a visible light induced cationic polymerization of epoxides with a titanium-complex free radical photoinitiator and diaryliodonium salts. Sangermano and coworkers140 performed cationic polymerizations using different onium salt photoinitiators. Kerem et al.141 used sulfonium salt photoinitiators. For a cationic ring-opening photo-polymerization of epoxide monomers and epoxide functional oligomers an efficient three-component visible light sensitive photoinitiator system was developed. The use of camphorquinone in combination with a benzyl alcohol generates radicals by visible light absorbance.142 Combining free radical and cationic polymerizations of vinyl monomers and cyclic ethers, initiated by visible light (400–500 nm), conjugated microporous polymeric networks containing thioxanthone groups were obtained.143
Besides these examples of cationic polymerizations, combined with free radical photoinitiators, it is also possible to combine free radical promoted cationic polymerization with controlled radical polymerizations: For example, the group of Yagci144 used Mn2(CO)10 as radical source and synthesized block copolymers by the combination from ATRP with visible light induced free radical promoted cationic polymerization. In a further example, the group of Yagci145 synthesized amphiphilic hyperbranched macromolecular structures in presence of Mn2(CO)10 by using the visible light induced self-condensing vinyl copolymerization of three methacrylates. Mn2(CO)10 for visible light induced polymerizations was not only applied in solution, furthermore surface functionalization was achieved: Xiong et al.146 described a visible light induced surface grafting polymerization with the use of Mn2(CO)10 on gold surfaces. The polymer-film thickness was controlled by variation of the irradiation time. The group of Chen147 reported the surface functionalization of poly(dimethylsiloxane) by visible light induced polymerization with Mn2(CO)10. Combining a visible light-induced free radical polymerization with a ROMP and hydrobromination the group of Yagci148,149 prepared a copolymer by grafting from using Mn2(CO)10 and visible light irradiation. Mn2(CO)10 as photo catalyst was also used for a visible light induced iodine transfer polymerizations (ITP) at 463 nm by Koumura et al.150 ITP is an controlled radical polymerization, which involves vinyl monomer, a conventional initiator and an iodinated chain transfer agent.151 The general mechanism of the polymerization using Mn2(CO)10 is shown in Fig. 6a.151 The applications of visible light induced photo initiation based on Mn2(CO)10 was summarized by the group of Yagci in 2016.151 Furthermore, visible-light induced ITP have be realized using an Ir(III) complex (Fig. 6b).152 Fors and Hawker153 portrayed a visible light induced living radical polymerization with ppm amounts of the photocatalyst fac-[Ir(ppy)3]. The suggested mechanism of the polymerization is shown in Fig. 6c.
 | ||
| Fig. 6 (a) Mechanism of photo induced ITP with Mn2(CO)10/alkyl iodide.151 Reprinted with permission from Polym. Int., 2016, 65, 1001–1014.151 Copyright 2016 John Wiley & Sons, Inc. (b) Visible-light induced ITP with Ir(III) complex.152 Reprinted with permission from J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 3283–3291.152 Copyright 2014 John Wiley & Sons, Inc. (c) Suggested mechanisms of the visible light induced polymerization using an Ir-based photoredox catalyst.153 Reprinted with permission from Angew. Chem., Int. Ed., 2012, 51, 8850–8853.153 Copyright 2021 John Wiley & Sons, Inc. | ||
These examples represent a selection among the manifold studies on visible light induced polymerizations based on cationic polymerization, whereby especially polymerizations based on onium salts or Mn2(CO)10 are frequently used. Most of the studies on this type of polymerization were performed at wavelengths below 600 nm and in solution. Going towards red light irradiation, the group of Fouassier reported the development of cationic polymerizations and showed the possibility to use various wavelengths, including red light irradiation.154 Despite the plenty examples on visible light induced cationic polymerizations in solution and even on surfaces, studies on polymer functionalization of nanoporous materials using visible light induced cationic polymerizations remain unreached (Fig. 8).
Visible light induced ROMP
Besides visible-light induced cationic polymerizations and ITP visible-light induced ROMP represents another, complementary polymerization technique beyond radical polymerization. Only a few examples on visible light induced ROMP have been demonstrated. In general, visible light induced ROMP are still relatively scarce, and so far limited to solution polymerizations. In the traditional ROMP metal-complexes, e.g. Ru-, W-, or Mo-alkylidene complexes, are used as catalysts.155 Also organocatalyzed ROMPs have been reported, which use vinyl ethers as initiators156,157 In 2015 the group of Boydston156 reported the first metal free ROMP using blue-light emitting LEDs (450–480 nm). The used photoredox mediators compatible with this blue-light LED irradiation are shown in Fig. 7a. Theunissen et al.158 published a ROMP and photolithographic olefin metathesis polymerization (PLOMP) using blue light. Using divinyl ether initiators and a photoredox catalyst to polymerize norbornene upon 450–480 nm light irradiation, the group of Boydston157 successfully performed a visible light induced ROMP. Fig. 7b shows the used divinyl ethers (1 and 2) and the pyrylium photocatalyst (4). Recently Eivig et al. reported ROMP under 420 nm irradiation as suitable for 3D applications.159 Catalysts with the general structure shown in Fig. 7c, absorb at 450 nm and can also be used for visible light control of ROMP.160 | ||
| Fig. 7 (a) Photoredox mediators for metal free ROMP under blue light irradiation by the group of Boydston.156 Reprinted with the permission from J. Am. Chem. Soc., 2015, 137, 1400–1403. Copyright 2015 American Chemical Society. (b) Photo catalyzed ROMP by the group of Boydston157 Reprinted with the permission from J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2977–2982.157 Copyright 2017 John Wiley & Sons, Inc. (c) General structure of visible light catalysts for ROMP.160 Reprinted with permission from Synlett, 2016, 27, 203–214.160 Copyright 2016, Rights Managed by Georg Thieme Verlag KG. | ||
Examples on nanoporous material functionalization using classical ROMP remain scarce and examples on visible light induced ROMP in nanopores have not yet been reported. For classical ROMP Mohite et al.161 polymerized free norbornene using ROMP in nanoporous silica nanoparticles, whereby it was bound to surface-grafted norbornene by using a crosslinking mechanism. Our research group reported on ROMP being used to functionalize nanopores with 5-norbornene-2-carboxylic acid pentafluorophenyl ester162 as well as with spiropyran and spirooxazine substituted polynorbornene homopolymers.163 Plenio and colleagues164 showed an alternative approach when covalently binding the catalyst to a silica nanoparticle surface followed by a SI-ROMP in presence of monomer in solution.
Conclusions
In summary, research on visible light induced polymerizations has been dynamically evolving over the last years. In particular, many examples of visible-light induced cationic polymerizations, ATRP, and RAFT in solution and on planar surfaces have been reported mainly within the last two decades (Fig. 8a). Main research activities within the last years have been focused on broadening the wavelengths range to cover the entire range of visible light, e.g. using metal photo catalysts. Subsequently, the number of reported studies on visible light induced polymerization increased and the wavelength range was shifted significantly into the visible wavelength range. Furthermore, the reduction of these metal photo catalysts in the reaction was strongly investigated resulting in synthesis strategies allowing metal-free visible light controlled polymerizations with increasing number of reported studies in recent years. Although the number of examples is still limited, visible light induced controlled radical polymerizations such as visible-light induced ATRP, visible-light induced PET-RAFT, dye-sensitized polymerizations, and visible light induced iniferter initiated polymerizations were successfully transferred to nanopore functionalization (Fig. 8b). ATRP is the most frequently used visible light induced polymerization in nanopores, with a contribution of 47% of the cited references in this review. Nevertheless, visible light induced PET-RAFT is especially significant when considering that only very recently in 2022 the first examples of PET-RAFT in nanopores was reported. The differences between the polymerization techniques in solution and on planar surfaces as compared to nanopores as well clearly demonstrates the challenges in nanopore visible-light polymerization with respect to ionic and ROMP polymerization. Visible light induced controlled radical polymerizations are envisioned to be of special interest with respect to automated and advanced material fabrication and functionalization strategies. Here in particular the potential enabling high local control on polymer placement in nanoporous materials is of interest. Mechanistically, the question of how confinement, local concentration variations as well as nanopore accessibility and transport influence such visible light induced polymerization still bares many open questions. In this context recent work on confinement controlled catalysis165–167 together with theoretical work on ion concentration profiles along nanopore cross sections14 points towards deeper understanding of confinement influences on reactions and represents a first starting point towards a rational confinement-controlled design. However, compared to the many examples in solution and even on planar surfaces, the development of visible light induced polymerizations for nanopore functionalization is still in its infancy (Fig. 8) but inherits great potential especially for automated polymer writing, highly precise polymer placement, or additive manufacturing.Author contributions
Claire Förster: writing original draft, visualization, investigation. Annette-Andrieu-Brunsen: acquisition of funding, supervision and assistance with manuscript writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No 803758).References
- W. Lu, Y. Cao and G. Qing, Recent Advances in the Modification and Characterization of Solid-State Nanopores, Chem. – Asian J., 2022, e202200675 CAS.
- R. Brilmayer, C. Förster, L. Zhao and A. Andrieu-Brunsen, Recent trends in nanopore polymer functionalization, Curr. Opin. Biotechnol, 2020, 63, 200–209 CrossRef CAS PubMed.
- R. Pardehkhorram and A. Andrieu-Brunsen, Pushing the limits of nanopore transport performance by polymer functionalization, Chem. Commun., 2022, 58, 5188–5204 RSC.
- L. Fu and J. Zhai, Biomimetic stimuli-responsive nanochannels and their applications, Electrophoresis, 2019, 40, 2058–2074 CrossRef CAS PubMed.
- Z. Zhu, D. Wang, Y. Tian and L. Jiang, Ion/Molecule Transportation in Nanopores and Nanochannels: From Critical Principles to Diverse Functions, J. Am. Chem. Soc., 2019, 141, 8658–8669 CrossRef CAS PubMed.
- Y. A. Perez Sirkin, M. Tagliazucchi and I. Szleifer, Transport in nanopores and nanochannels: some fundamental challenges and nature-inspired solutions, Mater. Today Adv., 2020, 5, 100047 CrossRef.
- S. Alberti, G. J. A. A. Soler-Illia and O. Azzaroni, Gated supramolecular chemistry in hybrid mesoporous silica nanoarchitectures: controlled delivery and molecular transport in response to chemical, physical and biological stimuli, Chem. Commun., 2015, 51, 6050–6075 RSC.
- Z. Zhang, L. Wen and L. Jiang, Bioinspired smart asymmetric nanochannel membranes, Chem. Soc. Rev., 2018, 47, 322–356 RSC.
- H. Zhang, Y. Tian and L. Jiang, From symmetric to asymmetric design of bio-inspired smart single nanochannels, Chem. Commun., 2013, 49, 10048–10063 RSC.
- X. Hou, W. Guo and L. Jiang, Biomimetic smart nanopores and nanochannels, Chem. Soc. Rev., 2011, 40, 2385–2401 RSC.
- L. Wen, X. Hou, Y. Tian, F.-Q. Nie, Y. Song, J. Zhai and L. Jiang, Bioinspired smart gating of nanochannels toward photoelectric-conversion systems, Adv. Mater., 2010, 22, 1021–1024 CrossRef CAS PubMed.
- Ç. K. Söz, S. Trosien and M. Biesalski, Janus Interface Materials: A Critical Review and Comparative Study, ACS Mater. Lett., 2020, 2, 336–357 CrossRef.
- S. Qin, K. Huang and I. Szleifer, Design of Multifunctional Nanopore Using Polyampholyte Brush with Composition Gradient, ACS Nano, 2021, 15(11), 17678–17688 CrossRef CAS PubMed.
- K. Huang and I. Szleifer, Design of multifunctional nanogate in response to multiple external stimuli using amphiphilic diblock copolymer, J. Am. Chem. Soc., 2017, 139, 6422–6430 CrossRef CAS PubMed.
- M. Szwarc, ‘Living’ Polymers, Nature, 1956, 178, 1168–1169 CrossRef CAS.
- K. Matyjaszewski, Atom Transfer Radical Polymerization: From Mechanisms to Applications, Isr. J. Chem., 2012, 52, 206–220 CrossRef CAS.
- M. Marin-Suarez, A. L. Medina-Castillo, J. F. Fernandez-Sanchez and A. Fernandez-Gutierrez, Atom-Transfer Radical Polymerisation (ATRP) as a Tool for the Development of Optical Sensing Phases, Isr. J. Chem., 2012, 52, 264–275 CrossRef CAS.
- J. Chiefari, Y. K. B. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process, Macromolecules, 1998, 5559–5562 CrossRef CAS.
- C. J. Hawker, Molecular Weight Control by a “Living” Free-Radical Polymerization Process, J. Am. Chem. Soc., 1994, 116, 11185–11186 CrossRef CAS.
- U. Meyer, F. Svec, J. M. J. Fréchet, C. J. Hawker and K. Irgum, Use of Stable Free Radicals for the Sequential Preparation and Surface Grafting of Functionalized Macroporous Monoliths, Macromolecules, 2000, 33, 7769–7775 CrossRef CAS.
- T. Otsu, M. Yoshida and T. Tazaki, A model for living radical polymerization, Die Makromol. Chem., Rapid Commun., 1982, 3, 133–140 CrossRef CAS.
- J. C. Tom, R. Brilmayer, J. Schmidt and A. Andrieu-Brunsen, Optimisation of Surface-Initiated Photoiniferter-Mediated Polymerisation under Confinement, and the Formation of Block Copolymers in Mesoporous Films, Polymers, 2017, 9, 539 CrossRef PubMed.
- L. Silies, H. Didzoleit, C. Hess, B. Stühn and A. Andrieu-Brunsen, Mesoporous Thin Films, Zwitterionic Monomers, and Iniferter-Initiated Polymerization: Polymerization in a Confined Space, Chem. Mater., 2015, 27, 1971–1981 CrossRef CAS.
- K. Parkatzidis, H. S. Wang, N. P. Truong and A. Anastasaki, Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update, Chem, 2020, 6, 1575–1588 CAS.
- M. Rubens, P. Latsrisaeng and T. Junkers, Visible light-induced iniferter polymerization of methacrylates enhanced by continuous flow, Polym. Chem., 2017, 8, 6496–6505 RSC.
- G. E. M. Crisenza and P. Melchiorre, Chemistry glows green with photoredox catalysis, Nat. Commun., 2020, 11, 803 CrossRef PubMed.
- T. P. Yoon, M. A. Ischay and J. Du, Visible light photocatalysis as a greener approach to photochemical synthesis, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed.
- C. A. Boyer and G. M. Miyake, Polymers and Light, Macromol. Rapid Commun., 2017, 38, 1700327 CrossRef PubMed.
- T. G. McKenzie, Q. Fu, E. H. H. Wong, D. E. Dunstan and G. G. Qiao, Visible Light Mediated Controlled Radical Polymerization in the Absence of Exogenous Radical Sources or Catalysts, Macromolecules, 2015, 48, 3864–3872 CrossRef CAS.
- J. Yeow, O. R. Sugita and C. Boyer, Visible Light-Mediated Polymerization-Induced Self-Assembly in the Absence of External Catalyst or Initiator, ACS Macro Lett., 2016, 5, 558–564 CrossRef CAS PubMed.
- N. Corrigan, S. Shanmugam, J. Xu and C. Boyer, Photocatalysis in organic and polymer synthesis, Chem. Soc. Rev., 2016, 45, 6165–6212 RSC.
- P. R. Judzewitsch, N. Corrigan, F. Trujillo, J. Xu, G. Moad, C. J. Hawker, E. H. H. Wong and C. Boyer, High-Throughput Process for the Discovery of Antimicrobial Polymers and Their Upscaled Production via Flow Polymerization, Macromolecules, 2020, 53, 631–639 CrossRef CAS.
- S. Oliver, L. Zhao, A. J. Gormley, R. Chapman and C. Boyer, Living in the Fast Lane—High Throughput Controlled/Living Radical Polymerization, Macromolecules, 2019, 52, 3–23 CrossRef CAS.
- Z. Li, Z. Han, M. H. Stenzel and R. Chapman, A High Throughput Approach for Designing Polymers That Mimic the TRAIL Protein, Nano Lett., 2022, 22, 2660–2666 CrossRef CAS PubMed.
- R. Li, S. Zhang, Q. Li, G. G. Qiao and Z. An, An Atom-Economic Enzymatic Cascade Catalysis for High-Throughput RAFT Synthesis of Ultrahigh Molecular Weight Polymers, Angew. Chem., 2022, 134, e202213396 Search PubMed.
- G. Ng, J. Yeow, R. Chapman, N. Isahak, E. Wolvetang, J. J. Cooper-White and C. Boyer, Pushing the Limits of High Throughput PET-RAFT Polymerization, Macromolecules, 2018, 51, 7600–7607 CrossRef CAS.
- R. Upadhya, A. Punia, M. J. Kanagala, L. Liu, M. Lamm, T. A. Rhodes and A. J. Gormley, Automated PET-RAFT Polymerization Towards Pharmaceutical Amorphous Solid Dispersion Development, ACS Appl. Polym. Mater., 2021, 3, 1525–1536 CrossRef CAS PubMed.
- Q. Liu, L. Fang, G. Yu, D. Wang, C.-L. Xiao and K. Wang, Detection of DNA base modifications by deep recurrent neural network on Oxford Nanopore sequencing data, Nat. Commun., 2019, 10, 2449 CrossRef PubMed.
- K. Matyjaszewski and T. P. Davis, Handbook of Radical Polymerization, John Wiley & Sons, Inc, Hoboken, NJ, USA, 2002 Search PubMed.
- H. J. Hageman, Photoinitiators for free radical polymerization, Prog. Org. Coat., 1985, 13, 123–150 CrossRef CAS.
- S. M. Müller, S. Schlögl, T. Wiesner, M. Haas and T. Griesser, Recent Advances in Type I Photoinitiators for Visible Light Induced Photopolymerization, ChemPhotoChem, 2022, 6, e202200091 CrossRef.
- X. He, Y. Gao, J. Nie and F. Sun, Methyl Benzoylformate Derivative Norrish Type I Photoinitiators for Deep-Layer Photocuring under Near-UV or Visible LED, Macromolecules, 2021, 54, 3854–3864 CrossRef CAS.
- J. Maier, S. M. Müller, A. Torvisco, G. Glotz, R. C. Fischer, T. Griesser, A.-M. Kelterer and M. Haas, Isolable Stannenolates Enable the Synthesis of Visible-Light Photoinitiators, ChemPhotoChem, 2022, 6, e202100213 CrossRef CAS.
- J. Lalevée and J. P. Fouassier, Dyes and Chomophores in Polymer Science, Wiley, Hoboken, 2015 Search PubMed.
- C. Deeb, C. Ecoffet, R. Bachelot, J. Plain, A. Bouhelier and O. Soppera, Plasmon-Based Free-Radical Photopolymerization: Effect of Diffusion on Nanolithography Processes, J. Am. Chem. Soc., 2011, 133, 10535–10542 CrossRef CAS PubMed.
- J. P. Fouassier and L. Jacques, Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency, Wiley-VCH Verlag GmbH & Co. KGaA, 2012 Search PubMed.
- J. Kabatc and J. Pączkowski, Monomeric asymmetric two- and three-cationic monomethine cyanine dyes as novel photoinitiators for free-radical polymerization, Dyes Pigm., 2010, 86, 133–142 CrossRef CAS.
- N. Herzog, J. Kind, C. Hess and A. Andrieu-Brunsen, Surface plasmon & visible light for polymer functionalization of mesopores and manipulation of ionic permselectivity, Chem. Commun., 2015, 51, 11697–11700 RSC.
- K. Sun, C. Pigot, Y. Zhang, T. Borjigin, F. Morlet-Savary, B. Graff, M. Nechab, P. Xiao, F. Dumur and J. Lalevée, Sunlight Induced Polymerization Photoinitiated by Novel Push–Pull Dyes: Indane-1,3-Dione, 1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione and 4-Dimethoxyphenyl-1-Allylidene Derivatives, Macromol. Chem. Phys., 2022, 223, 2100439 CrossRef CAS.
- F. Kameche, W. Heni, S. Telitel, L. Vidal, S. Marguet, L. Douillard, C. Fiorini-Debuisschert, R. Bachelot and O. Soppera, Probing Plasmon-Induced Chemical Mechanisms by Free-Radical Nanophotopolymerization, J. Phys. Chem. C, 2021, 125, 8719–8731 CrossRef CAS.
- D. John, R. Mohammadi, N. Vogel and A. Andrieu-Brunsen, Surface-Plasmon- and Green-Light-Induced Polymerization in Mesoporous Thin Silica Films, Langmuir, 2020, 36, 1671–1679 CrossRef CAS PubMed.
- M. Stanzel, L. Zhao, R. Mohammadi, R. Pardehkhorram, U. Kunz, N. Vogel and A. Andrieu-Brunsen, Simultaneous Nanolocal Polymer and In Situ Readout Unit Placement in Mesoporous Separation Layers, Anal. Chem., 2021, 93, 5394–5402 CrossRef CAS PubMed.
- B. Harke, P. Bianchini, F. Brandi and A. Diaspro, Photopolymerization inhibition dynamics for sub-diffraction direct laser writing lithography, ChemPhysChem, 2012, 13, 1429–1434 CrossRef CAS PubMed.
- J. B. Mueller, J. Fischer, F. Mayer, M. Kadic and M. Wegener, Polymerization kinetics in three-dimensional direct laser writing, Adv. Mater., 2014, 26, 6566–6571 CrossRef CAS PubMed.
- A. Selimis, V. Mironov and M. Farsari, Direct laser writing: Principles and materials for scaffold 3D printing, Microelectron. Eng., 2015, 132, 83–89 CrossRef CAS.
- Y. Wu, M. C. Simpson and J. Jin, 3D Printing of Thiol-Yne Photoresins through Visible Light Photoredox Catalysis, ChemistrySelect, 2022, 7, e202200319 CAS.
- F. Dumur, Recent advances on visible light Thiophene-based photoinitiators of polymerization, Eur. Polym. J., 2022, 169, 111120 CrossRef CAS.
- Y. Xu, G. Noirbent, D. Brunel, Z. Ding, D. Gigmes, B. Graff, P. Xiao, F. Dumur and J. Lalevée, Novel ketone derivative-based photoinitiating systems for free radical polymerization under mild conditions and 3D printing, Polym. Chem., 2020, 11, 5767–5777 RSC.
- X. Zhang, S. Keck, Y. Qi, S. Baudis and Y. Zhao, Study on Modified Dealkaline Lignin as Visible Light Macromolecular Photoinitiator for 3D Printing, ACS Sustainable Chem. Eng., 2020, 8(29), 10959–10970 CAS.
- J. Zhang, K. Launay, N. S. Hill, D. Zhu, N. Cox, J. Langley, J. Lalevée, M. H. Stenzel, M. L. Coote and P. Xiao, Disubstituted Aminoanthraquinone-Based Photoinitiators for Free Radical Polymerization and Fast 3D Printing under Visible Light, Macromolecules, 2018, 51, 10104–10112 CrossRef CAS.
- M. S. Eisen, Controlled Radical Polymerizations, Isr. J. Chem., 2012, 52, 204–205 CrossRef CAS.
- X. Huang and M. J. Wirth, Surface-Initiated Radical Polymerization on Porous Silica, Anal. Chem., 1997, 69, 4577–4580 CrossRef CAS.
- M. Kruk, Surface-Initiated Controlled Radical Polymerization in Ordered Mesoporous Silicas, Isr. J. Chem., 2012, 52, 246–255 CrossRef CAS.
- Q. Fu, G. V. R. Rao, L. K. Ista, Y. Wu, B. P. Andrzejewski, L. A. Sklar, T. L. Ward and G. P. López, Control of Molecular Transport Through Stimuli-Responsive Ordered Mesoporous Materials, Adv. Mater., 2003, 15, 1262–1266 CrossRef CAS.
- H. Bayat, M. Raoufi, I. Zamrik and H. Schönherr, Poly(diethylene glycol methylether methacrylate) Brush-Functionalized Anodic Alumina Nanopores: Curvature-Dependent Polymerization Kinetics and Nanopore Filling, Langmuir, 2020, 36, 2663–2672 CrossRef CAS PubMed.
- L. Cao, T. Man, J. Zhuang and M. Kruk, Poly(N-isopropylacrylamide) and poly(2-(dimethylamino)ethyl methacrylate) grafted on an ordered mesoporous silica surface using atom transfer radical polymerization with activators regenerated by electron transfer, J. Mater. Chem., 2012, 22, 6939 RSC.
- A. Martín, G. Morales, F. Martínez, R. van Grieken, L. Cao and M. Kruk, Acid hybrid catalysts from poly(styrenesulfonic acid) grafted onto ultra-large-pore SBA-15 silica using atom transfer radical polymerization, J. Mater. Chem., 2010, 20, 8026 RSC.
- A. Calvo, B. Yameen, F. J. Williams, O. Azzaroni and G. J. A. A. Soler-Illia, Facile molecular design of hybrid functional assemblies with controllable transport properties: mesoporous films meet polyelectrolyte brushes, Chem. Commun., 2009, 2553–2555 RSC.
- M. Kruk, B. Dufour, E. B. Celer, T. Kowalewski, M. Jaroniec and K. Matyjaszewski, Grafting Monodisperse Polymer Chains from Concave Surfaces of Ordered Mesoporous Silicas, Macromolecules, 2008, 41, 8584–8591 CrossRef CAS.
- S. Alberti, J. Giussi, O. Azzaroni and G. J. A. A. Soler-Illia, A Comparative Study of PMETAC-Modified Mesoporous Silica and Titania Thin Films for Molecular Transport Manipulation, Polymers, 2022, 14, 4823 CrossRef CAS PubMed.
- M. Barsbay and O. Güven, Grafting in confined spaces: Functionalization of nanochannels of track-etched membranes, Radiat. Phys. Chem., 2014, 105, 26–30 CrossRef CAS.
- K. Daumann, S. Frost and M. Ulbricht, Tunable and switchable nanoparticle separation with thermo-responsive track-etched membranes prepared by controlled surface-initiated polymerization of poly(N-isopropylacrylamide), RSC Adv., 2020, 10, 21028–21038 RSC.
- A. Friebe and M. Ulbricht, Controlled pore functionalization of poly(ethylene terephthalate) track-etched membranes via surface-initiated atom transfer radical polymerization, Langmuir, 2007, 23, 10316–10322 CrossRef CAS PubMed.
- D. A. Corbin and G. M. Miyake, Photoinduced Organocatalyzed Atom Transfer Radical Polymerization (O-ATRP): Precision Polymer Synthesis Using Organic Photoredox Catalysis, Chem. Rev., 2022, 122, 1830–1874 CrossRef CAS PubMed.
- D. Konkolewicz, K. Schröder, J. Buback, S. Bernhard and K. Matyjaszewski, Visible Light and Sunlight Photoinduced ATRP with ppm of Cu Catalyst, ACS Macro Lett., 2012, 1, 1219–1223 CrossRef CAS PubMed.
- M. Ciftci, M. A. Tasdelen and Y. Yagci, Sunlight induced atom transfer radical polymerization by using dimanganese decacarbonyl, Polym. Chem., 2014, 5, 600–606 RSC.
- X. Pan, N. Malhotra, A. Simakova, Z. Wang, D. Konkolewicz and K. Matyjaszewski, Photoinduced Atom Transfer Radical Polymerization with ppm-Level Cu Catalyst by Visible Light in Aqueous Media, J. Am. Chem. Soc., 2015, 137, 15430–15433 CrossRef CAS PubMed.
- N. J. Treat, B. P. Fors, J. W. Kramer, M. Christianson, C.-Y. Chiu, J. Read de Alaniz and C. J. Hawker, Controlled Radical Polymerization of Acrylates Regulated by Visible Light, ACS Macro Lett., 2014, 3, 580–584 CrossRef CAS PubMed.
- X. Liu, Y. Ni, J. Wu, H. Jiang, Z. Zhang, L. Zhang, Z. Cheng and X. Zhu, A sustainable photocontrolled ATRP strategy: facile separation and recycling of a visible-light-mediated catalyst fac-[Ir(ppy)3], Polym. Chem., 2018, 9, 584–592 RSC.
- G. Szczepaniak, J. Jeong, K. Kapil, S. Dadashi-Silab, S. S. Yerneni, P. Ratajczyk, S. Lathwal, D. J. Schild, S. R. Das and K. Matyjaszewski, Open-air green-light-driven ATRP enabled by dual photoredox/copper catalysis, Chem. Sci., 2022, 13, 11540–11550 RSC.
- N. J. Treat, H. Sprafke, J. W. Kramer, P. G. Clark, B. E. Barton, J. Read de Alaniz, B. P. Fors and C. J. Hawker, Metal-free atom transfer radical polymerization, J. Am. Chem. Soc., 2014, 136, 16096–16101 CrossRef CAS PubMed.
- G. M. Miyake and J. C. Theriot, Perylene as an Organic Photocatalyst for the Radical Polymerization of Functionalized Vinyl Monomers through Oxidative Quenching with Alkyl Bromides and Visible Light, Macromolecules, 2014, 47, 8255–8261 CrossRef CAS.
- M. A. Tasdelen, M. Ciftci and Y. Yagci, Visible Light-Induced Atom Transfer Radical Polymerization, Macromol. Chem. Phys., 2012, 213, 1391–1396 CrossRef CAS.
- X.-D. Liu, L. Zhang, Z.-P. Cheng and X. Zhu, Metal-Free Photoinduced Electron Transfer - Atom Transfer Radical Polymerization (PET-ATRP) via a Visible Light Organic Photocatalyst, Polym. Chem., 2016, 7, 689–700 RSC.
- Y. Yagci, G. Yilmaz, C. Kütahya and S. Aykac, LED and Visible Light-induced Metal Free ATRP Using Reducable Dyes in the Presence of Amines, Polym. Chem., 2016, 7, 6094–6098 RSC.
- L. Wang, R. Li and K. A. I. Zhang, Atom Transfer Radical Polymerization (ATRP) Catalyzed by Visible Light-Absorbed Small Molecule Organic Semiconductors, Macromol. Rapid Commun., 2018, 39, 1800466 CrossRef PubMed.
- Y. Xu, G. Li, Y. Hu and Y. Wang, Synthesis of Poly(N -isopropylacrylamide)- Block -Poly(tert -Butyl Methacrylate) Block Copolymer by Visible Light-Induced Metal-Free Atom Transfer Polymerization, Macromol. Chem. Phys., 2018, 219, 1800192 CrossRef.
- L. Qiao, M. Zhou, G. Shi, Z. Cui, X. Zhang, P. Fu, M. Liu, X. Qiao, Y. He and X. Pang, Ultrafast Visible-Light-Induced ATRP in Aqueous Media with Carbon Quantum Dots as the Catalyst and Its Application for 3D Printing, J. Am. Chem. Soc., 2022, 144, 9817–9826 CrossRef CAS PubMed.
- S. Dadashi-Silab, K. Kim, F. Lorandi, G. Szczepaniak, S. Kramer, L. Peteanu and K. Matyjaszewski, Red-Light-Induced, Copper-Catalyzed Atom Transfer Radical Polymerization, ACS Macro Lett., 2022, 11, 376–381 CrossRef CAS PubMed.
- C. Kütahya, C. Schmitz, V. Strehmel, Y. Yagci and B. Strehmel, Near-Infrared Sensitized Photoinduced Atom-Transfer Radical Polymerization (ATRP) with a Copper(II) Catalyst Concentration in the ppm Range, Angew. Chem., Int. Ed., 2018, 57, 7898–7902 CrossRef PubMed.
- A. Bansal, A. Kumar, P. Kumar, S. Bojja, A. K. Chatterjee, S. S. Ray and S. L. Jain, Visible light-induced surface initiated atom transfer radical polymerization of methyl methacrylate on titania/reduced graphene oxide nanocomposite, RSC Adv., 2015, 5, 21189–21196 RSC.
- X. Jiang, M. Xi, L. Bai, W. Wang, L. Yang, H. Chen, Y. Niu, Y. Cui, H. Yang and D. Wei, Surface-initiated PET-ATRP and mussel-inspired chemistry for surface engineering of MWCNTs and application in self-healing nanocomposite hydrogels, Mater. Sci. Eng., C, 2020, 109, 110553 CrossRef CAS PubMed.
- D. Fan, G. Wang, A. Ma, W. Wang, H. Chen, L. Bai, H. Yang, D. Wei and L. Yang, Surface Engineering of Porous Carbon for Self-Healing Nanocomposite Hydrogels by Mussel-Inspired Chemistry and PET-ATRP, ACS Appl. Mater. Interfaces, 2019, 11, 38126–38135 CrossRef CAS PubMed.
- E. H. Discekici, C. W. Pester, N. J. Treat, J. Lawrence, K. M. Mattson, B. Narupai, E. P. Toumayan, Y. Luo, A. J. McGrath, P. G. Clark, J. Read de Alaniz and C. J. Hawker, Simple Benchtop Approach to Polymer Brush Nanostructures Using Visible-Light-Mediated Metal-Free Atom Transfer Radical Polymerization, ACS Macro Lett., 2016, 5, 258–262 CrossRef CAS PubMed.
- J. Yan, X. Pan, M. Schmitt, Z. Wang, M. R. Bockstaller and K. Matyjaszewski, Enhancing Initiation Efficiency in Metal-Free Surface-Initiated Atom Transfer Radical Polymerization (SI-ATRP), ACS Macro Lett., 2016, 5, 661–665 CrossRef CAS PubMed.
- L. Huang, M. Liu, L. Mao, D. Xu, Q. Wan, G. Zeng, Y. Shi, Y. Wen, X. Zhang and Y. Wei, Preparation and controlled drug delivery applications of mesoporous silica polymer nanocomposites through the visible light induced surface-initiated ATRP, Appl. Surf. Sci., 2017, 412, 571–577 CrossRef CAS.
- H.-L. Su, L. Xu, X.-J. Hu, F.-F. Chen, G. Li, Z.-K. Yang, L.-P. Wang and H.-L. Li, Polymer grafted mesoporous SBA-15 material synthesized via metal-free ATRP as pH-sensitive drug carrier for quercetin, Eur. Polym. J., 2021, 148, 110354 CrossRef CAS.
- L. Ma, N. Li, J. Zhu and X. Chen, Visible Light-Induced Metal Free Surface Initiated Atom Transfer Radical Polymerization of Methyl Methacrylate on SBA-15, Polymers, 2017, 9, 58 CrossRef CAS PubMed.
- S. Jockusch and Y. Yagci, The active role of excited states of phenothiazines in photoinduced metal free atom transfer radical polymerization: singlet or triplet excited states?, Polym. Chem., 2016, 7, 6039–6043 RSC.
- X. Xu, J. He, Y. Zeng, C. Yu and F. Zhang, Controllable surface-initiated metal-free atom transfer radical polymerization of methyl methacrylate on mesoporous SBA-15 via reductive quenching, Eur. Polym. J., 2020, 131, 109724 CrossRef CAS.
- X. Xu, Y. Zou, J. He, Y. Zeng, C. Yu and F. Zhang, Insight into the effects of reaction conditions on metal-free surface-initiated atom-transfer radical polymerization of methyl methacrylate from SBA-15, J. Appl. Phys., 2020, 127, 115102 CrossRef CAS.
- J. Meng, J. Li, Y. Zhang and S. Ma, A novel controlled grafting chemistry fully regulated by light for membrane surface hydrophilization and functionalization, J. Membr. Sci., 2014, 455, 405–414 CrossRef CAS.
- F. Kameche, W. Heni, S. Telitel, D. Ge, L. Vidal, F. Dumur, D. Gigmes, J. Lalevée, S. Marguet, L. Douillard, C. Fiorini-Debuisschert, R. Bachelot and O. Soppera, Plasmon-triggered living photopolymerization for elaboration of hybrid polymer/metal nanoparticles, Mater. Today, 2020, 40, 38–47 CrossRef CAS.
- K. Skrabania, A. Miasnikova, A. M. Bivigou-Koumba, D. Zehm and A. Laschewsky, Examining the UV-vis absorption of RAFT chain transfer agents and their use for polymer analysis, Polym. Chem., 2011, 2, 2074–2083 RSC.
- S. Li, G. Han and W. Zhang, Photoregulated reversible addition–fragmentation chain transfer (RAFT) polymerization, Polym. Chem., 2020, 11, 1830–1844 RSC.
- T. G. McKenzie, L. P. D. M. Costa, Q. Fu, D. E. Dunstan and G. G. Qiao, Investigation into the photolytic stability of RAFT agents and the implications for photopolymerization reactions, Polym. Chem., 2016, 7, 4246–4253 RSC.
- J. Xu, K. Jung, A. Atme, S. Shanmugam and C. Boyer, A robust and versatile photoinduced living polymerization of conjugated and unconjugated monomers and its oxygen tolerance, J. Am. Chem. Soc., 2014, 136, 5508–5519 CrossRef CAS PubMed.
- S. Perrier, 50th Anniversary Perspective: RAFT Polymerization—A User Guide, Macromolecules, 2017, 50, 7433–7447 CrossRef CAS.
- M. Li, M. Fromel, D. Ranaweera, S. Rocha, C. Boyer and C. W. Pester, SI-PET-RAFT: Surface-Initiated Photoinduced Electron Transfer-Reversible Addition–Fragmentation Chain Transfer Polymerization, ACS Macro Lett., 2019, 8, 374–380 CrossRef CAS PubMed.
- T. Otsu, Iniferter concept and living radical polymerization, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2121–2136 CrossRef CAS.
- T. Otsu and K. Nayatani, Vinyl polymerization XXVIII. The polymerization of styrene initiated by tetralkyl thiuram disulfides, Makromol. Chem., 1958, 27, 149–156 CrossRef CAS.
- T. Schulte, Entwicklung neuer Alkoxyamine für die Nitroxid-vermittelte kontrollierte lebende radikalische Polymerisation. Zugl.: Marburg, Univ., Diss., Cuvillier, Göttingen, 1st edn, 2004 Search PubMed.
- S. Shanmugam, J. Xu and C. Boyer, Light-Regulated Polymerization under Near-Infrared/Far-Red Irradiation Catalyzed by Bacteriochlorophyll a, Angew. Chem., Int. Ed., 2016, 55, 1036–1040 CrossRef CAS PubMed.
- S. M. Clouthier, J. Tanaka and W. You, Photomediated RAFT step-growth polymerization with maleimide monomers, Polym. Chem., 2022, 13, 6114–6119 RSC.
- A. J. Gormley, J. Yeow, G. Ng, Ó. Conway, C. Boyer and R. Chapman, An Oxygen-Tolerant PET-RAFT Polymerization for Screening Structure-Activity Relationships, Angew. Chem., Int. Ed., 2018, 57, 1557–1562 CrossRef CAS PubMed.
- S. V. Wanasinghe, M. Sun, K. Yehl, J. Cuthbert, K. Matyjaszewski and D. Konkolewicz, PET-RAFT Increases Uniformity in Polymer Networks, ACS Macro Lett., 2022, 11, 1156–1161 CrossRef CAS PubMed.
- G. Ng, J. Yeow, J. Xu and C. Boyer, Application of oxygen tolerant PET-RAFT to polymerization-induced self-assembly, Polym. Chem., 2017, 8, 2841–2851 RSC.
- A. Bagheri, J. Yeow, H. Arandiyan, J. Xu, C. Boyer and M. Lim, Polymerization of a Photocleavable Monomer Using Visible Light, Macromol. Rapid Commun., 2016, 37, 905–910 CrossRef CAS PubMed.
- N. Corrigan, D. Rosli, J. W. J. Jones, J. Xu and C. Boyer, Oxygen Tolerance in Living Radical Polymerization: Investigation of Mechanism and Implementation in Continuous Flow Polymerization, Macromolecules, 2016, 49, 6779–6789 CrossRef CAS.
- S. Shanmugam, J. Xu and C. Boyer, Exploiting Metalloporphyrins for Selective Living Radical Polymerization Tunable over Visible Wavelengths, J. Am. Chem. Soc., 2015, 137, 9174–9185 CrossRef CAS PubMed.
- J. Zhou, C. Hong and C. Pan, The photo-controlled polymerization-induced self-assembly and reorganization process for fabrication of polymeric nanomaterials, Mater. Chem. Front., 2017, 1, 1200–1206 RSC.
- J. Yeow, J. Xu and C. Boyer, Polymerization-Induced Self-Assembly Using Visible Light Mediated Photoinduced Electron Transfer–Reversible Addition–Fragmentation Chain Transfer Polymerization, ACS Macro Lett., 2015, 4, 984–990 CrossRef CAS PubMed.
- A. Bagheri, C. W. A. Bainbridge, K. E. Engel, G. G. Qiao, J. Xu, C. Boyer and J. Jin, Oxygen Tolerant PET-RAFT Facilitated 3D Printing of Polymeric Materials under Visible LEDs, ACS Appl. Polym. Mater., 2020, 782–790 CrossRef CAS.
- A. R. Kuzmyn, L. W. Teunissen, P. Fritz, B. van Lagen, M. M. J. Smulders and H. Zuilhof, Diblock and Random Antifouling Bioactive Polymer Brushes on Gold Surfaces by Visible-Light-Induced Polymerization (SI-PET-RAFT) in Water, Adv. Mater. Interfaces, 2022, 9, 2101784 CrossRef CAS.
- J. Xu, S. Shanmugam, H. T. Duong and C. Boyer, Organo-photocatalysts for photoinduced electron transfer-reversible addition–fragmentation chain transfer (PET-RAFT) polymerization, Polym. Chem., 2015, 6, 5615–5624 RSC.
- V. Bellotti and R. Simonutti, New Light in Polymer Science: Photoinduced Reversible Addition-Fragmentation Chain Transfer Polymerization (PET-RAFT) as Innovative Strategy for the Synthesis of Advanced Materials, Polymers, 2021, 13, 1119 CrossRef CAS PubMed.
- T. G. McKenzie, E. H. H. Wong, Q. Fu, A. Sulistio, D. E. Dunstan and G. G. Qiao, Controlled Formation of Star Polymer Nanoparticles via Visible Light Photopolymerization, ACS Macro Lett., 2015, 4, 1012–1016 CrossRef CAS PubMed.
- P. Gao, H. Cao, Y. Ding, M. Cai, Z. Cui, X. Lu and Y. Cai, Synthesis of Hydrogen-Bonded Pore-Switchable Cylindrical Vesicles via Visible-Light-Mediated RAFT Room-Temperature Aqueous Dispersion Polymerization, ACS Macro Lett., 2016, 5, 1327–1331 CrossRef CAS PubMed.
- T. Joshi and L. Nebhani, Light-regulated growth of polymer chains from the surface of RAFT agent primed mesoporous silica nanoparticles, Surf. Interfaces, 2022, 29, 101764 CrossRef CAS.
- C. Förster, L. Veith and A. Andrieu-Brunsen, Visible light induced RAFT for asymmetric functionalization of silica mesopores, RSC Adv., 2022, 12, 27109–27113 RSC.
- M. Stanzel, U. Kunz and A. Andrieu-Brunsen, Layer-selective functionalisation in mesoporous double layer via iniferter initiated polymerisation for nanoscale step gradient formation, Eur. Polym. J., 2021, 156, 110604 CrossRef CAS.
- D. John, M. Stanzel and A. Andrieu-Brunsen, Surface Plasmons and Visible Light Iniferter Initiated Polymerization for Nanolocal Functionalization of Mesoporous Separation Layers, Adv. Funct. Mater., 2021, 31, 2009732 CrossRef CAS.
- Q. Michaudel, V. Kottisch and B. P. Fors, Kationische Polymerisation: von der Photoinitiierung zur Steuerung durch Licht, Angew. Chem., 2017, 129, 9798–9808 CrossRef.
- J. V. Crivello, The discovery and development of onium salt cationic photoinitiators, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 4241–4254 CrossRef CAS.
- G. Yilmaz, S. Beyazit and Y. Yagci, Visible light induced free radical promoted cationic polymerization using thioxanthone derivatives, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1591–1596 CrossRef CAS.
- E. Sari, M. Mitterbauer, R. Liska and Y. Yagci, Visible light induced free radical promoted cationic polymerization using acylsilanes, Prog. Org. Coat., 2019, 132, 139–143 CrossRef CAS.
- G. Yilmaz, B. Iskin, F. Yilmaz and Y. Yagci, Visible Light-Induced Cationic Polymerization Using Fullerenes, ACS Macro Lett., 2012, 1, 1212–1215 CrossRef CAS PubMed.
- K. Kaya, M. Seba, T. Fujita, S. Yamago and Y. Yagci, Visible light-induced free radical promoted cationic polymerization using organotellurium compounds, Polym. Chem., 2018, 9, 5639–5643 RSC.
- J. V. Crivello, Radical-Promoted Visible Light Photoinitiated Cationic Polymerization of Epoxides, J. Macromol. Sci., Part A: Pure Appl. Chem., 2009, 46, 474–483 CrossRef CAS.
- J. V. Crivello and M. Sangermano, Visible and long-wavelength photoinitiated cationic polymerization, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 343–356 CrossRef CAS.
- K. Kaya, J. Kreutzer and Y. Yagci, A Charge-Transfer Complex of Thioxanthonephenacyl Sulfonium Salt as a Visible-Light Photoinitiator for Free Radical and Cationic Polymerizations, ChemPhotoChem, 2019, 3, 1187–1192 CrossRef CAS.
- J. V. Crivello, A new visible light sensitive photoinitiator system for the cationic polymerization of epoxides, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 866–875 CrossRef CAS.
- S. Dadashi-Silab, H. Bildirir, R. Dawson, A. Thomas and Y. Yagci, Microporous Thioxanthone Polymers as Heterogeneous Photoinitiators for Visible Light Induced Free Radical and Cationic Polymerizations, Macromolecules, 2014, 47, 4607–4614 CrossRef CAS.
- M. U. Kahveci, G. Acik and Y. Yagci, Synthesis of Block Copolymers by Combination of Atom Transfer Radical Polymerization and Visible Light-Induced Free Radical Promoted Cationic Polymerization, Macromol. Rapid Commun., 2012, 33, 309–313 CrossRef CAS PubMed.
- C. Aydogan, M. Ciftci, A. M. Asiri and Y. Yagci, Visible light induced one-pot synthesis of amphiphilic hyperbranched copolymers, Polymer, 2018, 158, 90–95 CrossRef CAS.
- X. Xiong, W. Liu, Y. Luan, J. Du, Z. Wu and H. Chen, A Versatile, Fast, and Efficient Method of Visible-Light-Induced Surface Grafting Polymerization, Langmuir, 2014, 30, 5474–5480 CrossRef CAS PubMed.
- X. Xiong, Z. Wu, J. Pan, L. Xue, Y. Xu and H. Chen, A facile approach to modify poly(dimethylsiloxane) surfaces via visible light-induced grafting polymerization, J. Mater. Chem. B, 2015, 3, 629–634 RSC.
- M. Ciftci, P. Batat, A. L. Demirel, G. Xu, M. Buchmeiser and Y. Yagci, Visible Light-Induced Grafting from Polyolefins, Macromolecules, 2013, 46, 6395–6401 CrossRef CAS.
- M. Ciftci, S. Kork, G. Xu, M. R. Buchmeiser and Y. Yagci, Polyethylene- g -poly(cyclohexene oxide) by Mechanistic Transformation from ROMP to Visible Light-Induced Free Radical Promoted Cationic Polymerization, Macromolecules, 2015, 48, 1658–1663 CrossRef CAS.
- K. Koumura, K. Satoh and M. Kamigaito, Mn2(CO)10-induced controlled/living radical copolymerization of vinyl acetate and methyl acrylate: Spontaneous formation of block copolymers consisting of gradient and homopolymer segments, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1343–1353 CrossRef CAS.
- M. Ciftci, M. A. Tasdelen and Y. Yagci, Macromolecular design and application using Mn2(CO)10-based visible light photoinitiating systems, Polym. Int., 2016, 65, 1001–1014 CrossRef CAS.
- Q. Liu, L. Liu, Y. Ma, C. Zhao and W. Yang, Visible light-induced controlled radical polymerization of methacrylates with perfluoroalkyl iodide as the initiator in conjugation with a photoredox catalyst fac-[Ir(ppy)]3, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 3283–3291 CrossRef CAS.
- B. P. Fors and C. J. Hawker, Control of a Living Radical Polymerization of Methacrylates by Light, Angew. Chem., Int. Ed., 2012, 51, 8850–8853 CrossRef CAS PubMed.
- J. Lalevée, H. Mokbel and J.-P. Fouassier, Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources, Molecules, 2015, 20, 7201–7221 CrossRef PubMed.
- A. E. Goetz and A. J. Boydston, Metal-Free Preparation of Linear and Cross-Linked Polydicyclopentadiene, J. Am. Chem. Soc., 2015, 137, 7572–7575 CrossRef CAS PubMed.
- K. A. Ogawa, A. E. Goetz and A. J. Boydston, Metal-Free Ring-Opening Metathesis Polymerization, J. Am. Chem. Soc., 2015, 137, 1400–1403 CrossRef CAS PubMed.
- P. Lu, N. M. Alrashdi and A. J. Boydston, Bidirectional metal-free ROMP from difunctional organic initiators, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2977–2982 CrossRef CAS.
- C. Theunissen, M. A. Ashley and T. Rovis, Visible-Light-Controlled Ruthenium-Catalyzed Olefin Metathesis, J. Am. Chem. Soc., 2019, 141, 6791–6796 CrossRef CAS PubMed.
- O. Eivgi, A. Vaisman, N. B. Nechmad, M. Baranov and N. G. Lemcoff, Latent Ruthenium Benzylidene Phosphite Complexes for Visible-Light-Induced Olefin Metathesis, ACS Catal., 2020, 10, 2033–2038 CrossRef CAS.
- K. A. Ogawa, A. E. Goetz and A. J. Boydston, Developments in Externally Regulated Ring-Opening Metathesis Polymerization, Synlett, 2016, 203–214 CAS.
- D. P. Mohite, Z. J. Larimore, H. Lu, J. T. Mang, C. Sotiriou-Leventis and N. Leventis, Monolithic Hierarchical Fractal Assemblies of Silica Nanoparticles Cross-Linked with Polynorbornene via ROMP: A Structure–Property Correlation from Molecular to Bulk through Nano, Chem. Mater., 2012, 24, 3434–3448 CrossRef CAS.
- M. Ochs, R. Mohammadi, N. Vogel and A. Andrieu-Brunsen, Wetting-Controlled Localized Placement of Surface Functionalities within Nanopores, Small, 2020, 16, e1906463 CrossRef PubMed.
- F. Krohm, J. Kind, R. Savka, M. Alcaraz Janßen, D. Herold, H. Plenio, C. M. Thiele and A. Andrieu-Brunsen, Photochromic spiropyran- and spirooxazine-homopolymers in mesoporous thin films by surface initiated ROMP, J. Mater. Chem. C, 2016, 4, 4067–4076 RSC.
- J. Elbert, J. Mersini, N. Vilbrandt, C. Lederle, M. Kraska, M. Gallei, B. Stühn, H. Plenio and M. Rehahn, Reversible Activity Modulation of Surface-Attached Grubbs Second Generation Type Catalysts Using Redox-Responsive Polymers, Macromolecules, 2013, 46, 4255–4267 CrossRef CAS.
- F. Ziegler, H. Kraus, M. J. Benedikter, D. Wang, J. R. Bruckner, M. Nowakowski, K. Weißer, H. Solodenko, G. Schmitz, M. Bauer, N. Hansen and M. R. Buchmeiser, Confinement Effects for Efficient Macrocyclization Reactions with Supported Cationic Molybdenum Imido Alkylidene N -Heterocyclic Carbene Complexes, ACS Catal., 2021, 11, 11570–11578 CrossRef CAS.
- F. Ziegler, T. Roider, M. Pyschik, C. P. Haas, D. Wang, U. Tallarek and M. R. Buchmeiser, Olefin Ring-closing Metathesis under Spatial Confinement and Continuous Flow, ChemCatChem, 2021, 13, 2234–2241 CrossRef CAS.
- F. Ziegler, J. Teske, I. Elser, M. Dyballa, W. Frey, H. Kraus, N. Hansen, J. Rybka, U. Tallarek and M. R. Buchmeiser, Olefin Metathesis in Confined Geometries: A Biomimetic Approach toward Selective Macrocyclization, J. Am. Chem. Soc., 2019, 141, 19014–19022 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |



