Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of spirocyclic 1,2-diamines by dearomatising intramolecular diamination of phenols†
Anthony
Aimon
 a,
Mark J.
Dow
a,
Mark J.
Dow
 a,
Abigail R.
Hanby
a,
Ephraim A.
Okolo
a,
Christopher M.
Pask
a,
Adam
Nelson
a,
Abigail R.
Hanby
a,
Ephraim A.
Okolo
a,
Christopher M.
Pask
a,
Adam
Nelson
 *ab and
Stephen P.
Marsden
*ab and
Stephen P.
Marsden
 *a
*a
aSchool of Chemistry, University of Leeds, Leeds, LS2 9JT, UK. E-mail: s.p.marsden@leeds.ac.uk; a.s.nelson@leeds.ac.uk
bAstbury Centre for Structural Molecular Biology, University of Leeds, Leeds, LS2 9JT, UK
First published on 12th December 2022
Abstract
The stereocontrolled synthesis of complex spirotricyclic systems containing an embedded syn-1,2-diaminocyclohexane unit is reported, based upon a dearomatising oxidation of phenols bearing pendant ureas capable of acting as double nucleophiles. This complexity-generating transformation yields products with rich functionality suitable for application in the synthesis of potentially bioactive compounds.
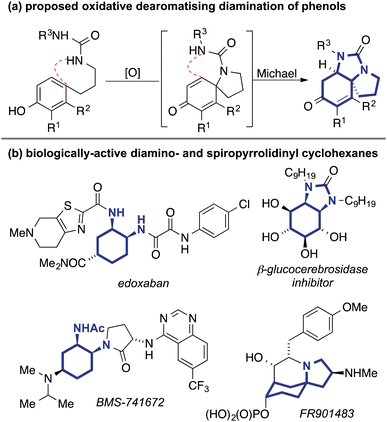
Dearomatisation reactions can be powerful tools to rapidly increase molecular complexity and access highly three-dimensional, sp3-rich molecular scaffolds from simple sp2-rich monocyclic precursors.1,2 In order to create diverse sp3-rich scaffolds for the synthesis of lead-like molecules3 and fragments,4 we were interested in developing a method that would allow the concise synthesis of highly functionalised 1,2-diaminocyclohexanes, motifs that are embedded in a wide range of natural and non-natural bioactive skeleta.
The formation of nitrogen-containing spirocycles by dearomatisation reactions5 may be triggered by reaction of phenol with external oxidants6 or electrophilic reagents7 followed by attack by a pendant nitrogen nucleophile, or alternatively by direct intramolecular attack of the arene on an electrophilic nitrogen species.8 We envisioned that by performing an oxidative cyclisation using a urea as the nucleophile, we might be able to effect a subsequent aza-Michael addition on the resulting spirocyclic dienone derivative, leading to formation of a spirotricyclic derivative containing an embedded 1,2-diamine in a single operation (Fig. 1a). While the intramolecular aza-Michael reaction of amides, carbamates and sulfonamides to cyclohexadienones has been widely exploited in biomimetic and other syntheses,9,10 there is to our knowledge only one example of a urea acting as a nucleophile, and this involved sequential dearomatisation and urea formation/asymmetric cyclisation reactions.11
The intended products contain substructures that are embedded in a number of biologically-relevant skeleta. Vicinal 1,2-diamines are common motifs in many drugs and functional molecules,12 and the 1,2-diaminocyclohexane structure is found in small molecule bioactives such as the anti-coagulant edoxaban13 and the CCR2 antagonist BMS-74167214 (Fig. 1b), while derived ureas15 show activity as, for example, inhibitors of β-glucocerebrosidase.16 Additionally, the tricycles contain an embedded 1-azaspiro[4.5]decane, a structure found in myriad bioactive products17,18 such as the immunosuppressive agent FR-901483.19 We therefore envisaged that the spirotricyclic products might be useful starting points for the synthesis of potentially bioactive compounds, and report herein their successful synthesis through the single-step oxidative dearomatisation.
Investigations started by subjecting the (4-hydroxyphenylpropyl) urea 1a to proposed dearomatisation conditions, using phenyliodine(III) bis(acetate)/bis(trifluoroacetate) (PIDA/PIFA respectively) as oxidants (Table 1) in hexafluoroisopropanol (HFIP).6b,e While no reaction occurred with PIDA under the conditions shown, we were pleased to see that using PIFA direct conversion to the desired tricyclic structure 2a was observed by NMR analysis (entry 2). The reaction was also successful in other fluorinated solvents (entries 3 and 4) and while lower conversions were observed in cheaper non-fluorinated solvents (entries 5 and 6), the use of a mixed HFIP/dichloromethane solvent system at higher (0.2 M) concentration gave acceptable yields. The reaction was carried out on a 7 mmol preparative scale to yield 2a in 68% isolated yield (>1 g).
| Entrya | I(III) source | Solvent | Yieldb (%) |
|---|---|---|---|
| a Method: substrate (1.0 eq.), PIDA/PIFA (1.1 eq.), 0.1 M in solvent, 0 °C (2 h) then rt. b NMR conversion. c Reaction carried out at 0.2 M concentration. d Isolated yield, 7 mmol scale. | |||
| 1 | PhI(OAc)2 | HFIP | 0 |
| 2 | PhI(O2CCF3)2 | HFIP | 49 |
| 3 | PhI(O2CCF3)2 | TFE | 48 |
| 4 | PhI(O2CCF3)2 | TFA | 56 |
| 5 | PhI(O2CCF3)2 | MeCN | 25 |
| 6 | PhI(O2CCF3)2 | DCM | 12 |
| 7 | PhI(O2CCF3)2 | HFIP/DCMc | 60 (68)d |
The substrate scope of the reaction was then probed, and the results are shown Table 2. We first examined the effect of different urea substituents using ureas 1b–j, typically prepared from the reaction between 3-(4-hydroxyphenyl)propylamine and the relevant isocyanate (ESI†). Under the optimised reaction conditions, a range of N-alkyl ureas gave the tricyclic derivatives 2b–2e in moderate to good yield (panel a). The presence of an electron-withdrawing N-sulfonyl group resulted in a slightly lower yield (2f, 23%), but pleasingly the primary urea 1g cyclised to give the free N–H tricyclic urea 2g in 64% yield. Finally, a range of N-aryl urea substituents were also tolerated in the reaction, yielding tricycles 2h–j. The compounds were formed exclusively as the cis-fused ureas in all cases, as expected: the structure of 2f was confirmed by X-ray crystallography (ESI†).
Variation in the phenolic component was next investigated (panel b). The use of 3-methoxy-4-hydroxyphenyl groups also gave good yields of the tricyclic products 2k–2n; notably these were formed as single regioisomers, with Michael addition occurring at the more electrophilic alkene in the presumed intermediate cyclohexadienone. Hydroxynaphthalenes could also be cyclised effectively, leading to polycyclic tetralone derivatives 2o–r. Finally, cyclisation of an 8-hydroxytetrahydroquinoline-containing substrate gave the tetracycle enamine 2s in good yield.
We also investigated the behaviour of some alternative substrates (Scheme 1). Activation of ortho-substituted phenol 3 with PIFA under the standard conditions did not produce a regioisomeric spirocycle to products 2, but rather gave fused tricyclic urea 4 in 19% yield. The precise order of events leading to 4 is unknown, but such motifs have previously been prepared by intramolecular oxidative cyclisation of tetrahydroquinolinyl ureas using hypervalent iodine reagents.20
The potential to employ nucleophiles other than ureas in the Michael addition step was also probed. Attempted cyclisation of the thiourea variant of 2a or of Boc-glycinyl amides (in place of the urea, designed to give a six-membered ring in the Michael addition) both gave complex mixtures, but success was achieved using β-amidoester 5.21 Oxidation to the spirocyclodienone occurred smoothly but the expected conjugate addition did not occur under the mildly acidic reaction conditions; instead, cyclisation could be effected as a separate step under basic conditions using cesium carbonate. The product was again formed as a single regioisomer via attack at the less-substituted alkene, and as a single diastereomer whose stereochemistry was determined by X-ray crystallography (ESI†).
The products of the oxidative dearomatising diamination are richly-functionalised small scaffolds, and we show some illustrative functional group transformations on these products in Scheme 2. Reduction of the alkene function of the enone may be achieved either by conjugate reduction with triethylsilane and Wilkinson's catalyst (to give 7), or by hydrogenation with Pd(OH)2/C (to give 8 or 9). Reduction of the resulting ketones could be achieved with LiAlH4 but was found to be more diastereoselective using NaBH4 and CeCl3 at low temperature (e.g. d.r. of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 vs. 100
7 vs. 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 for formation of 10). Stereoselective installation of an amine group was effected by sequential one-pot imine formation/reduction to give 13. Highly stereoselective reduction of enone 2c to an allylic alcohol can be achieved under Luche conditions to give 14. Finally, cleavage of the N-tosyl urea can be achieved by treatment of the saturated ketone 8 with sodium methoxide, giving spirobicyclic product 15. The stereochemistry of compounds 8, 14 and a crystalline derivative of 10 was confirmed by X-ray crystallography, while that of 13 was confirmed by nOe experiments (ESI†).
0 for formation of 10). Stereoselective installation of an amine group was effected by sequential one-pot imine formation/reduction to give 13. Highly stereoselective reduction of enone 2c to an allylic alcohol can be achieved under Luche conditions to give 14. Finally, cleavage of the N-tosyl urea can be achieved by treatment of the saturated ketone 8 with sodium methoxide, giving spirobicyclic product 15. The stereochemistry of compounds 8, 14 and a crystalline derivative of 10 was confirmed by X-ray crystallography, while that of 13 was confirmed by nOe experiments (ESI†).
In summary we have described and exemplified a new one-pot intramolecular dearomatisation/aza-Michael process that installs a cis-1,2-diaminocyclohexane motif that can be readily further functionalized. The rapid generation of structural and stereochemical complexity from simple achiral precursors reinforces the power of dearomatisation reactions in synthesis. The small polycyclic products have the potential to act as scaffolds for the synthesis of putative bioactive compounds: over 400 compounds based on scaffolds such as 13 have been prepared and contributed to the Public Compound Collection of the Joint European Compound Library within the European Lead Factory project,22 and results based on our own exploitation of these scaffolds will be reported in due course.
We acknowledge support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115489, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies in kind contribution. We also acknowledge funding from the Tertiary Education Trust Fund (TETFund) of Nigeria for a PhD scholarship (to EAO), the Royal Society of Chemistry (Undergraduate Research Bursary to ARH) and EPSRC for an Established Career Fellowship (to A. N.; EP/N025652/1).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. P. Roche and J. A. Porco Jr., Angew. Chem., Int. Ed., 2011, 50, 4068–4093 CrossRef CAS PubMed; (b) F. López Ortiz, M. J. Iglesias, I. Fernández, C. M. Andújar Sánchez and G. Ruiz Gómez, Chem. Rev., 2007, 107, 1580–1691 CrossRef PubMed; (c) A. Pape, K. P. Kaliappan and E. P. Kündig, Chem. Rev., 2000, 100, 2917–2940 CrossRef CAS PubMed.
- Dearoamative diamination via formal [4+2] cycloadditions: (a) 1,2-diamination: C. W. Davis, T. W. Bingham, M. Okumura and D. Sarlah, Synthesis, 2021, 4290–4296 CAS; (b) 1,4-diamination: W. C. Wertjes, M. Okumura and D. Sarlah, J. Am. Chem. Soc., 2019, 141, 163–167 CrossRef CAS PubMed.
- (a) Review: A. Nadin, C. Hattotuwagama and I. Churcher, Angew. Chem., Int. Ed., 2012, 51, 1114–1122 CrossRef CAS PubMed . For recent examples, see: ; (b) M. Uguen, G. Davison, L. J. Sprenger, J. H. Hunter, M. P. Martin, S. Turberville, J. E. Watt, B. T. Golding, M. E. M. Noble, H. L. Stewart and M. J. Waring, J. Med. Chem., 2022, 65, 11322–11399 CrossRef CAS PubMed; (c) S. M. Wales, E. G. Merisor, H. V. Adcock, C. A. Pearce, I. R. Strutt, W. Lewis, D. Hamza and C. J. Moody, Chem. – Eur. J., 2018, 24, 8233–8239 CrossRef CAS PubMed; (d) D. J. Foley, P. G. E. Craven, P. M. Collins, R. G. Doveston, A. Aimon, R. Talon, I. Churcher, F. von Delft, S. P. Marsden and A. Nelson, Chem. – Eur. J., 2017, 23, 15227–15232 CrossRef CAS PubMed.
- (a) Review of sp3-rich fragments: A. D. Morley, A. Pugliese, K. Birchall, J. Bower, P. Brennan, N. Brown, T. Chapman, M. Drysdale, I. H. Gilbert, S. Hoelder, A. Jordan, S. V. Ley, A. Merritt, D. Miller, M. E. Swarbrick and P. G. Wyatt, Drug Discovery Today, 2013, 18, 1221–1227 CrossRef PubMed; (b) For some recent examples, see: T. D. Downes, S. P. Jones, H. F. Klein, M. C. Wheldon, M. Atobe, P. S. Bond, J. D. Firth, N. S. Chan, L. Waddelove, R. E. Hubbard, D. C. Blakemore, C. de Fusco, S. D. Roughley, L. R. Vidler, M. A. Whatton, A. J.-A. Woolford, G. L. Wrigley and P. O’Brien, Chem. – Eur. J., 2020, 26, 8969–8975 CrossRef CAS PubMed; (c) N. S. Troelsen, E. Shanina, D. Gonzalez-Romero, D. Dankova, I. S. A. Jensen, K. J. Sniady, F. Nami, H. Zhang, C. Rademacher, A. Cuenda, C. H. Gotfredsen and M. H. Clausen, Angew. Chem., Int. Ed., 2020, 132, 2224–2230 CrossRef; (d) A. Sveiczer, A. J. P. North, N. Mateu, S. L. Kidd, H. F. Sore and D. R. Spring, Org. Lett., 2019, 21, 4600–4604 CrossRef CAS PubMed.
- C. Jing, J. J. Farndon and J. F. Bower, Chem. Rec., 2021, 21, 2909–2926 CrossRef CAS PubMed.
- Initial reports: (a) N. A. Braun, M. A. Ciufolini, K. Peters and E.-M. Peters, Tetrahedron Lett., 1998, 39, 4667–4670 CrossRef CAS; (b) G. Scheffler, H. Seike and E. J. Sorensen, Angew. Chem., Int. Ed., 2000, 39, 4593–4596 CrossRef CAS ; For more recent examples, see: ; (c) H. Liang and M. A. Ciufolini, Chem. – Eur. J., 2010, 16, 13262–13270 CrossRef CAS PubMed; (d) T. Kasahara and M. A. Ciufolini, Can. J. Chem., 2013, 91, 82–90 CrossRef CAS; (e) M. Paladino, J. Zaifman and M. A. Ciufolini, Org. Lett., 2015, 17, 3422–3425 CrossRef CAS PubMed; (f) N. Jain and M. A. Ciufolini, Synlett, 2015, 631–634 CAS; (g) D. Sarkar and N. Rout, Org. Lett., 2019, 21, 4132–4136 CrossRef CAS PubMed.
- S. R. Sahoo and D. Sarkar, Eur. J. Org. Chem., 2020, 397–401 CrossRef CAS.
- For select recent examples, see: (a) Z.-L. Xia, C. Zheng, R.-Q. Xu and S.-L. You, Nat. Commun., 2019, 10, 3150 CrossRef PubMed; (b) B.-H. Zhu, W.-T. Guo, Q. Sun, P.-C. Qian, L.-W. Ye and L. Li, Adv. Synth. Catal., 2022, 364, 314–318 CrossRef CAS; (c) J. J. Farndon, X. Ma and J. F. Bower, J. Am. Chem. Soc., 2017, 139, 14005–14008 CrossRef CAS PubMed; (d) X. Ma, J. J. Farndon, T. A. Young, N. Fey and J. F. Bower, Angew. Chem., Int. Ed., 2017, 56, 14531–14535 CrossRef CAS PubMed; (e) E. Lee, Y. Hwang, Y. B. Kim, D. Kim and S. Chang, J. Am. Chem. Soc., 2021, 143, 6363–6369 CrossRef CAS PubMed; (f) V. K. Soni, H. S. Hwang, Y. K. Moon, S.-W. Park, Y. You and E. J. Cho, J. Am. Chem. Soc., 2019, 141, 10538–10545 CrossRef CAS PubMed.
- (a) P. Wipf and Y. Kim, Tetrahedron Lett., 1992, 33, 5477–5480 CrossRef CAS; (b) K. Endo, K. Seya and H. Hikino, J. Chem. Soc., Chem. Commun., 1988, 934–935 RSC; (c) Selected examples: T. T. Kim, C. Lee, S. Heo, H.-S. Lee and S. Han, Chem. – Asian J., 2021, 16, 3882–3885 CrossRef CAS PubMed; (d) M. A. Beaulieu, X. Ottenwaelder and S. Canesi, Chem. – Eur. J., 2014, 20, 7581–7584 CrossRef CAS PubMed.
- Selected recent examples on skeleta other than tyrosine, see: (a) K. Hosoya, K. Iida, M. Odagi and K. Nagasawa, J. Org. Chem., 2020, 85, 11980–11988 CAS; (b) J. A. Rossi-Ashton, A. K. Clarke, R. J. K. Taylor and W. P. Unsworth, Org. Lett., 2020, 22, 1175–1181 CrossRef CAS PubMed; (c) M. Odagi, Y. Yamamoto and K. Nagasawa, Angew. Chem., Int. Ed., 2018, 57, 2229–2232 CrossRef CAS PubMed; (d) J.-Y. Du, C. Zeng, X.-J. Han, H. Qu, X.-H. Zhao, X.-T. An and C. A. Fan, J. Am. Chem. Soc., 2015, 137, 4267–4273 CrossRef CAS PubMed.
- K.-W. Hu, X. You, J.-Z. Wang, X. Wen, H. Sun, Q.-L. Xu and Z. Lai, Org. Lett., 2021, 23, 7873–7877 CrossRef CAS PubMed.
- D. Lucet, T. Le Gall and C. Mioskowski, Angew. Chem., Int. Ed., 1998, 37, 2580–2627 CrossRef CAS PubMed.
- T. Furugohri, K. Isobe, Y. Honda, C. Kamisato-Matsumoto, N. Sugiyama, T. Nagahara, Y. Morishima and T. Shibano, J. Thromb. Haemostasis, 2008, 6, 1542–1549 CAS.
- M. G. Yang, Z. Xiao, R. J. Cherney, A. J. Tebben, D. G. Batt, G. D. Brown, J. Chen, M. E. Cvijic, M. Dabros, J. V. Duncia, M. Galella, D. S. Gardner, P. Khandelwal, S. S. Ko, M. F. Malley, R. Mo, J. Pang, A. V. Rose, J. B. Santella, H. Shi, A. Srivastava, S. C. Traeger, B. Wang., S. Xu, R. Zhao, J. C. Barrish, S. Mandlekar, Q. Zhao and P. H. Carter, ACS Med. Chem. Lett., 2019, 10, 300–305 CrossRef CAS PubMed.
- A. K. Ghosh and M. Brindisi, J. Med. Chem., 2020, 63, 2751–22788 CrossRef CAS PubMed.
- A. Trapero and A. Llebaria, ACS Med. Chem. Lett., 2011, 2, 614–619 CrossRef CAS PubMed.
- (a) K. Hiesinger, D. Dar’in, E. Proschak and M. Krasavin, J. Med. Chem., 2021, 64, 150–183 CrossRef CAS PubMed; (b) Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS PubMed.
- G. Dake, Tetrahedron, 2006, 62, 3467–3492 CrossRef CAS.
- K. Sakamoto, E. Tsujii, F. Abe, T. Nakanishi, M. Yamashita, N. Shigematsu, S. Izumi and M. Okuhara, J. Antibiot., 1996, 49, 37–44 CrossRef CAS PubMed.
- (a) O. Familiar, H. Munier-Lehmann, J. A. Aínsa, M.-J. Camarasa and M.-J. Pérez-Pérez, Eur. J. Med. Chem., 2010, 45, 5910–5918 CrossRef CAS PubMed; (b) A. G. Romero, W. H. Darlington, E. J. Jacobsen and J. W. Mickelson, Tetrahedron Lett., 1996, 37, 2361–2364 CrossRef CAS; (c) K. Yasuo and K. Masami, Chem. Lett., 1990, 581–582 Search PubMed.
- For an intramolecular Michael addition of a dicarbonyl to a spirocyclic cyclohexadienone, see: J. Liang, J. Chen, J. Liu, L. Li and H. Zhang, Chem. Commun., 2010, 46, 3666–3668 RSC.
- R. Morgentin, M. Dow, A. Aimon, G. Karageorgis, T. Kalliokoski, D. Roche, S. Marsden and A. Nelson, Drug Discovery Today, 2018, 23, 1578–1583 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full preparative experimental procedures and compound characterisation data for all numbered compounds presented in the paper; copies of 1H and 13C NMR spectra of all compounds; and X-ray crystallographic data for relevant compounds. CCDC 2174965, 2174962, 2174967, 2175094 and 2174963. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06137f |
| This journal is © The Royal Society of Chemistry 2023 |